

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50008805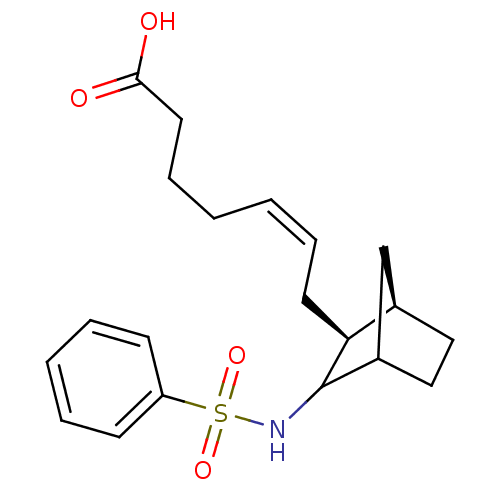 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (GUINEA PIG) | BDBM50008805 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50060462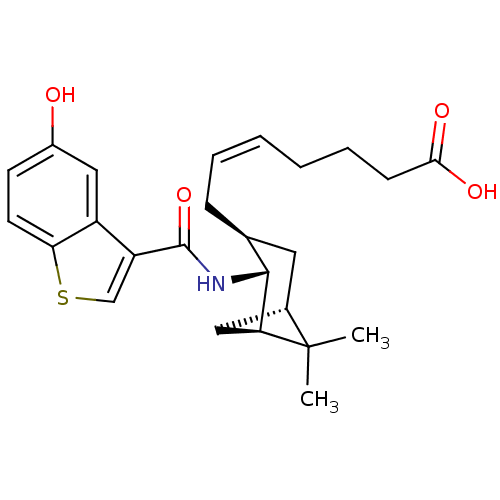 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (GUINEA PIG) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM85347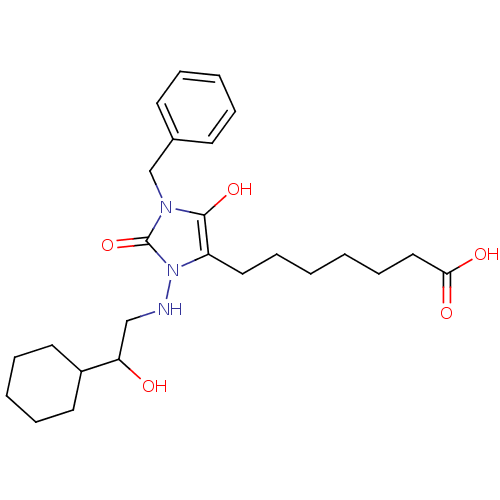 (BWA868C | CAS_122021 | NSC_122021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50008805 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50060458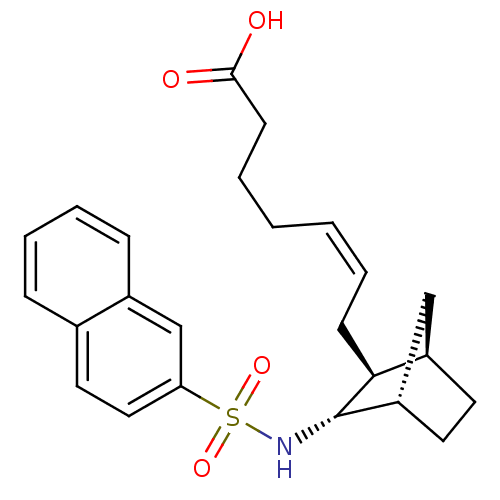 ((Z)-7-[(1R,2S,3S,4S)-3-(Naphthalene-2-sulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]- (+)-S-145 specific binding to human platelet membranes in TXA2 receptor (TP) assay | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060454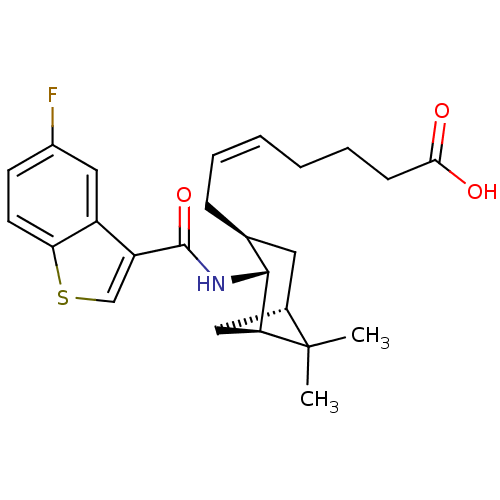 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Fluoro-benzo[b]thiophen...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128750 (7-{2-[(5-Fluoro-benzo[b]thiophene-3-carbonyl)-amin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128768 (7-{2-[(5-Hydroxy-benzo[b]thiophene-3-carbonyl)-ami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060453 ((Z)-7-{(1R,2R,3S,5S)-2-[(6-Hydroxy-benzo[b]thiophe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060454 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Fluoro-benzo[b]thiophen...