 Found 21785 hits with Last Name = 'hou' and Initial = 'j'
Found 21785 hits with Last Name = 'hou' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1B
(GUINEA PIG) | BDBM50590649
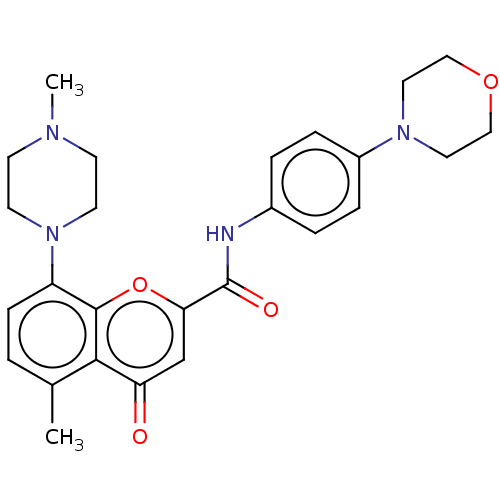
(CHEMBL5209320)Show SMILES Cc1ccc(N2CCN([11CH3])CC2)c2oc(cc(=O)c12)C(=O)Nc1ccc(cc1)N1CCOCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCL2 (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50507816
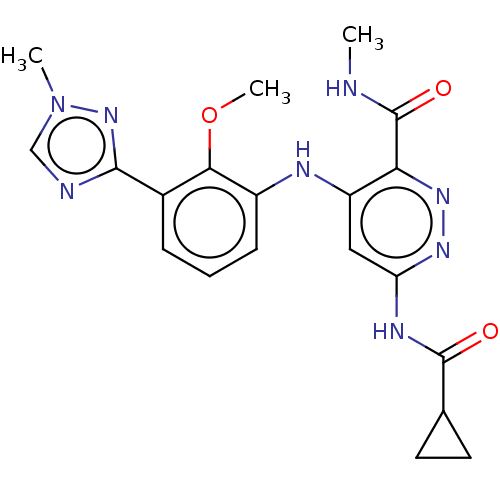
(Bms-986165 | Deucravacitinib)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1cccc(-c2ncn(C)n2)c1OC Show InChI InChI=1S/C20H22N8O3/c1-21-20(30)16-14(9-15(25-26-16)24-19(29)11-7-8-11)23-13-6-4-5-12(17(13)31-3)18-22-10-28(2)27-18/h4-6,9-11H,7-8H2,1-3H3,(H,21,30)(H2,23,24,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 in human Jurkat cells assessed as reduction in IFN-alpha stimulated TYK2 phosphorylation by caspase3/7 reagent-based Western blot ... |
Eur J Med Chem 163: 413-427 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.072
BindingDB Entry DOI: 10.7270/Q2736V7K |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50400098
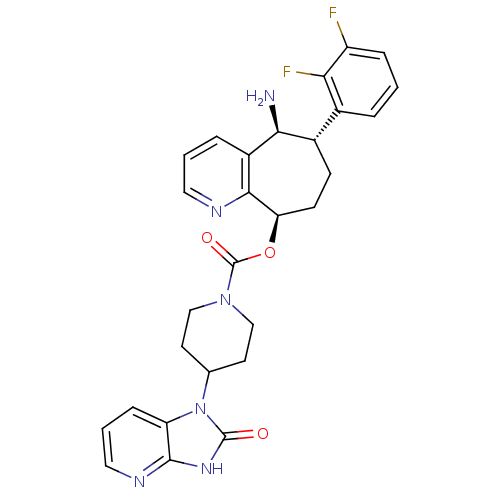
(CHEMBL2178422)Show SMILES N[C@H]1[C@@H](CC[C@@H](OC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)c2ncccc12)c1cccc(F)c1F |r| Show InChI InChI=1S/C28H28F2N6O3/c29-20-6-1-4-17(23(20)30)18-8-9-22(25-19(24(18)31)5-2-12-32-25)39-28(38)35-14-10-16(11-15-35)36-21-7-3-13-33-26(21)34-27(36)37/h1-7,12-13,16,18,22,24H,8-11,14-15,31H2,(H,33,34,37)/t18-,22+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis |
J Med Chem 55: 10644-51 (2012)
Article DOI: 10.1021/jm3013147
BindingDB Entry DOI: 10.7270/Q2M046M8 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50576898
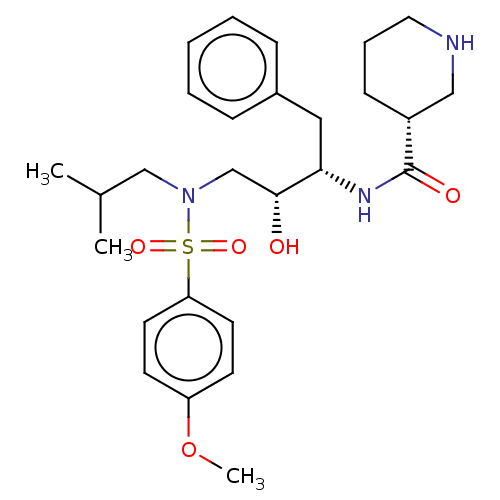
(CHEMBL4877646)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCNC1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113450
BindingDB Entry DOI: 10.7270/Q2571GTH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514220
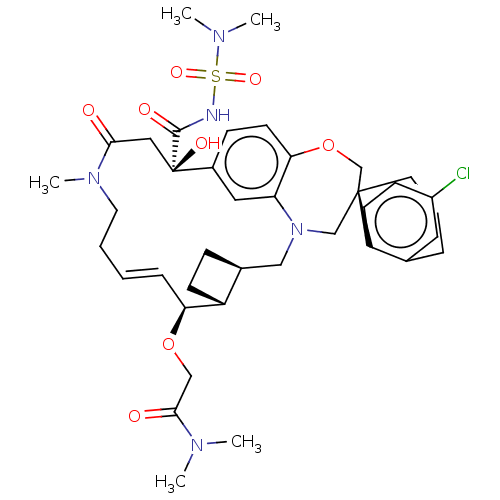
(CHEMBL4535151 | US11274105, Example 188)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCC(=O)N(C)C)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:15| Show InChI InChI=1S/C39H52ClN5O8S/c1-42(2)36(47)23-52-33-10-6-7-18-44(5)35(46)21-39(49,37(48)41-54(50,51)43(3)4)28-12-16-34-32(20-28)45(22-27-11-14-30(27)33)24-38(25-53-34)17-8-9-26-19-29(40)13-15-31(26)38/h6,10,12-13,15-16,19-20,27,30,33,49H,7-9,11,14,17-18,21-25H2,1-5H3,(H,41,48)/b10-6+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50576910

