

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523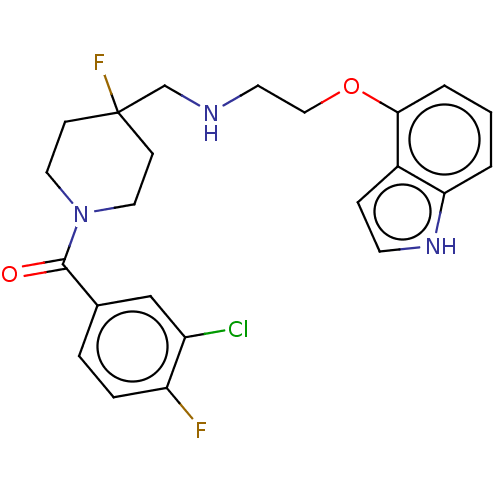 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433523 (US10562853, Compound 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease L10F/L19I/K20R/L33F/E35D/M36I/R41K/F53L/I54V/L63P/H69K/A71V/T74P/I84V/L89M/L90M/I93L mutant expressed in Esch... | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279 (CHEMBL4436207) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease L10F/L19I/K20R/L33F/E35D/M36I/R41K/F53L/I54V/L63P/H69K/A71V/T74P/I84V/L89M/L90M/I93L mutant expressed in Esch... | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease L10F/L19I/K20R/L33F/E35D/M36I/R41K/F53L/I54V/L63P/H69K/A71V/T74P/I84V/L89M/L90M/I93L mutant expressed in Esch... | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50246899 ((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17129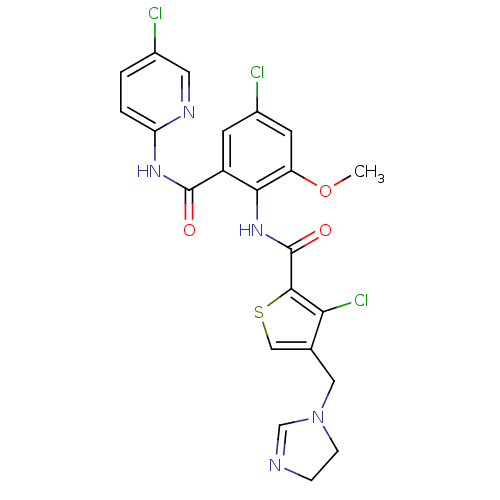 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17122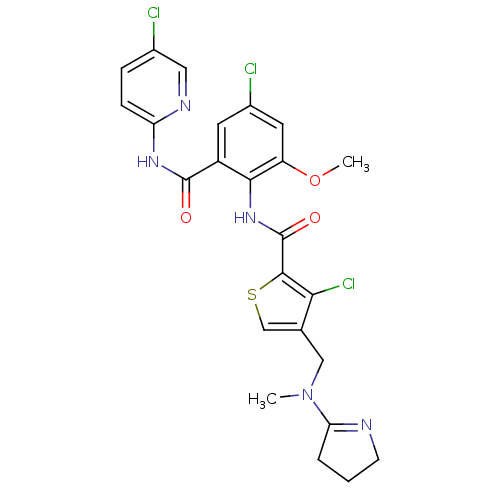 