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128768 (7-{2-[(5-Hydroxy-benzo[b]thiophene-3-carbonyl)-ami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128767 (7-{2-[(6-Hydroxy-benzo[b]thiophene-3-carbonyl)-ami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060453 ((Z)-7-{(1R,2R,3S,5S)-2-[(6-Hydroxy-benzo[b]thiophe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128750 (7-{2-[(5-Fluoro-benzo[b]thiophene-3-carbonyl)-amin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060459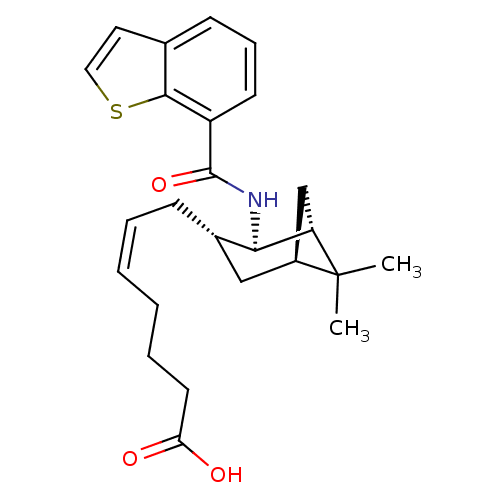 ((Z)-7-{(1R,2R,3S,5S)-2-[(Benzo[b]thiophene-7-carbo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128762 (7-{2-[(Benzo[b]thiophene-7-carbonyl)-amino]-6,6-di...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060465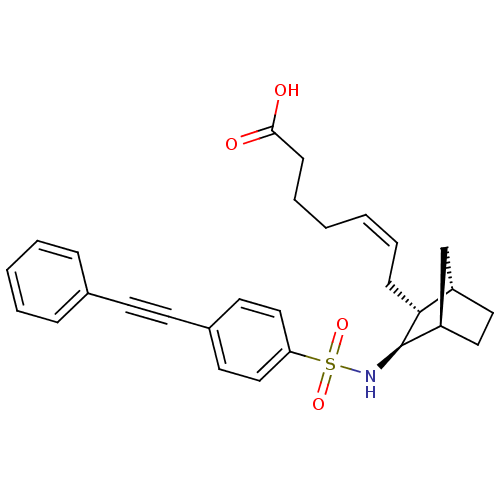 ((+) 7-[3-(4-Phenylethynyl-benzenesulfonylamino)-bi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060465 ((+) 7-[3-(4-Phenylethynyl-benzenesulfonylamino)-bi...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128757 (7-{6,6-Dimethyl-2-[(5-methyl-thiophene-3-carbonyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128694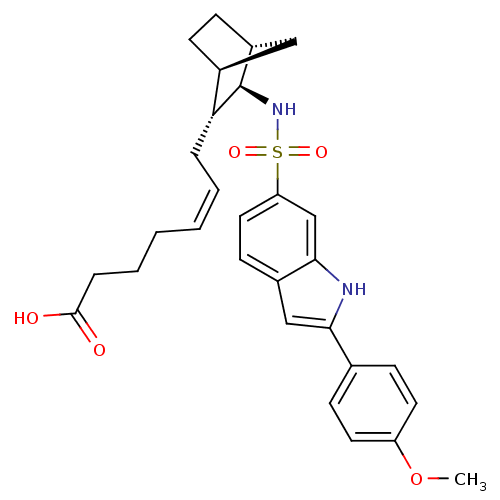 ((+) 7-{3-[2-(4-Methoxy-phenyl)-1H-indole-6-sulfony...