(CHEMBL4862758)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN(C)C1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113450
BindingDB Entry DOI: 10.7270/Q2571GTH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50472582

(CHEMBL45422)Show SMILES CNc1nc(CNCC2(F)CCN(CC2)C(=O)c2ccc(F)c(Cl)c2)ccc1C Show InChI InChI=1S/C21H25ClF2N4O/c1-14-3-5-16(27-19(14)25-2)12-26-13-21(24)7-9-28(10-8-21)20(29)15-4-6-18(23)17(22)11-15/h3-6,11,26H,7-10,12-13H2,1-2H3,(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50590640

(CHEMBL5177580)Show SMILES COc1ccccc1N1CCN(CCCCc2ccc3n(C)c(=O)oc3c2)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514222
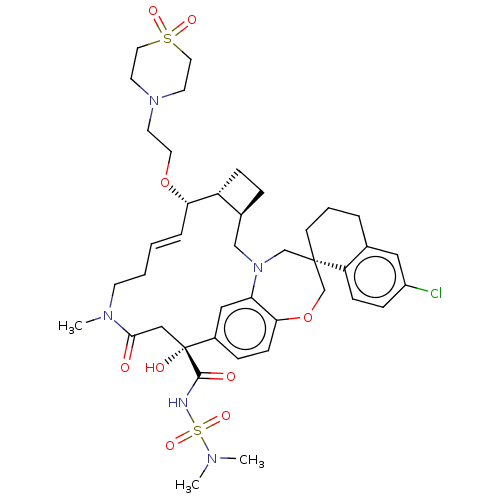
(CHEMBL4580244 | US11274105, Example 193)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCS(=O)(=O)CC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:20| Show InChI InChI=1S/C41H56ClN5O9S2/c1-44(2)58(53,54)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(55-20-17-46-18-21-57(51,52)22-19-46)33-12-9-30(33)26-47-27-40(28-56-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50274047

(CHEMBL3613796)Show SMILES OB(O)CNS(=O)(=O)c1ccc(cc1C(F)(F)F)-c1nnn[nH]1 Show InChI InChI=1S/C9H9BF3N5O4S/c11-9(12,13)6-3-5(8-15-17-18-16-8)1-2-7(6)23(21,22)14-4-10(19)20/h1-3,14,19-20H,4H2,(H,15,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCL School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli AmpC using CENTA as substrate by spectrometry based Lineweaver-Burk plot analysis |
Bioorg Med Chem 26: 2921-2927 (2018)
Article DOI: 10.1016/j.bmc.2018.04.055
BindingDB Entry DOI: 10.7270/Q25T3P0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514203
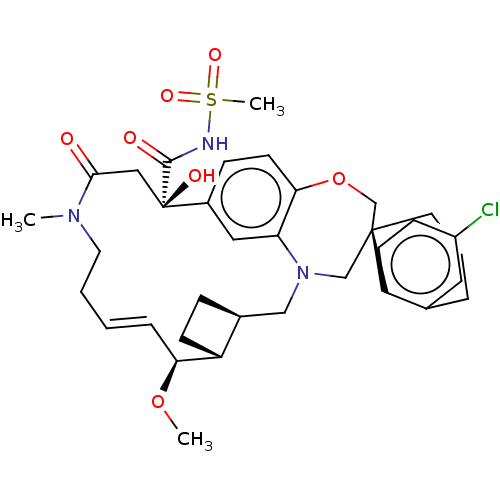
(CHEMBL4593361 | US11274105, Example 6)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(C)(=O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C35H44ClN3O7S/c1-38-16-5-4-8-30(45-2)27-12-9-24(27)20-39-21-34(15-6-7-23-17-26(36)11-13-28(23)34)22-46-31-14-10-25(18-29(31)39)35(42,19-32(38)40)33(41)37-47(3,43)44/h4,8,10-11,13-14,17-18,24,27,30,42H,5-7,9,12,15-16,19-22H2,1-3H3,(H,37,41)/b8-4+/t24-,27+,30-,34-,35+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514196
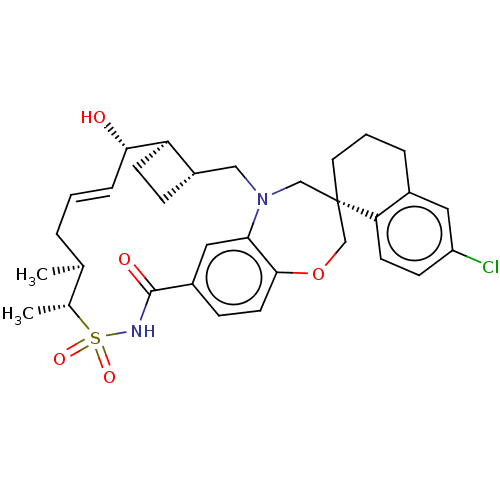
(CHEMBL4476472)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C32H39ClN2O5S/c1-20-5-3-7-29(36)26-11-8-24(26)17-35-18-32(14-4-6-22-15-25(33)10-12-27(22)32)19-40-30-13-9-23(16-28(30)35)31(37)34-41(38,39)21(20)2/h3,7,9-10,12-13,15-16,20-21,24,26,29,36H,4-6,8,11,14,17-19H2,1-2H3,(H,34,37)/b7-3+/t20-,21+,24-,26+,29-,32-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514202

