 Found 555 hits with Last Name = 'vidal' and Initial = 'j'
Found 555 hits with Last Name = 'vidal' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430967

(CHEMBL2337848)Show SMILES CC[C@H](C)C(=O)C(=O)N[C@H]1Cc2ccc(O)c(c2)-c2cccc3c2NC(=O)[C@@]3(O)[C@H](O)[C@H](NC(=O)[C@H](CC(N)=O)CC1=O)C(=O)N\C=C/C |r| Show InChI InChI=1S/C34H39N5O10/c1-4-11-36-31(46)27-29(44)34(49)21-8-6-7-19(26(21)39-33(34)48)20-12-17(9-10-23(20)40)13-22(37-32(47)28(43)16(3)5-2)24(41)14-18(15-25(35)42)30(45)38-27/h4,6-12,16,18,22,27,29,40,44,49H,5,13-15H2,1-3H3,(H2,35,42)(H,36,46)(H,37,47)(H,38,45)(H,39,48)/b11-4-/t16-,18-,22-,27-,29+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430968
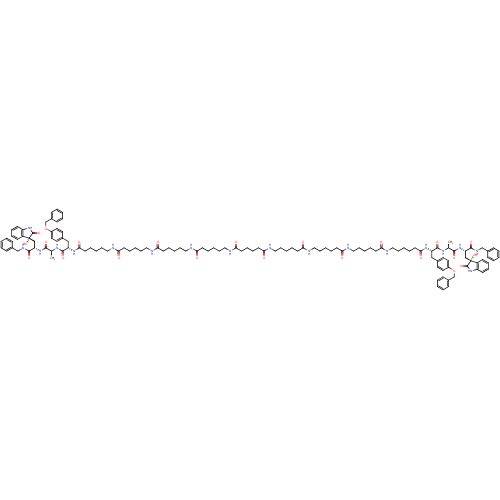
(CHEMBL2337847)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C128H172N18O22/c1-91(119(157)143-107(121(159)137-87-95-45-15-3-16-46-95)85-127(165)101-53-31-33-55-103(101)145-125(127)163)139-123(161)105(83-93-67-71-99(72-68-93)167-89-97-49-19-5-20-50-97)141-117(155)65-29-13-43-81-133-113(151)61-25-9-39-77-129-109(147)57-23-7-37-75-131-111(149)59-27-11-41-79-135-115(153)63-35-36-64-116(154)136-80-42-12-28-60-112(150)132-76-38-8-24-58-110(148)130-78-40-10-26-62-114(152)134-82-44-14-30-66-118(156)142-106(84-94-69-73-100(74-70-94)168-90-98-51-21-6-22-52-98)124(162)140-92(2)120(158)144-108(122(160)138-88-96-47-17-4-18-48-96)86-128(166)102-54-32-34-56-104(102)146-126(128)164/h3-6,15-22,31-34,45-56,67-74,91-92,105-108,165-166H,7-14,23-30,35-44,57-66,75-90H2,1-2H3,(H,129,147)(H,130,148)(H,131,149)(H,132,150)(H,133,151)(H,134,152)(H,135,153)(H,136,154)(H,137,159)(H,138,160)(H,139,161)(H,140,162)(H,141,155)(H,142,156)(H,143,157)(H,144,158)(H,145,163)(H,146,164)/t91-,92-,105-,106-,107-,108-,127+,128+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430962
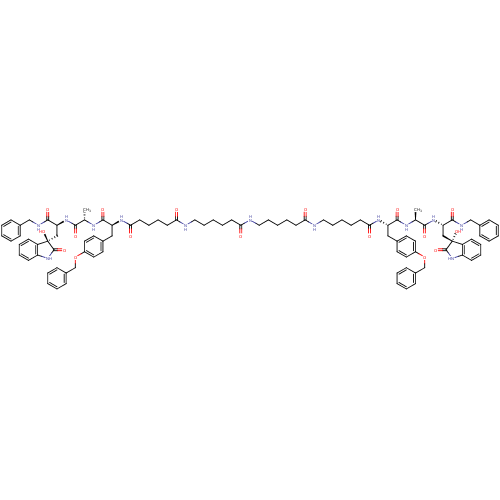
(CHEMBL2337843)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C98H117N13O17/c1-66(89(117)108-82(91(119)102-62-70-30-10-3-11-31-70)60-97(125)76-38-21-23-40-78(76)110-95(97)123)104-93(121)80(58-68-47-51-74(52-48-68)127-64-72-34-14-5-15-35-72)106-87(115)45-20-9-29-57-100-85(113)43-18-7-27-55-99-84(112)42-19-8-28-56-101-86(114)44-25-26-46-88(116)107-81(59-69-49-53-75(54-50-69)128-65-73-36-16-6-17-37-73)94(122)105-67(2)90(118)109-83(92(120)103-63-71-32-12-4-13-33-71)61-98(126)77-39-22-24-41-79(77)111-96(98)124/h3-6,10-17,21-24,30-41,47-54,66-67,80-83,125-126H,7-9,18-20,25-29,42-46,55-65H2,1-2H3,(H,99,112)(H,100,113)(H,101,114)(H,102,119)(H,103,120)(H,104,121)(H,105,122)(H,106,115)(H,107,116)(H,108,117)(H,109,118)(H,110,123)(H,111,124)/t66-,67-,80-,81-,82-,83-,97+,98+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430961

