

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50508727 ((+)-Aromoline | CHEMBL508781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Mixed-type inhibition of human BuChE assessed as enzyme-inhibitor complex using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analys... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50508727 ((+)-Aromoline | CHEMBL508781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Mixed-type inhibition of human BuChE assessed as enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrate by Lineweaver-Burk p... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50038879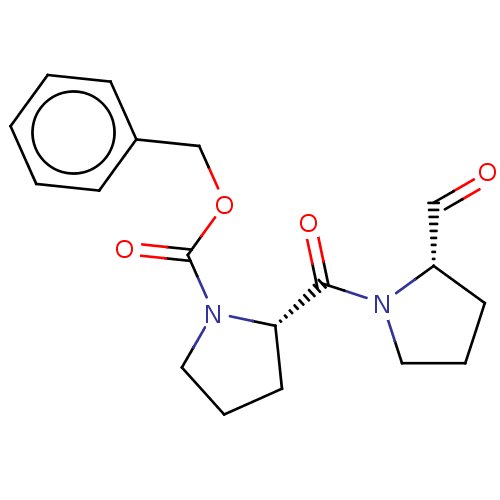 ((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50199522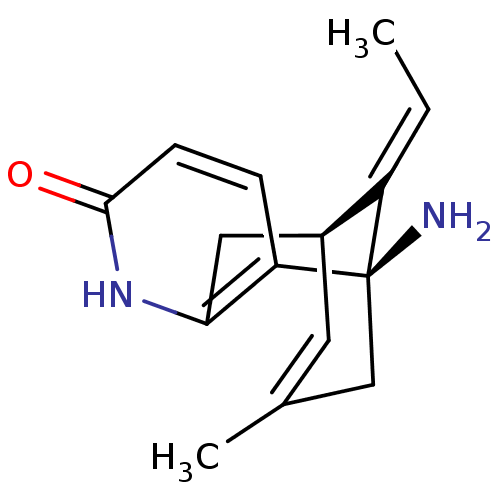 ((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50508727 ((+)-Aromoline | CHEMBL508781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50292332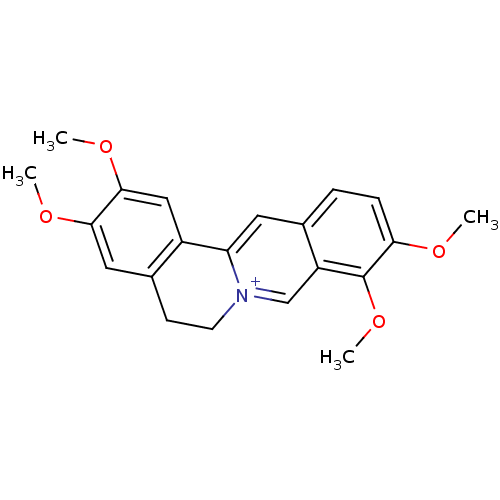 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50508729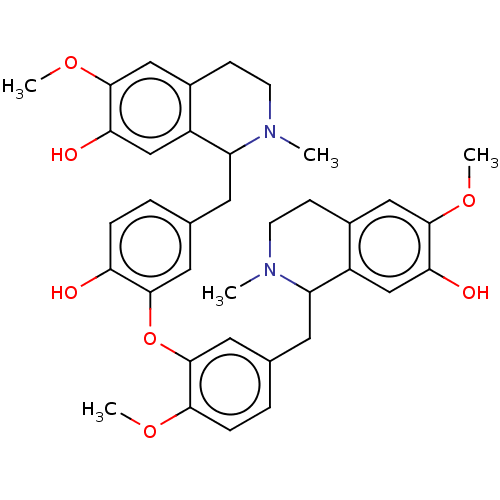 (CHEMBL4548293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50508729 (CHEMBL4548293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508728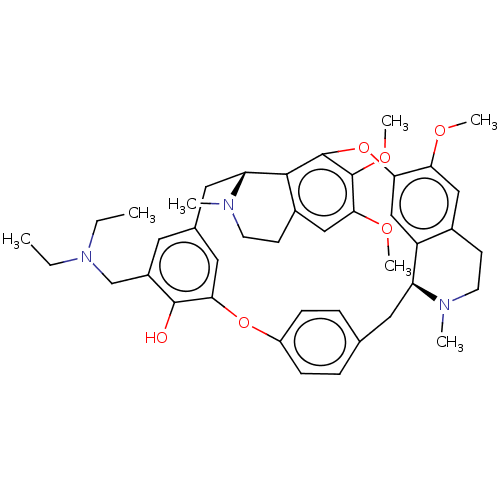 (CHEMBL4590539) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50508731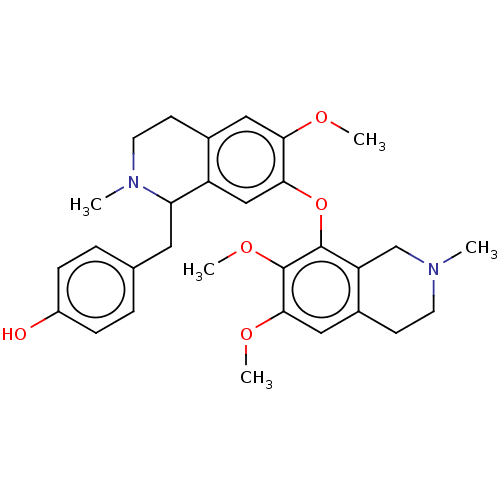 (CHEMBL4585711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50508730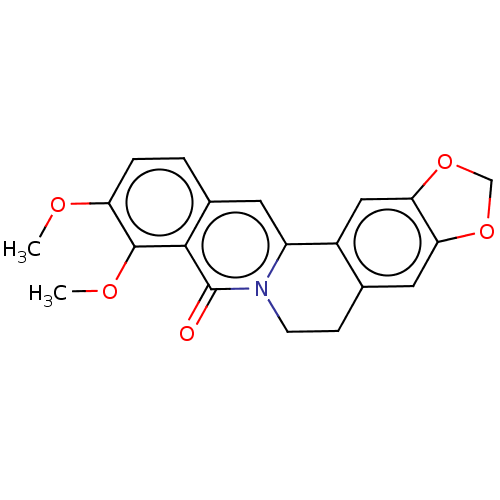 (Oxyberberine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50508728 (CHEMBL4590539) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50508726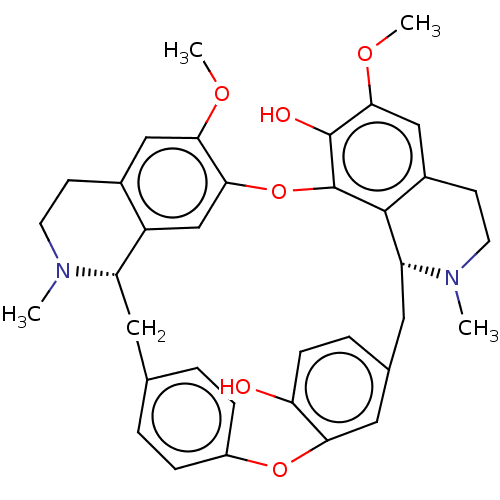 (CHEBI:7714 | NSC-251534 | OBAMEGINE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50508730 (Oxyberberine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50508731 (CHEMBL4585711) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50241654 ((+)-berbamine | Berbamine | CHEMBL504323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50508726 (CHEBI:7714 | NSC-251534 | OBAMEGINE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50199522 ((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50508727 ((+)-Aromoline | CHEMBL508781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50508728 (CHEMBL4590539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241654 ((+)-berbamine | Berbamine | CHEMBL504323) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508727 ((+)-Aromoline | CHEMBL508781) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508730 (Oxyberberine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50241654 ((+)-berbamine | Berbamine | CHEMBL504323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508726 (CHEBI:7714 | NSC-251534 | OBAMEGINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50508731 (CHEMBL4585711) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 BindingDB Entry DOI: 10.7270/Q2NZ8BXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||