(CHEMBL4446369 | US11274105, Example 179)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C40H52ClF2N5O7S/c1-45(2)56(52,53)44-37(50)40(51)21-36(49)46(3)16-5-4-8-34(54-18-17-47-24-39(42,43)25-47)31-12-9-28(31)22-48-23-38(26-55-35-14-10-29(40)20-33(35)48)15-6-7-27-19-30(41)11-13-32(27)38/h4,8,10-11,13-14,19-20,28,31,34,51H,5-7,9,12,15-18,21-26H2,1-3H3,(H,44,50)/b8-4+/t28-,31+,34-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50400099
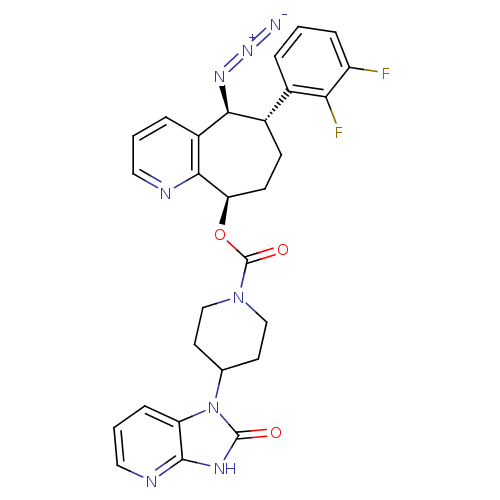
(CHEMBL2178420)Show SMILES Fc1cccc([C@@H]2CC[C@@H](OC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)c3ncccc3[C@H]2N=[N+]=[N-])c1F |r| Show InChI InChI=1S/C28H26F2N8O3/c29-20-6-1-4-17(23(20)30)18-8-9-22(25-19(5-2-12-32-25)24(18)35-36-31)41-28(40)37-14-10-16(11-15-37)38-21-7-3-13-33-26(21)34-27(38)39/h1-7,12-13,16,18,22,24H,8-11,14-15H2,(H,33,34,39)/t18-,22+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis |
J Med Chem 55: 10644-51 (2012)
Article DOI: 10.1021/jm3013147
BindingDB Entry DOI: 10.7270/Q2M046M8 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514199
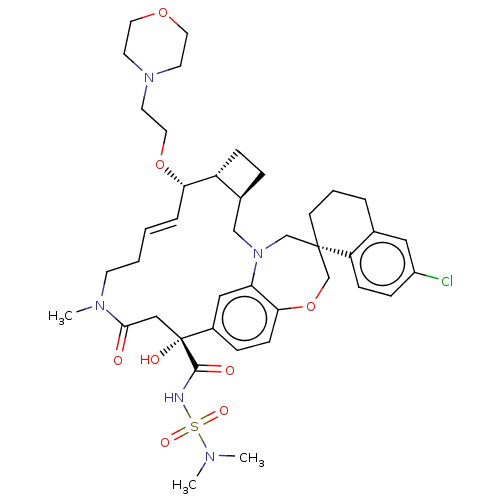
(CHEMBL4553660 | US11274105, Example 182)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CCOCC1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)25-38(48)45(3)16-5-4-8-36(54-22-19-46-17-20-53-21-18-46)33-12-9-30(33)26-47-27-40(28-55-37-14-10-31(41)24-35(37)47)15-6-7-29-23-32(42)11-13-34(29)40/h4,8,10-11,13-14,23-24,30,33,36,50H,5-7,9,12,15-22,25-28H2,1-3H3,(H,43,49)/b8-4+/t30-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514200
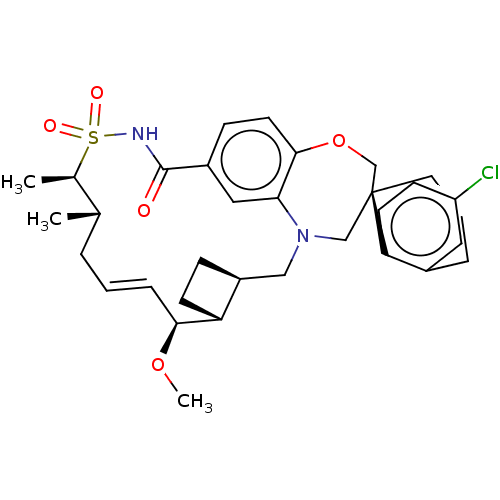
(CHEMBL4446378 | US10703733, Comparative Example 1)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\C[C@H](C)[C@@H](C)S(=O)(=O)NC(=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C33H41ClN2O5S/c1-21-6-4-8-30(40-3)27-12-9-25(27)18-36-19-33(15-5-7-23-16-26(34)11-13-28(23)33)20-41-31-14-10-24(17-29(31)36)32(37)35-42(38,39)22(21)2/h4,8,10-11,13-14,16-17,21-22,25,27,30H,5-7,9,12,15,18-20H2,1-3H3,(H,35,37)/b8-4+/t21-,22+,25-,27+,30-,33-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50241199
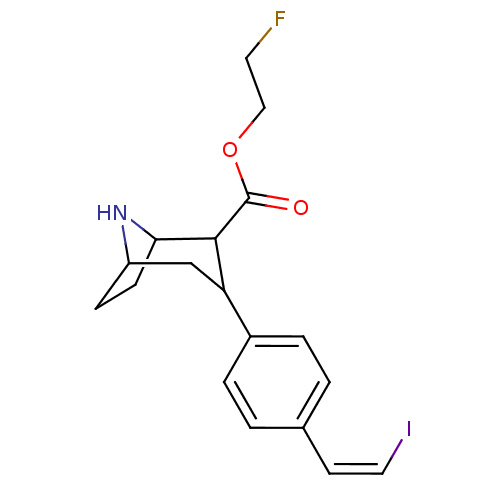
((Z)-2-[18F]fluoroethyl 3-(4-(2-iodovinyl)phenyl)-8...)Show SMILES FCCOC(=O)C1C2CCC(CC1c1ccc(\C=C/I)cc1)N2 |TLB:13:12:22:9.8,THB:4:6:22:9.8| Show InChI InChI=1S/C18H21FINO2/c19-8-10-23-18(22)17-15(11-14-5-6-16(17)21-14)13-3-1-12(2-4-13)7-9-20/h1-4,7,9,14-17,21H,5-6,8,10-11H2/b9-7- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H](R,S)citalopram.HBr from human SERT transfected in human HEK293 cells |