(CHEMBL2337844)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C104H128N14O18/c1-71(95(125)115-87(97(127)109-67-75-33-11-3-12-34-75)65-103(133)81-41-23-25-43-83(81)117-101(103)131)111-99(129)85(63-73-51-55-79(56-52-73)135-69-77-37-15-5-16-38-77)113-93(123)49-22-10-32-62-107-91(121)47-20-8-30-60-105-89(119)45-19-7-29-59-106-90(120)46-21-9-31-61-108-92(122)48-27-28-50-94(124)114-86(64-74-53-57-80(58-54-74)136-70-78-39-17-6-18-40-78)100(130)112-72(2)96(126)116-88(98(128)110-68-76-35-13-4-14-36-76)66-104(134)82-42-24-26-44-84(82)118-102(104)132/h3-6,11-18,23-26,33-44,51-58,71-72,85-88,133-134H,7-10,19-22,27-32,45-50,59-70H2,1-2H3,(H,105,119)(H,106,120)(H,107,121)(H,108,122)(H,109,127)(H,110,128)(H,111,129)(H,112,130)(H,113,123)(H,114,124)(H,115,125)(H,116,126)(H,117,131)(H,118,132)/t71-,72-,85-,86-,87-,88-,103+,104+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430970

(CHEMBL2337845)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C110H139N15O19/c1-76(101(133)122-92(103(135)116-72-80-36-12-3-13-37-80)70-109(141)86-44-25-27-46-88(86)124-107(109)139)118-105(137)90(68-78-55-59-84(60-56-78)143-74-82-40-16-5-17-41-82)120-99(131)53-24-11-35-67-114-97(129)51-22-9-33-65-112-95(127)49-20-7-31-63-111-94(126)48-21-8-32-64-113-96(128)50-23-10-34-66-115-98(130)52-29-30-54-100(132)121-91(69-79-57-61-85(62-58-79)144-75-83-42-18-6-19-43-83)106(138)119-77(2)102(134)123-93(104(136)117-73-81-38-14-4-15-39-81)71-110(142)87-45-26-28-47-89(87)125-108(110)140/h3-6,12-19,25-28,36-47,55-62,76-77,90-93,141-142H,7-11,20-24,29-35,48-54,63-75H2,1-2H3,(H,111,126)(H,112,127)(H,113,128)(H,114,129)(H,115,130)(H,116,135)(H,117,136)(H,118,137)(H,119,138)(H,120,131)(H,121,132)(H,122,133)(H,123,134)(H,124,139)(H,125,140)/t76-,77-,90-,91-,92-,93-,109+,110+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430969