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17127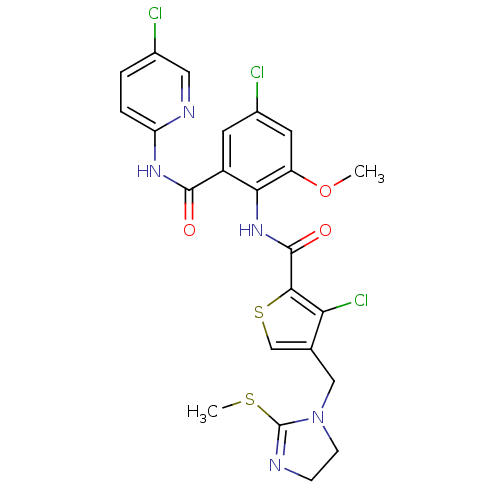 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044868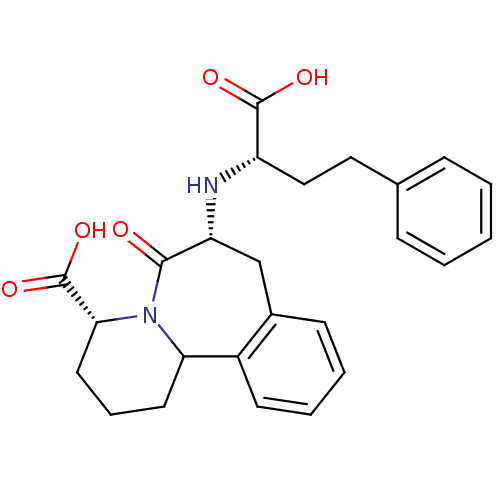 (7-(1-Carboxy-3-phenyl-propylamino)-6-oxo-1,2,3,4,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17135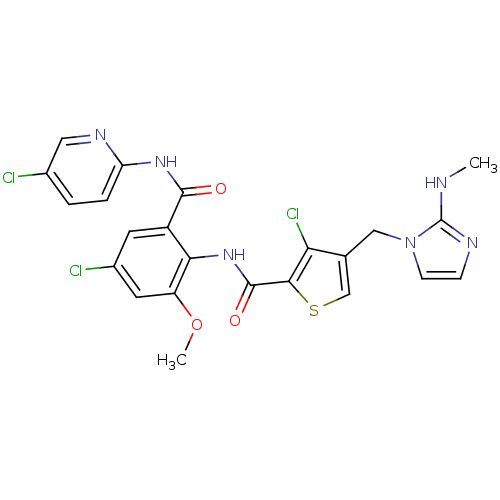 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17136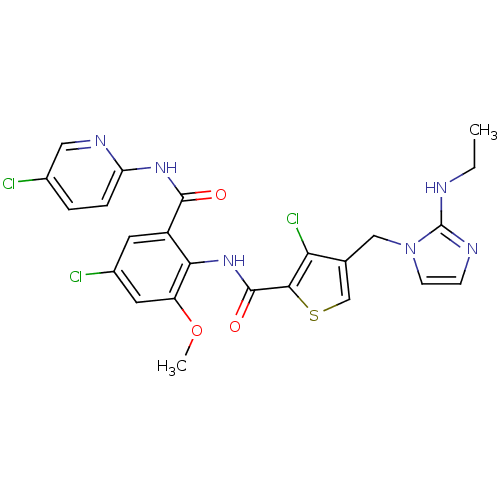 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17134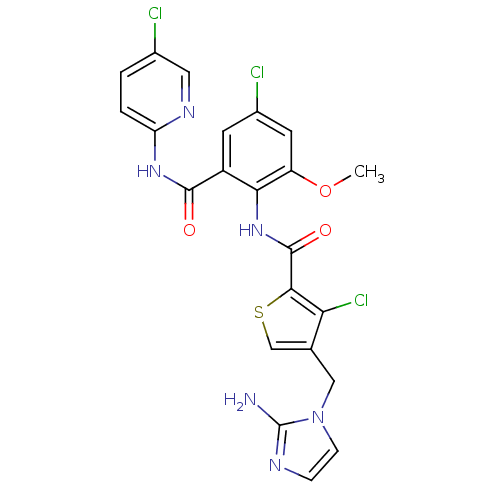 (4-[(2-amino-1H-imidazol-1-yl)methyl]-3-chloro-N-{4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17111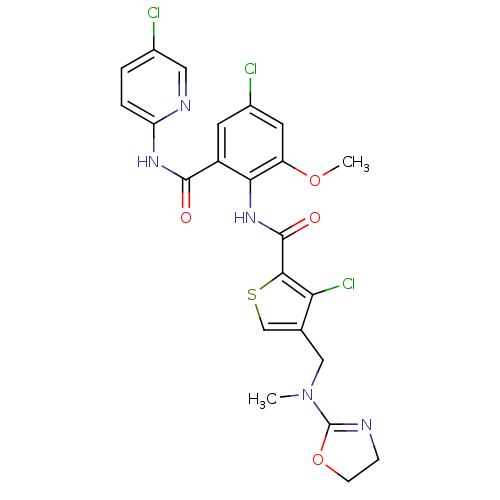 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17137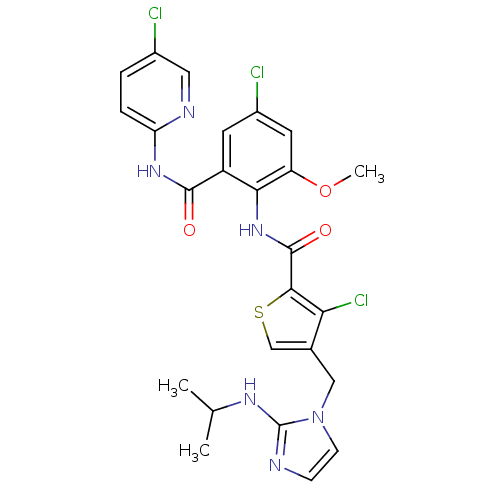 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537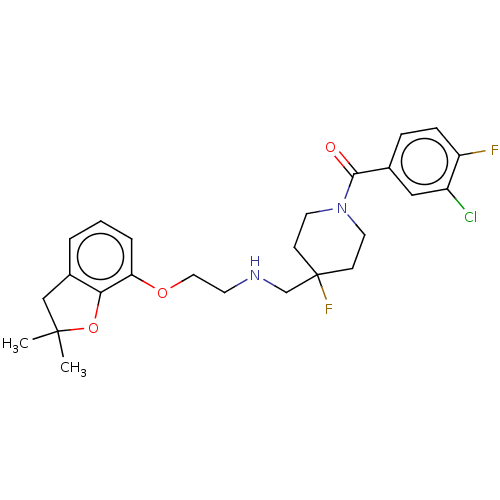 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089452 (CHEMBL3578201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089452 (CHEMBL3578201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50089452 (CHEMBL3578201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of antiTEV-tagged GCP2 extracellular portion (aa 44-750) (unknown origin) using folyl-di-L-glutamate as substrate by HPLC-based enzymatic ... | J Med Chem 58: 4357-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b00278 BindingDB Entry DOI: 10.7270/Q2DR2X70 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50466554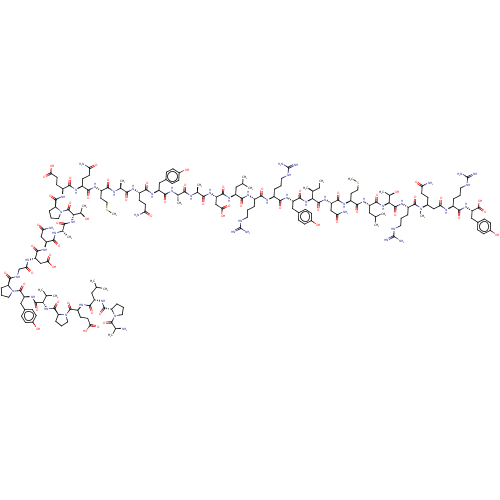 (CHEMBL4286615) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-PP from human Y4R expressed in CHO-K1 cell membranes after 2 hrs by scintillation proximity assay | J Med Chem 61: 10519-10530 (2018) Article DOI: 10.1021/acs.jmedchem.8b01046 BindingDB Entry DOI: 10.7270/Q2ZC85JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50466543 (CHEMBL4276961) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-PP from human Y4R expressed