J Med Chem 51: 7788-99 (2008)
Article DOI: 10.1021/jm800781a
BindingDB Entry DOI: 10.7270/Q21C1WRQ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50219561
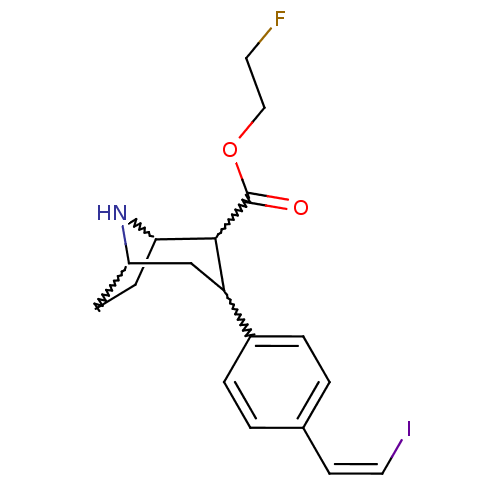
(2-beta-carbo(2-fluoroethoxy)-3-beta-(4'-((Z)-2-iod...)Show SMILES FCCOC(=O)C1C2CCC(CC1c1ccc(\C=C/I)cc1)N2 |w:10.9,12.13,7.24,6.5,TEB:4:6:8.9:22,13:12:8.9:22| Show InChI InChI=1S/C18H21FINO2/c19-8-10-23-18(22)17-15(11-14-5-6-16(17)21-14)13-3-1-12(2-4-13)7-9-20/h1-4,7,9,14-17,21H,5-6,8,10-11H2/b9-7- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Binding affinity to human SERT expressed in HEK293 cells |
J Med Chem 50: 4553-60 (2007)
Article DOI: 10.1021/jm061303s
BindingDB Entry DOI: 10.7270/Q2ZP45V0 |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50546262
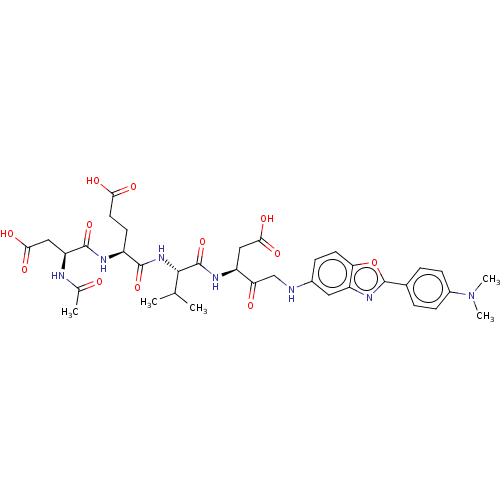
(CHEMBL4751195)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)CNc1ccc2oc(nc2c1)-c1ccc(cc1)N(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50400102

(CHEMBL2178424)Show SMILES O[C@H]1[C@@H](CC[C@@H](OC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)c2ncccc12)c1cccc(F)c1F |r| Show InChI InChI=1S/C28H27F2N5O4/c29-20-6-1-4-17(23(20)30)18-8-9-22(24-19(25(18)36)5-2-12-31-24)39-28(38)34-14-10-16(11-15-34)35-21-7-3-13-32-26(21)33-27(35)37/h1-7,12-13,16,18,22,25,36H,8-11,14-15H2,(H,32,33,37)/t18-,22+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from CGRP receptor in human SK-N-MC cells after 2 hrs by gamma scintillation counter analysis |
J Med Chem 55: 10644-51 (2012)
Article DOI: 10.1021/jm3013147
BindingDB Entry DOI: 10.7270/Q2M046M8 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514215
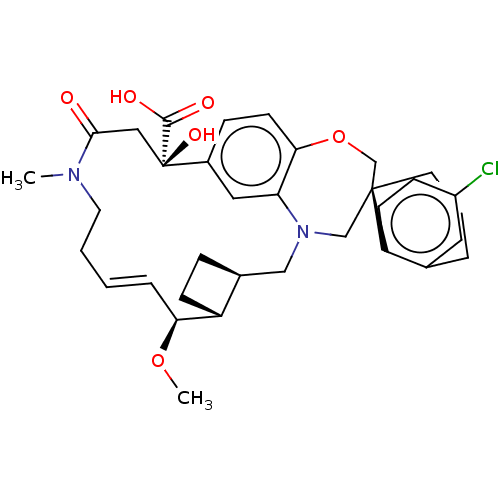
(CHEMBL4577379 | US11274105, Example 4)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-3-7-29(42-2)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(41,32(39)40)18-31(36)38/h3,7,9-10,12-13,16-17,23,26,29,41H,4-6,8,11,14-15,18-21H2,1-2H3,(H,39,40)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50541871
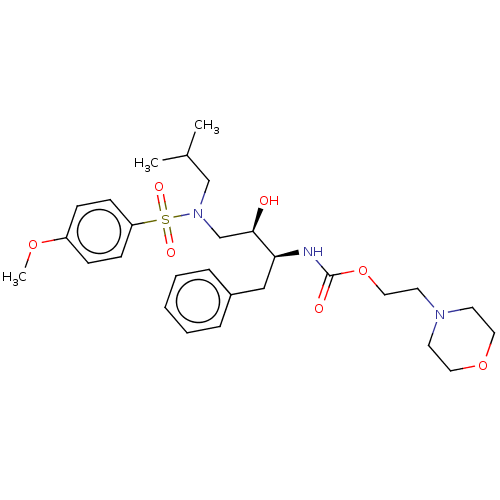
(CHEMBL4640442)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OCCN1CCOCC1 |r| Show InChI InChI=1S/C28H41N3O7S/c1-22(2)20-31(39(34,35)25-11-9-24(36-3)10-12-25)21-27(32)26(19-23-7-5-4-6-8-23)29-28(33)38-18-15-30-13-16-37-17-14-30/h4-12,22,26-27,32H,13-21H2,1-3H3,(H,29,33)/t26-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease expressed in Escherichia coli using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrat... |
ACS Med Chem Lett 11: 1196-1204 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00043