(CHEMBL2337846)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)NCCCCCC(=O)N[C@@H](Cc1ccc(OCc2ccccc2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C116H150N16O20/c1-81(107(141)129-97(109(143)123-77-85-39-13-3-14-40-85)75-115(149)91-47-27-29-49-93(91)131-113(115)147)125-111(145)95(73-83-59-63-89(64-60-83)151-79-87-43-17-5-18-44-87)127-105(139)57-25-11-37-71-119-101(135)53-21-7-33-67-117-99(133)51-23-9-35-69-121-103(137)55-31-32-56-104(138)122-70-36-10-24-52-100(134)118-68-34-8-22-54-102(136)120-72-38-12-26-58-106(140)128-96(74-84-61-65-90(66-62-84)152-80-88-45-19-6-20-46-88)112(146)126-82(2)108(142)130-98(110(144)124-78-86-41-15-4-16-42-86)76-116(150)92-48-28-30-50-94(92)132-114(116)148/h3-6,13-20,27-30,39-50,59-66,81-82,95-98,149-150H,7-12,21-26,31-38,51-58,67-80H2,1-2H3,(H,117,133)(H,118,134)(H,119,135)(H,120,136)(H,121,137)(H,122,138)(H,123,143)(H,124,144)(H,125,145)(H,126,146)(H,127,139)(H,128,140)(H,129,141)(H,130,142)(H,131,147)(H,132,148)/t81-,82-,95-,96-,97-,98-,115+,116+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50463046
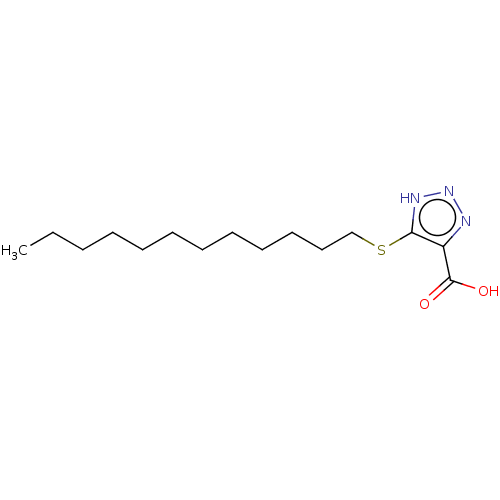
(CHEMBL1229989)Show InChI InChI=1S/C15H27N3O2S/c1-2-3-4-5-6-7-8-9-10-11-12-21-14-13(15(19)20)16-18-17-14/h2-12H2,1H3,(H,19,20)(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged glycolate oxidase expressed in C41(D43) Escherichia coli using glycolate as substrate preincubated for 30 m... |
J Med Chem 61: 7144-7167 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00399
BindingDB Entry DOI: 10.7270/Q23B62SD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430963
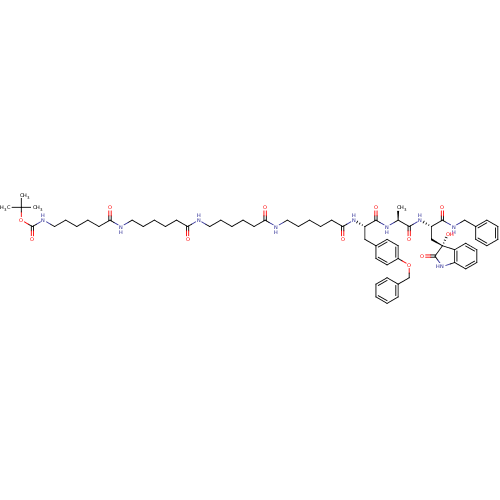
(CHEMBL2337842)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C66H91N9O12/c1-47(60(80)74-55(61(81)71-45-49-25-11-5-12-26-49)44-66(85)52-29-19-20-30-53(52)75-63(66)83)72-62(82)54(43-48-35-37-51(38-36-48)86-46-50-27-13-6-14-28-50)73-59(79)34-18-10-23-41-69-57(77)32-16-8-21-39-67-56(76)31-15-7-22-40-68-58(78)33-17-9-24-42-70-64(84)87-65(2,3)4/h5-6,11-14,19-20,25-30,35-38,47,54-55,85H,7-10,15-18,21-24,31-34,39-46H2,1-4H3,(H,67,76)(H,68,78)(H,69,77)(H,70,84)(H,71,81)(H,72,82)(H,73,79)(H,74,80)(H,75,83)/t47-,54-,55-,66+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50196043