in CHO-K1 cell membranes after 2 hrs by scintillation proximity assay | J Med Chem 61: 10519-10530 (2018) Article DOI: 10.1021/acs.jmedchem.8b01046 BindingDB Entry DOI: 10.7270/Q2ZC85JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030759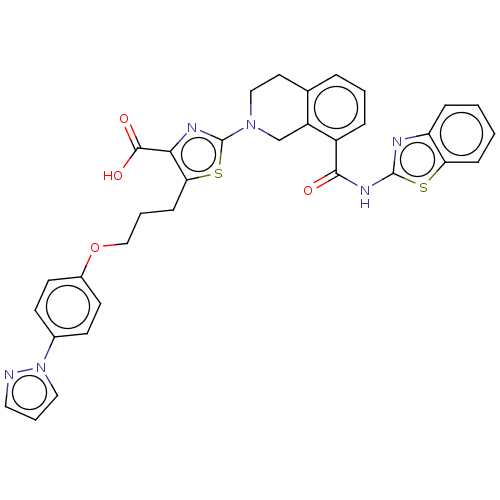 (CHEMBL3342194) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030758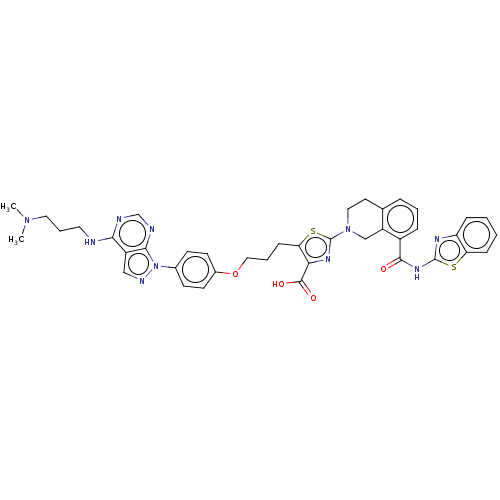 (CHEMBL3342195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030757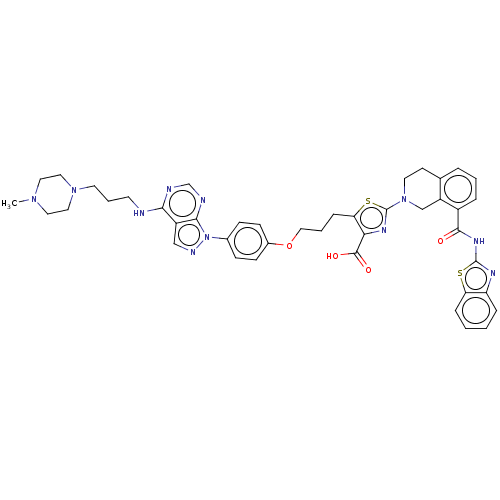 (CHEMBL3342196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50466543 (CHEMBL4276961) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-PP from human Y4R expressed in CHO-K1 cell membranes after 2 hrs by scintillation proximity assay | J Med Chem 61: 10519-10530 (2018) Article DOI: 10.1021/acs.jmedchem.8b01046 BindingDB Entry DOI: 10.7270/Q2ZC85JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17123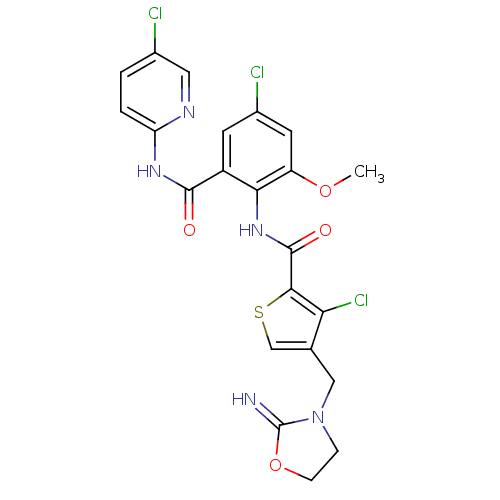 