BindingDB Entry DOI: 10.7270/Q23200F2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50590648
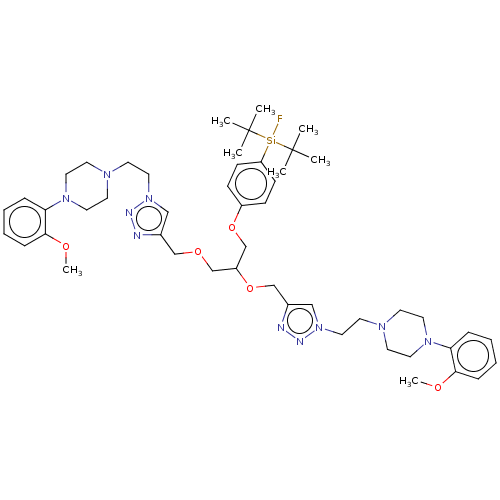
(CHEMBL5202019)Show SMILES [#6]-[#8]-c1ccccc1-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-n2cc(-[#6]-[#8]-[#6]-[#6](-[#6]-[#8]-c3ccc(cc3)[Si;v4]([18F])(C([#6])([#6])[#6])C([#6])([#6])[#6])-[#8]-[#6]-c3cn(-[#6]-[#6]-[#7]-4-[#6]-[#6]-[#7](-[#6]-[#6]-4)-c4ccccc4-[#8]-[#6])nn3)nn2)-[#6]-[#6]-1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514218
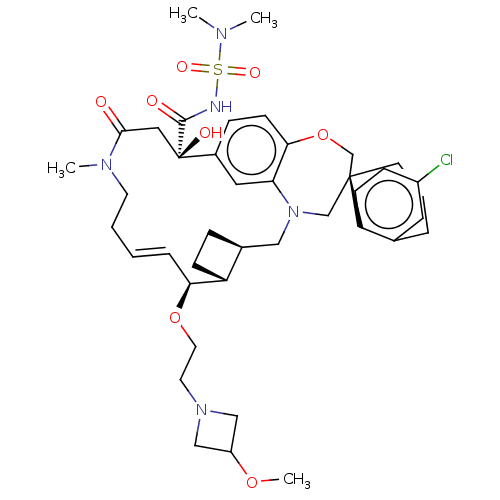
(CHEMBL4539543 | US11274105, Example 197)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(C1)OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:18| Show InChI InChI=1S/C41H56ClN5O8S/c1-44(2)56(51,52)43-39(49)41(50)22-38(48)45(3)17-6-5-9-36(54-19-18-46-24-32(25-46)53-4)33-13-10-29(33)23-47-26-40(27-55-37-15-11-30(41)21-35(37)47)16-7-8-28-20-31(42)12-14-34(28)40/h5,9,11-12,14-15,20-21,29,32-33,36,50H,6-8,10,13,16-19,22-27H2,1-4H3,(H,43,49)/b9-5+/t29-,33+,36-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514219

(CHEMBL4438074 | US11274105, Example 181)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCN1CC(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:17| Show InChI InChI=1S/C40H53ClFN5O7S/c1-44(2)55(51,52)43-38(49)40(50)21-37(48)45(3)16-5-4-8-35(53-18-17-46-23-31(42)24-46)32-12-9-28(32)22-47-25-39(26-54-36-14-10-29(40)20-34(36)47)15-6-7-27-19-30(41)11-13-33(27)39/h4,8,10-11,13-14,19-20,28,31-32,35,50H,5-7,9,12,15-18,21-26H2,1-3H3,(H,43,49)/b8-4+/t28-,32+,35-,39-,40+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514216
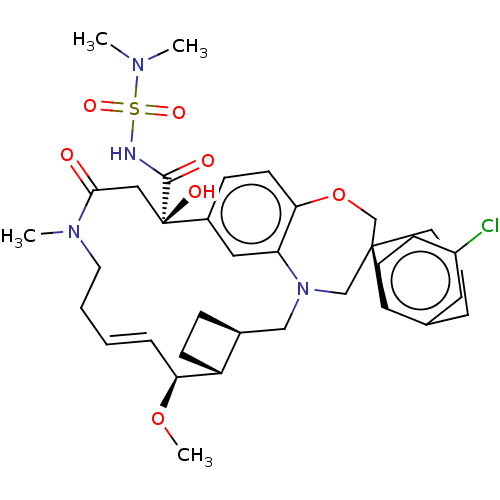
(CHEMBL4528051 | US11274105, Example 5)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OC)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:10| Show InChI InChI=1S/C36H47ClN4O7S/c1-39(2)49(45,46)38-34(43)36(44)20-33(42)40(3)17-6-5-9-31(47-4)28-13-10-25(28)21-41-22-35(23-48-32-15-11-26(36)19-30(32)41)16-7-8-24-18-27(37)12-14-29(24)35/h5,9,11-12,14-15,18-19,25,28,31,44H,6-8,10,13,16-17,20-23H2,1-4H3,(H,38,43)/b9-5+/t25-,28+,31-,35-,36+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514214

(CHEMBL4542646 | US11274105, Example 41)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C33H39ClN2O6/c1-35-14-3-2-6-28(37)25-10-7-22(25)18-36-19-32(13-4-5-21-15-24(34)9-11-26(21)32)20-42-29-12-8-23(16-27(29)36)33(41,31(39)40)17-30(35)38/h2,6,8-9,11-12,15-16,22,25,28,37,41H,3-5,7,10,13-14,17-20H2,1H3,(H,39,40)/b6-2+/t22-,25+,28-,32-,33+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514201