(3-N-(L-ArgNO2)-trans-3-amino-L-proline-NH2 ditrifl...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(=O)N[C@H]1CCN[C@@H]1C(N)=O |w:8.8| Show InChI InChI=1S/C11H22N8O4/c12-6(2-1-4-16-11(14)18-19(22)23)10(21)17-7-3-5-15-8(7)9(13)20/h6-8,15H,1-5,12H2,(H2,13,20)(H,17,21)(H3,14,16,18)/t6-,7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS |
J Med Chem 49: 6254-63 (2006)
Article DOI: 10.1021/jm0604124
BindingDB Entry DOI: 10.7270/Q2W37VZ8 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM21961
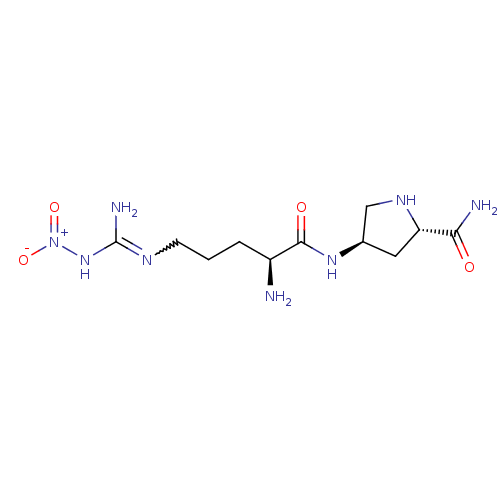
((2S,4R)-4-[(2S)-2-amino-5-(1-nitrocarbamimidamido)...)Show SMILES N[C@@H](CCCN=C(N)N[N+]([O-])=O)C(=O)N[C@H]1CN[C@@H](C1)C(N)=O |w:5.4| Show InChI InChI=1S/C11H22N8O4/c12-7(2-1-3-15-11(14)18-19(22)23)10(21)17-6-4-8(9(13)20)16-5-6/h6-8,16H,1-5,12H2,(H2,13,20)(H,17,21)(H3,14,15,18)/t6-,7+,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 100 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Struct Mol Biol 11: 54-9 (2004)
Article DOI: 10.1038/nsmb704
BindingDB Entry DOI: 10.7270/Q27942ZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50138976

((2S)-4-[((2S)-2-amino-5-{[(E)-imino(nitroamino)met...)Show SMILES N[C@@H](CCCNC(=N)N[N+]([O-])=O)C(=O)N[C@H]1CN[C@@H](C1)C(N)=O Show InChI InChI=1S/C11H22N8O4/c12-7(2-1-3-15-11(14)18-19(22)23)10(21)17-6-4-8(9(13)20)16-5-6/h6-8,16H,1-5,12H2,(H2,13,20)(H,17,21)(H3,14,15,18)/t6-,7+,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Binding affinity towards neuronal nitric oxide synthase (nNOS) |
J Med Chem 47: 703-10 (2004)
Article DOI: 10.1021/jm030297m
BindingDB Entry DOI: 10.7270/Q2MC90SQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430964
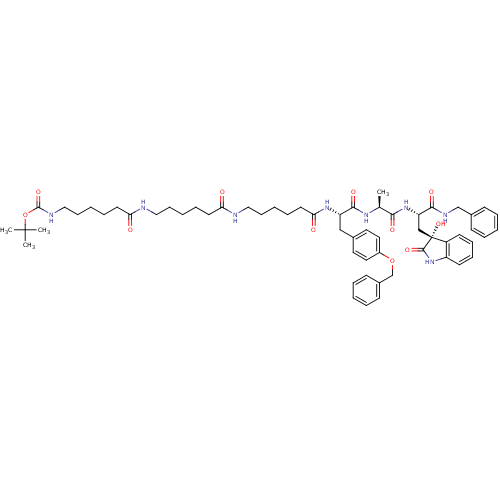
(CHEMBL2337841)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C60H80N8O11/c1-42(54(72)67-50(55(73)64-40-44-22-10-5-11-23-44)39-60(77)47-26-17-18-27-48(47)68-57(60)75)65-56(74)49(38-43-31-33-46(34-32-43)78-41-45-24-12-6-13-25-45)66-53(71)30-16-9-20-36-62-51(69)28-14-7-19-35-61-52(70)29-15-8-21-37-63-58(76)79-59(2,3)4/h5-6,10-13,17-18,22-27,31-34,42,49-50,77H,7-9,14-16,19-21,28-30,35-41H2,1-4H3,(H,61,70)(H,62,69)(H,63,76)(H,64,73)(H,65,74)(H,66,71)(H,67,72)(H,68,75)/t42-,49-,50-,60+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM22030

((2S)-2-amino-N-[(1S)-3-amino-1-(aminocarbonyl)prop...)Show SMILES NCC[C@H](NC(=O)[C@@H](N)CCCN=C(N)N[N+]([O-])=O)C(N)=O |r,w:12.11| Show InChI InChI=1S/C10H22N8O4/c11-4-3-7(8(13)19)16-9(20)6(12)2-1-5-15-10(14)17-18(21)22/h6-7H,1-5,11-12H2,(H2,13,19)(H,16,20)(H3,14,15,17)/t6-,7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Binding affinity towards neuronal nitric oxide synthase (nNOS) |
J Med Chem 47: 703-10 (2004)
Article DOI: 10.1021/jm030297m
BindingDB Entry DOI: 10.7270/Q2MC90SQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM22030