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128738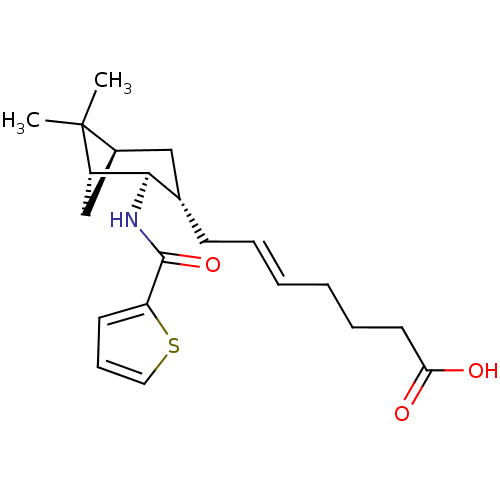 (7-{6,6-Dimethyl-2-[(thiophene-2-carbonyl)-amino]-b...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128736 (7-[2-(2-Furan-3-yl-acetylamino)-6,6-dimethyl-bicyc...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128711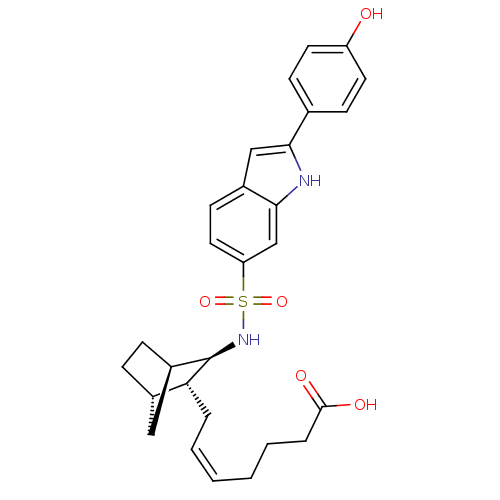 ((+) 7-{3-[2-(4-Hydroxy-phenyl)-1H-indole-6-sulfony...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128767 (7-{2-[(6-Hydroxy-benzo[b]thiophene-3-carbonyl)-ami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128744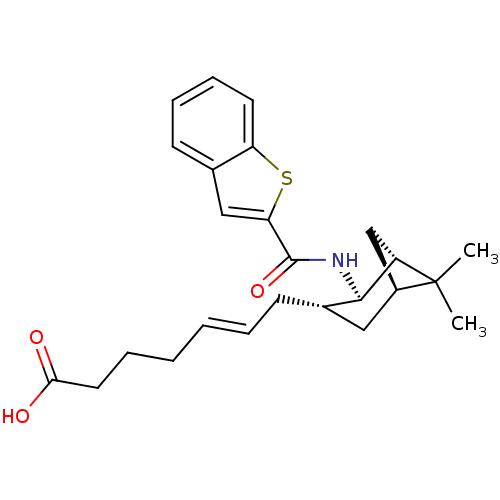 (7-{2-[(Benzo[b]thiophene-2-carbonyl)-amino]-6,6-di...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128721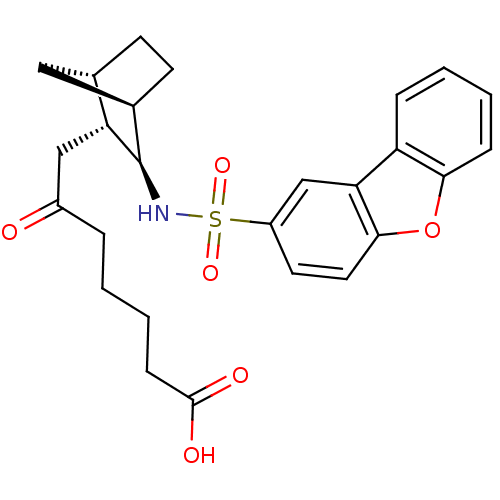 (7-[3-(Dibenzofuran-2-sulfonylamino)-bicyclo[2.2.1]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of cAMP formation evoked by prostaglandin D2 receptor in human platelets | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128751 (7-{6,6-Dimethyl-2-[(2-methyl-thiophene-3-carbonyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128725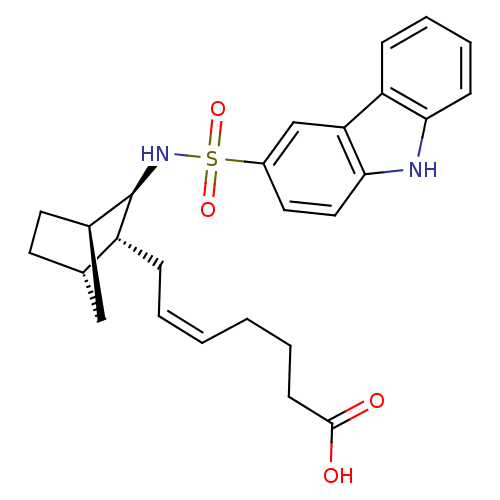 ((+) 7-[3-(9H-Carbazole-3-sulfonylamino)-bicyclo[2....) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128725 ((+) 7-[3-(9H-Carbazole-3-sulfonylamino)-bicyclo[2....) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of cAMP formation evoked by prostaglandin D2 receptor in human platelets | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128749 (7-{2-[(Benzo[b]thiophene-3-carbonyl)-amino]-6,6-di...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128693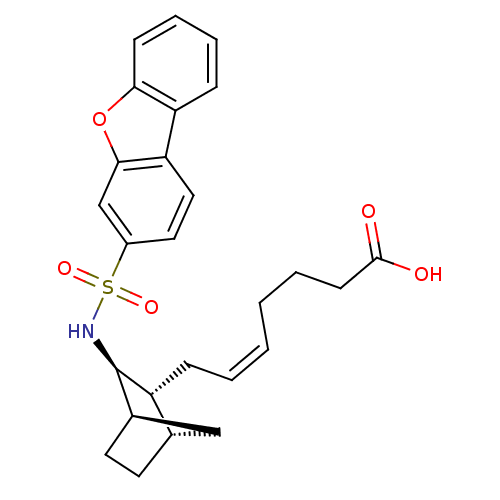 ((+) 7-[3-(Dibenzofuran-3-sulfonylamino)-bicyclo[2....) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060452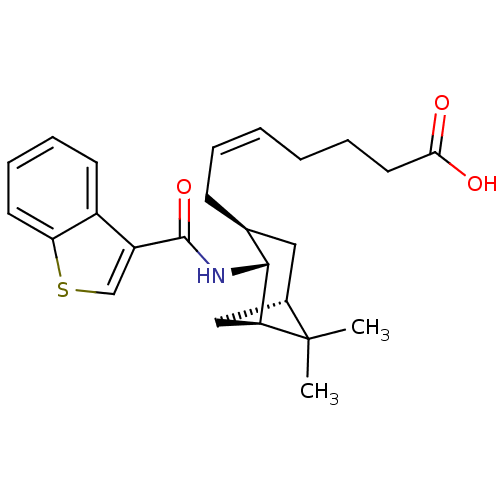 ((Z)-7-{(1R,2R,3S,5S)-2-[(Benzo[b]thiophene-3-carbo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060447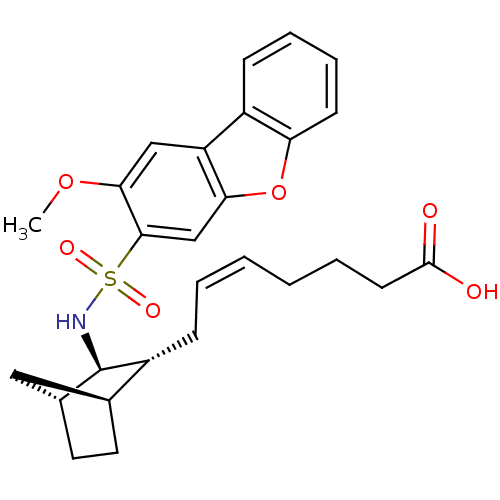 ((+) 7-[3-(2-Methoxy-dibenzofuran-3-sulfonylamino)-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128711 ((+) 7-{3-[2-(4-Hydroxy-phenyl)-1H-indole-6-sulfony...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of cAMP formation evoked by prostaglandin D2 receptor in human platelets | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128757 (7-{6,6-Dimethyl-2-[(5-methyl-thiophene-3-carbonyl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060463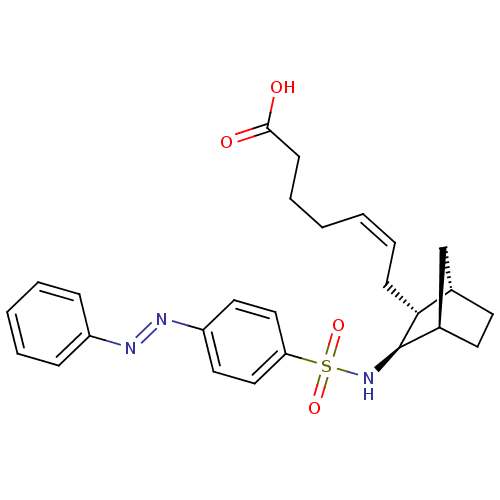 ((+) 7-[3-(4-Phenylazo-benzenesulfonylamino)-bicycl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060459 ((Z)-7-{(1R,2R,3S,5S)-2-[(Benzo[b]thiophene-7-carbo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128762 (7-{2-[(Benzo[b]thiophene-7-carbonyl)-amino]-6,6-di...