(CHEMBL4547370 | US11274105, Example 191)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCOCC(F)F)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:16| Show InChI InChI=1S/C39H51ClF2N4O8S/c1-44(2)55(50,51)43-37(48)39(49)21-36(47)45(3)16-5-4-8-33(53-18-17-52-23-35(41)42)30-12-9-27(30)22-46-24-38(25-54-34-14-10-28(39)20-32(34)46)15-6-7-26-19-29(40)11-13-31(26)38/h4,8,10-11,13-14,19-20,27,30,33,35,49H,5-7,9,12,15-18,21-25H2,1-3H3,(H,43,48)/b8-4+/t27-,30+,33-,38-,39+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50576913
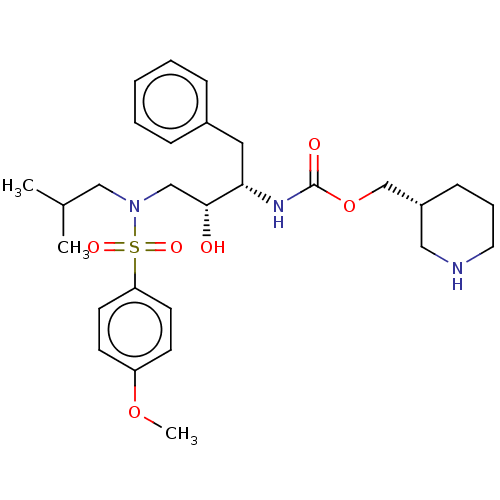
(CHEMBL4868812)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC[C@@H]1CCCNC1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113450
BindingDB Entry DOI: 10.7270/Q2571GTH |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514206
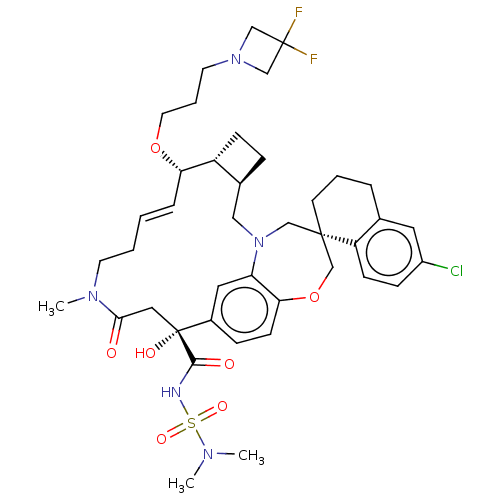
(CHEMBL4588330 | US11274105, Example 187)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](OCCCN1CC(F)(F)C1)\C=C\CCN(C)C(=O)C[C@](O)(C(=O)NS(=O)(=O)N(C)C)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:19| Show InChI InChI=1S/C41H54ClF2N5O7S/c1-46(2)57(53,54)45-38(51)41(52)22-37(50)47(3)17-5-4-9-35(55-19-7-18-48-25-40(43,44)26-48)32-13-10-29(32)23-49-24-39(27-56-36-15-11-30(41)21-34(36)49)16-6-8-28-20-31(42)12-14-33(28)39/h4,9,11-12,14-15,20-21,29,32,35,52H,5-8,10,13,16-19,22-27H2,1-3H3,(H,45,51)/b9-4+/t29-,32+,35-,39-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50438565
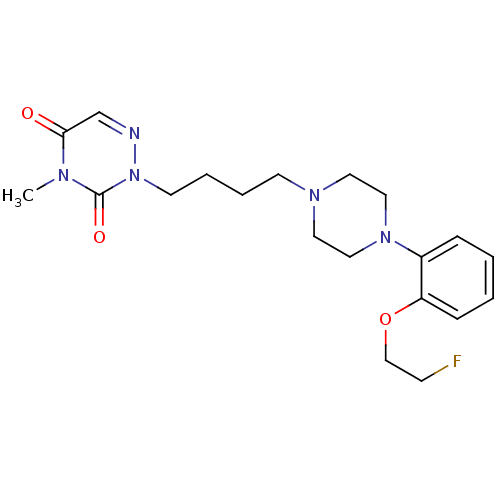
(CHEMBL2413153)Show SMILES Cn1c(=O)cnn(CCCCN2CCN(CC2)c2ccccc2OCCF)c1=O Show InChI InChI=1S/C20H28FN5O3/c1-23-19(27)16-22-26(20(23)28)10-5-4-9-24-11-13-25(14-12-24)17-6-2-3-7-18(17)29-15-8-21/h2-3,6-7,16H,4-5,8-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50039824
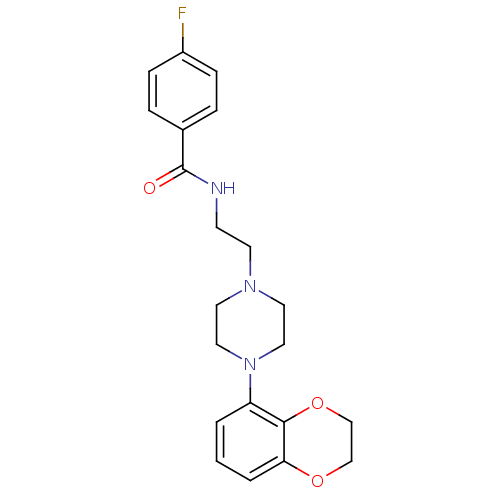
(CHEMBL81728 | N-{2-[4-(2,3-Dihydro-benzo[1,4]dioxi...)Show SMILES Fc1ccc(cc1)C(=O)NCCN1CCN(CC1)c1cccc2OCCOc12 Show InChI InChI=1S/C21H24FN3O3/c22-17-6-4-16(5-7-17)21(26)23-8-9-24-10-12-25(13-11-24)18-2-1-3-19-20(18)28-15-14-27-19/h1-7H,8-15H2,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50576900
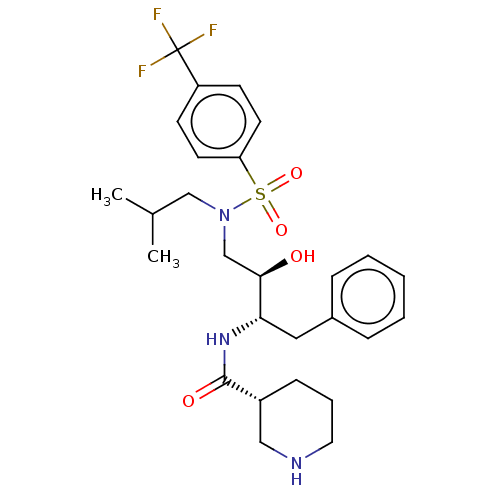
(CHEMBL4852688)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCNC1)S(=O)(=O)c1ccc(cc1)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113450
BindingDB Entry DOI: 10.7270/Q2571GTH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50092959
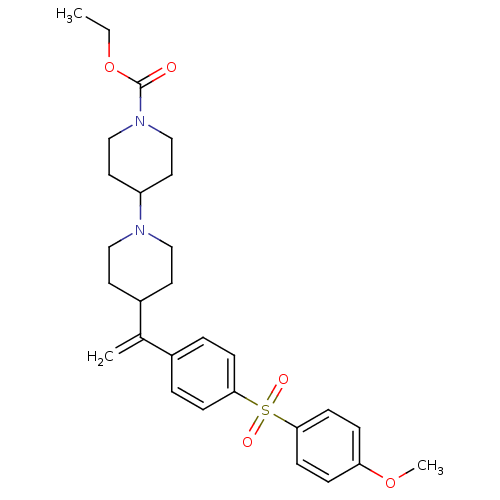