((2S)-2-amino-N-[(1S)-3-amino-1-(aminocarbonyl)prop...)Show SMILES NCC[C@H](NC(=O)[C@@H](N)CCCN=C(N)N[N+]([O-])=O)C(N)=O |r,w:12.11| Show InChI InChI=1S/C10H22N8O4/c11-4-3-7(8(13)19)16-9(20)6(12)2-1-5-15-10(14)17-18(21)22/h6-7H,1-5,11-12H2,(H2,13,19)(H,16,20)(H3,14,15,17)/t6-,7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against neuronal nitric oxide synthase (nNOS) |
J Med Chem 47: 703-10 (2004)
Article DOI: 10.1021/jm030297m
BindingDB Entry DOI: 10.7270/Q2MC90SQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM21960
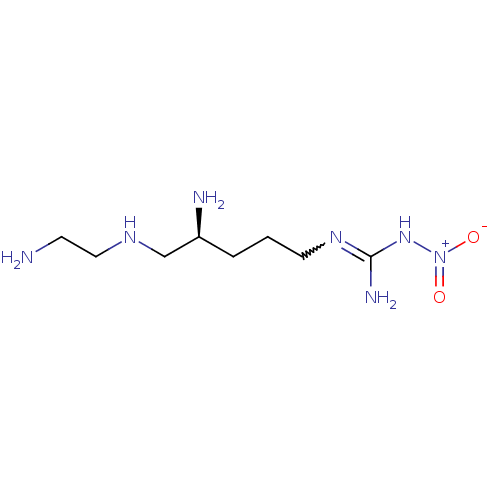
(3-[(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl]-1-n...)Show SMILES NCCNC[C@@H](N)CCCN=C(N)N[N+]([O-])=O |w:10.9| Show InChI InChI=1S/C8H21N7O2/c9-3-5-12-6-7(10)2-1-4-13-8(11)14-15(16)17/h7,12H,1-6,9-10H2,(H3,11,13,14)/t7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 150 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Struct Mol Biol 11: 54-9 (2004)
Article DOI: 10.1038/nsmb704
BindingDB Entry DOI: 10.7270/Q27942ZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430963
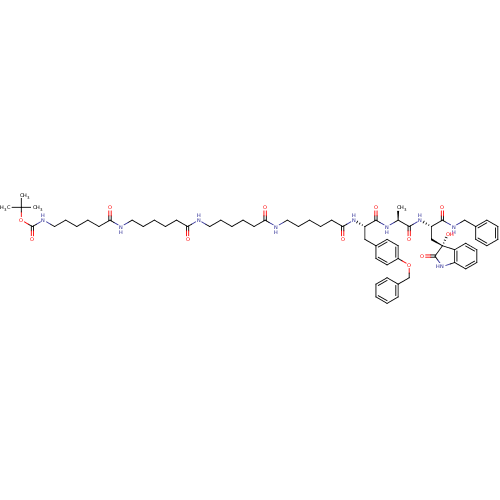
(CHEMBL2337842)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C66H91N9O12/c1-47(60(80)74-55(61(81)71-45-49-25-11-5-12-26-49)44-66(85)52-29-19-20-30-53(52)75-63(66)83)72-62(82)54(43-48-35-37-51(38-36-48)86-46-50-27-13-6-14-28-50)73-59(79)34-18-10-23-41-69-57(77)32-16-8-21-39-67-56(76)31-15-7-22-40-68-58(78)33-17-9-24-42-70-64(84)87-65(2,3)4/h5-6,11-14,19-20,25-30,35-38,47,54-55,85H,7-10,15-18,21-24,31-34,39-46H2,1-4H3,(H,67,76)(H,68,78)(H,69,77)(H,70,84)(H,71,81)(H,72,82)(H,73,79)(H,74,80)(H,75,83)/t47-,54-,55-,66+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM22030

((2S)-2-amino-N-[(1S)-3-amino-1-(aminocarbonyl)prop...)Show SMILES NCC[C@H](NC(=O)[C@@H](N)CCCN=C(N)N[N+]([O-])=O)C(N)=O |r,w:12.11| Show InChI InChI=1S/C10H22N8O4/c11-4-3-7(8(13)19)16-9(20)6(12)2-1-5-15-10(14)17-18(21)22/h6-7H,1-5,11-12H2,(H2,13,19)(H,16,20)(H3,14,15,17)/t6-,7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 300 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Struct Mol Biol 11: 54-9 (2004)
Article DOI: 10.1038/nsmb704
BindingDB Entry DOI: 10.7270/Q27942ZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430964