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060463 ((+) 7-[3-(4-Phenylazo-benzenesulfonylamino)-bicycl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060447 ((+) 7-[3-(2-Methoxy-dibenzofuran-3-sulfonylamino)-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128769 (7-{2-[(5-Methanesulfonylamino-benzo[b]thiophene-3-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128712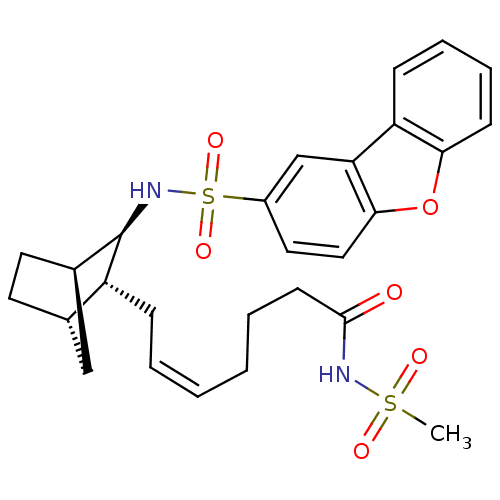 (CHEMBL309443 | Dibenzofuran-2-sulfonic acid [3-(7-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of cAMP formation evoked by prostaglandin D2 receptor in human platelets | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128749 (7-{2-[(Benzo[b]thiophene-3-carbonyl)-amino]-6,6-di...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060452 ((Z)-7-{(1R,2R,3S,5S)-2-[(Benzo[b]thiophene-3-carbo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128703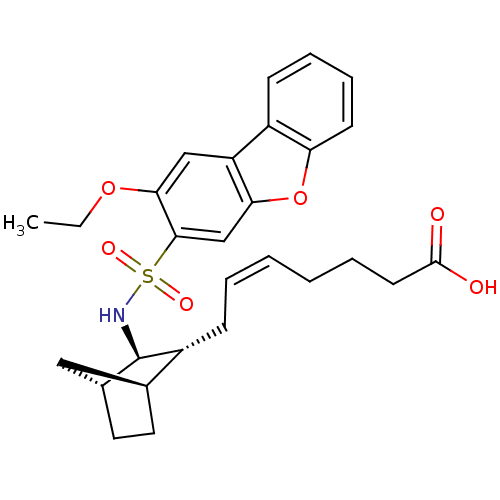 ((+) 7-[3-(2-Ethoxy-dibenzofuran-3-sulfonylamino)-b...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- PGD-2 radioligand binding to prostaglandin D2 receptor on human platelet membrane | J Med Chem 46: 2436-45 (2003) Article DOI: 10.1021/jm020517g BindingDB Entry DOI: 10.7270/Q2154GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 283 total ) | Next | Last >> |