(4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...)Show SMILES CCOC(=O)N1CCC(CC1)N1CCC(CC1)C(=C)c1ccc(cc1)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C28H36N2O5S/c1-4-35-28(31)30-19-15-24(16-20-30)29-17-13-23(14-18-29)21(2)22-5-9-26(10-6-22)36(32,33)27-11-7-25(34-3)8-12-27/h5-12,23-24H,2,4,13-20H2,1,3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
Bioorg Med Chem Lett 17: 2260-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.058
BindingDB Entry DOI: 10.7270/Q2668H0S |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50514204
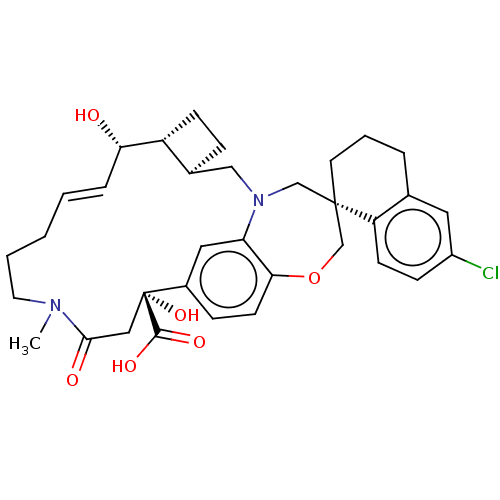
(CHEMBL4437832)Show SMILES [H][C@@]12CC[C@@]1([H])[C@@H](O)\C=C\CCCN(C)C(=O)C[C@](O)(C(O)=O)c1ccc3OC[C@]4(CCCc5cc(Cl)ccc45)CN(C2)c3c1 |r,t:9| Show InChI InChI=1S/C34H41ClN2O6/c1-36-15-4-2-3-7-29(38)26-11-8-23(26)19-37-20-33(14-5-6-22-16-25(35)10-12-27(22)33)21-43-30-13-9-24(17-28(30)37)34(42,32(40)41)18-31(36)39/h3,7,9-10,12-13,16-17,23,26,29,38,42H,2,4-6,8,11,14-15,18-21H2,1H3,(H,40,41)/b7-3+/t23-,26+,29-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His6x-tagged human Mcl-1 (171 to 327 residues) interaction with biotinylated human Bim (51 to 76 residues) incub... |
J Med Chem 62: 10258-10271 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01310
BindingDB Entry DOI: 10.7270/Q2TQ64WW |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at dopamine D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01192
BindingDB Entry DOI: 10.7270/Q2XD15CH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50113332
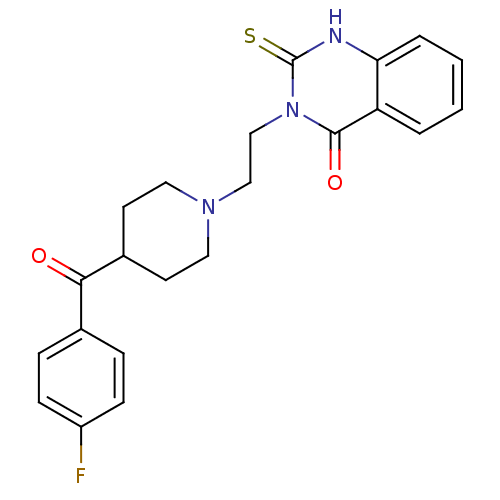
(3-(2-(4-(4-fluorobenzoyl)piperidin-1-yl)ethyl)-2-t...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=S)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O2S/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377656

(CHEMBL259534)Show SMILES Clc1ccc(NC(=O)c2ccccc2NC(=O)c2ccc(cc2)-n2ccccc2=O)nc1 Show InChI InChI=1S/C24H17ClN4O3/c25-17-10-13-21(26-15-17)28-24(32)19-5-1-2-6-20(19)27-23(31)16-8-11-18(12-9-16)29-14-4-3-7-22(29)30/h1-15H,(H,27,31)(H,26,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using acromogenic substrate S-2222 preincubated for 30 mins before substrate addition measured after 20 mins by spectr... |
Eur J Med Chem 95: 388-99 (2015)
Article DOI: 10.1016/j.ejmech.2015.03.052
BindingDB Entry DOI: 10.7270/Q29G5PH4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50182020
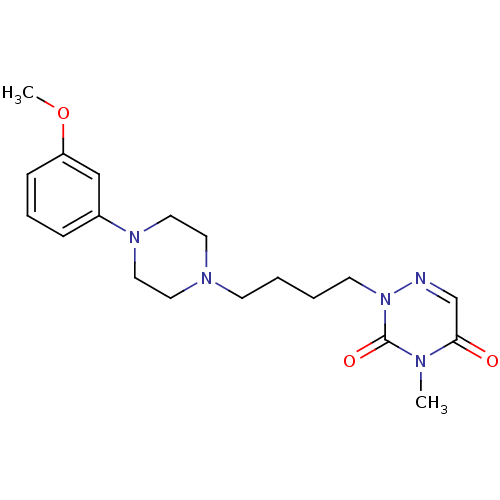
(2-(4-(4-(3-methoxyphenyl)piperazin-1-yl)butyl)-4-m...)Show SMILES COc1cccc(c1)N1CCN(CCCCn2ncc(=O)n(C)c2=O)CC1 Show InChI InChI=1S/C19H27N5O3/c1-21-18(25)15-20-24(19(21)26)9-4-3-8-22-10-12-23(13-11-22)16-6-5-7-17(14-16)27-2/h5-7,14-15H,3-4,8-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50092961