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17124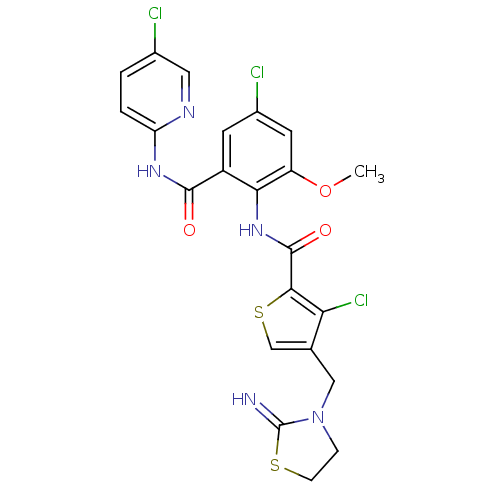 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17125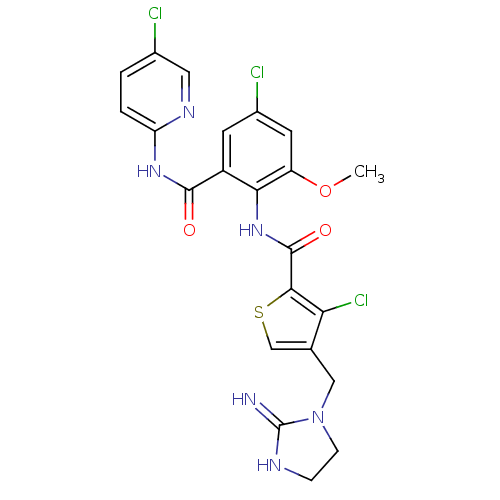 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17128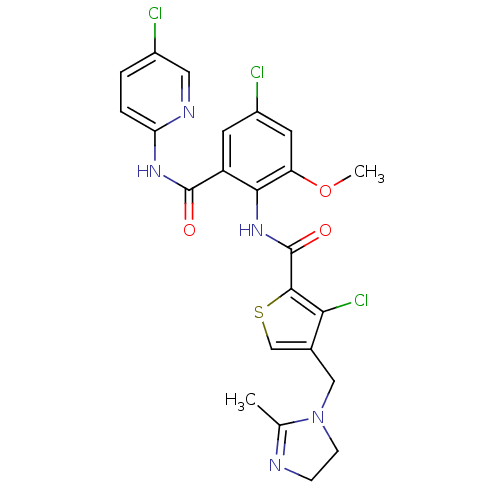 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17130 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17131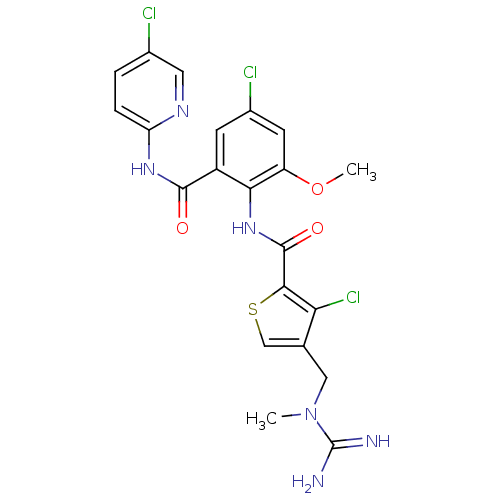 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17133 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | J Med Chem 50: 2967-80 (2007) Article DOI: 10.1021/jm070125f BindingDB Entry DOI: 10.7270/Q2J101F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433537 (US10562853, Compound 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50466554 (CHEMBL4286615) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-PP from human Y4R expressed in CHO-K1 cell membranes after 2 hrs by scintillation proximity assay | J Med Chem 61: 10519-10530 (2018) Article DOI: 