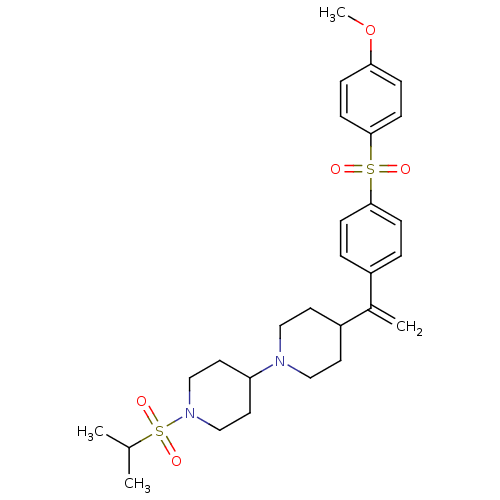
(1-(isopropylsulfonyl)-4-(4-(1-(4-(4-methoxyphenyls...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C(=C)C1CCN(CC1)C1CCN(CC1)S(=O)(=O)C(C)C Show InChI InChI=1S/C28H38N2O5S2/c1-21(2)37(33,34)30-19-15-25(16-20-30)29-17-13-24(14-18-29)22(3)23-5-9-27(10-6-23)36(31,32)28-11-7-26(35-4)8-12-28/h5-12,21,24-25H,3,13-20H2,1-2,4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
Bioorg Med Chem Lett 17: 2260-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.058
BindingDB Entry DOI: 10.7270/Q2668H0S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50011175
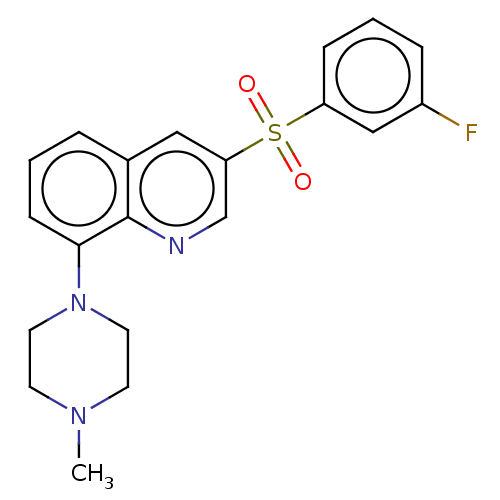
(CHEMBL3260313)Show SMILES CN1CCN(CC1)c1cccc2cc(cnc12)S(=O)(=O)c1cccc(F)c1 Show InChI InChI=1S/C20H20FN3O2S/c1-23-8-10-24(11-9-23)19-7-2-4-15-12-18(14-22-20(15)19)27(25,26)17-6-3-5-16(21)13-17/h2-7,12-14H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612995

(CHEMBL5276586)Show SMILES Cl.[H][C@@]1(OC(OC[C@H]1O)c1ccccc1[N+]([O-])=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50590673
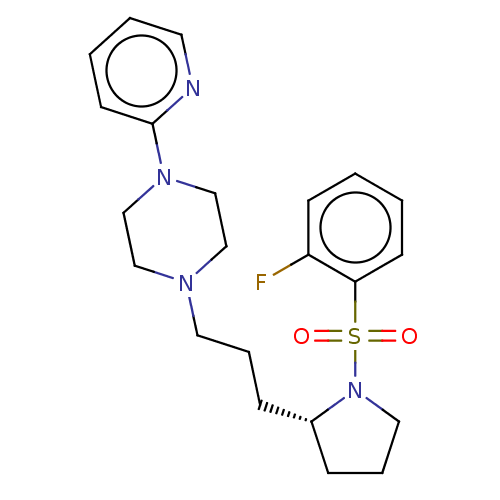
(CHEMBL5179460)Show SMILES Fc1ccccc1S(=O)(=O)N1CCC[C@@H]1CCCN1CCN(CC1)c1ccccn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113450
BindingDB Entry DOI: 10.7270/Q2571GTH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway
Curated by ChEMBL
| Assay Description
Displacement of [3H]OH-DPAT from human recombinant 5-HT1A receptor measured after 60 mins by scintillation counter method |
Eur J Med Chem 176: 292-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.064
BindingDB Entry DOI: 10.7270/Q2NP27V8 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612998
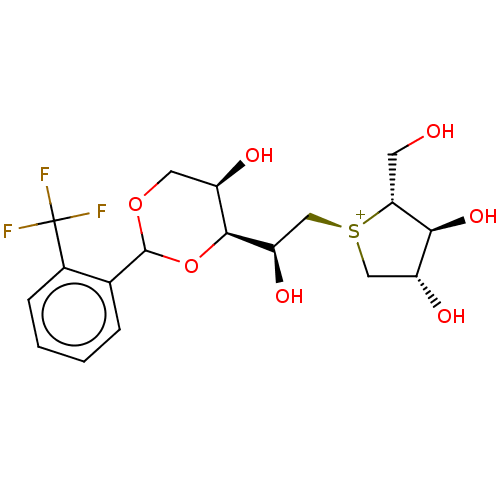
(CHEMBL5269400)Show SMILES [Cl-].[H][C@@]1(OC(OC[C@H]1O)c1ccccc1C(F)(F)F)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50576912
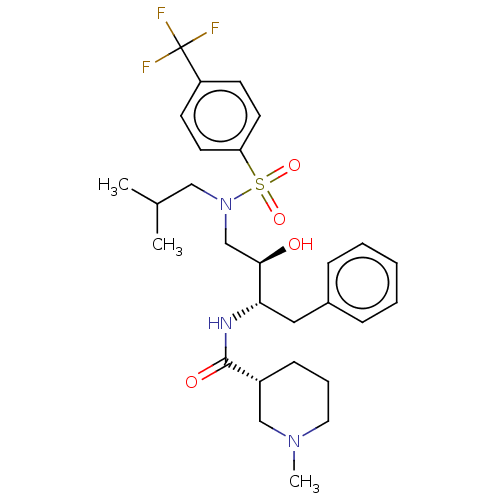
(CHEMBL4861507)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN(C)C1)S(=O)(=O)c1ccc(cc1)C(F)(F)F |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type HIV1 protease using Arg-Glu (EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-Lys(DABCYL)-Arg as substrate preincubated for 20 to 30 mi... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113450
BindingDB Entry DOI: 10.7270/Q2571GTH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50590637

(CHEMBL5189925) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00633
BindingDB Entry DOI: 10.7270/Q26M3BS8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data