10.1021/acs.jmedchem.8b01046 BindingDB Entry DOI: 10.7270/Q2ZC85JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105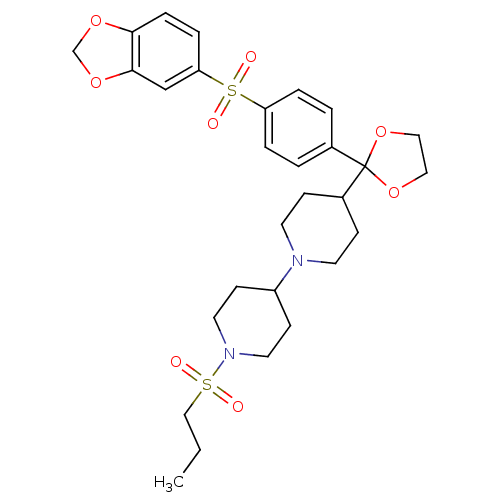 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030752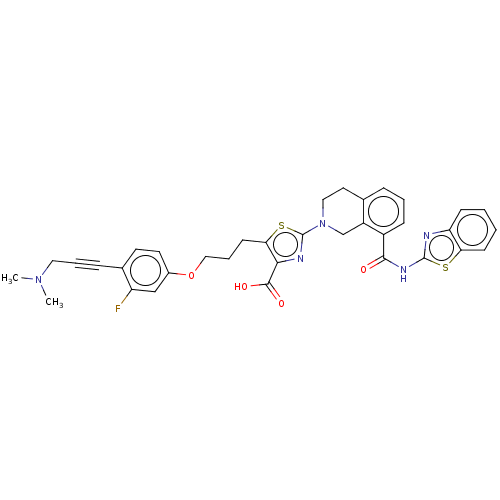 (CHEMBL3342333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030754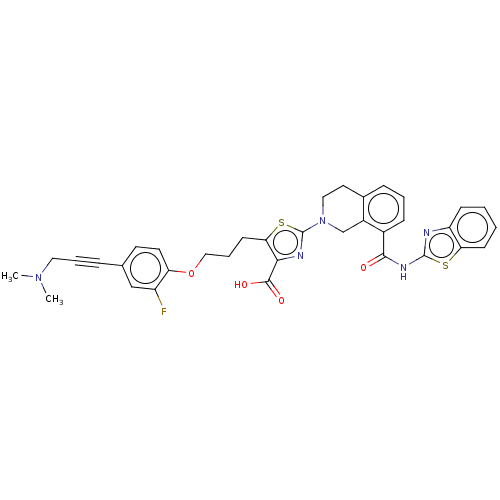 (CHEMBL3342332) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scytalone dehydratase (Magnaporthe grisea) | BDBM50486975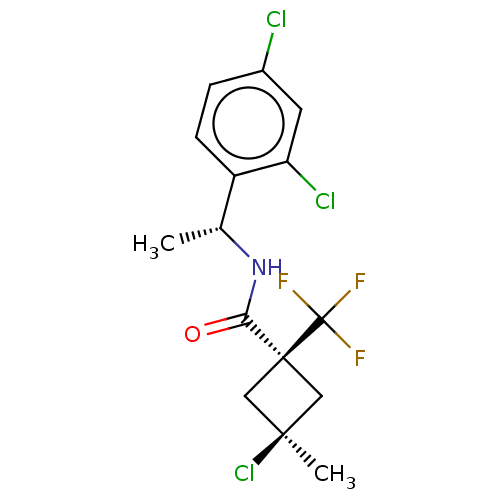 (CHEMBL2251221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stine Haskell Research Center Curated by ChEMBL | Assay Description Inhibition of Magnaporthe grisea scytalone dehydratase | Bioorg Med Chem 8: 897-907 (2000) Article DOI: 10.1016/s0968-0896(00)00034-1 BindingDB Entry DOI: 10.7270/Q2SX6H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease D30N/N88D mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 67779 total ) | Next | Last >> |