(CHEMBL2337841)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCCNC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C60H80N8O11/c1-42(54(72)67-50(55(73)64-40-44-22-10-5-11-23-44)39-60(77)47-26-17-18-27-48(47)68-57(60)75)65-56(74)49(38-43-31-33-46(34-32-43)78-41-45-24-12-6-13-25-45)66-53(71)30-16-9-20-36-62-51(69)28-14-7-19-35-61-52(70)29-15-8-21-37-63-58(76)79-59(2,3)4/h5-6,10-13,17-18,22-27,31-34,42,49-50,77H,7-9,14-16,19-21,28-30,35-41H2,1-4H3,(H,61,70)(H,62,69)(H,63,76)(H,64,73)(H,65,74)(H,66,71)(H,67,72)(H,68,75)/t42-,49-,50-,60+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50196042

(4-N-(L-ArgNO2)-Nalpha-benzyl-trans-4-amino-L-proli...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(=O)N[C@@H]1C[C@H](N(Cc2ccccc2)C1)C(N)=O |w:8.8| Show InChI InChI=1S/C18H28N8O4/c19-14(7-4-8-22-18(21)24-26(29)30)17(28)23-13-9-15(16(20)27)25(11-13)10-12-5-2-1-3-6-12/h1-3,5-6,13-15H,4,7-11,19H2,(H2,20,27)(H,23,28)(H3,21,22,24)/t13-,14+,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 328 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS |
J Med Chem 49: 6254-63 (2006)
Article DOI: 10.1021/jm0604124
BindingDB Entry DOI: 10.7270/Q2W37VZ8 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM20462
((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCc1ccc(O)c(O)c1 Show InChI InChI=1S/C28H41NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-28(32)29-23-22-25-20-21-26(30)27(31)24-25/h6-7,9-10,12-13,15-16,20-21,24,30-31H,2-5,8,11,14,17-19,22-23H2,1H3,(H,29,32)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS
Curated by ChEMBL
| Assay Description
Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... |
Bioorg Med Chem Lett 11: 447-9 (2001)
BindingDB Entry DOI: 10.7270/Q2VT1RBM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50096883
((5Z,8Z,11Z,14Z,17Z)-Icosa-5,8,11,14,17-pentaenoic ...)Show SMILES CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCc1ccc(O)c(O)c1 Show InChI InChI=1S/C28H39NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-28(32)29-23-22-25-20-21-26(30)27(31)24-25/h3-4,6-7,9-10,12-13,15-16,20-21,24,30-31H,2,5,8,11,14,17-19,22-23H2,1H3,(H,29,32)/b4-3-,7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS
Curated by ChEMBL
| Assay Description
Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... |
Bioorg Med Chem Lett 11: 447-9 (2001)
BindingDB Entry DOI: 10.7270/Q2VT1RBM |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM22988

((5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-...)Show InChI InChI=1S/C22H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(25)23-20-21-24/h6-7,9-10,12-13,15-16,24H,2-5,8,11,14,17-21H2,1H3,(H,23,25)/b7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS
Curated by ChEMBL
| Assay Description
Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... |
Bioorg Med Chem Lett 11: 447-9 (2001)
BindingDB Entry DOI: 10.7270/Q2VT1RBM |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594014
(CHEMBL5182027)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(Br)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50370361

(CHEMBL1169419)Show SMILES N[C@@H](CCCN=C(N)N[N+]([O-])=O)C(=O)N[C@@H]1CN[C@H](C1)C(N)=O |r,w:5.4| Show InChI InChI=1S/C11H22N8O4/c12-7(2-1-3-15-11(14)18-19(22)23)10(21)17-6-4-8(9(13)20)16-5-6/h6-8,16H,1-5,12H2,(H2,13,20)(H,17,21)(H3,14,15,18)/t6-,7-,8+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Binding affinity towards neuronal nitric oxide synthase (nNOS) |
J Med Chem 47: 703-10 (2004)
Article DOI: 10.1021/jm030297m
BindingDB Entry DOI: 10.7270/Q2MC90SQ |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50594014

(CHEMBL5182027)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(Br)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50096881

((10Z,13Z,16Z,19Z)-Docosa-7,10,13,16,19-pentaenoic ...)Show SMILES CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCCCC(=O)NCCc1ccc(O)c(O)c1 Show InChI InChI=1S/C30H43NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-30(34)31-25-24-27-22-23-28(32)29(33)26-27/h3-4,6-7,9-10,12-13,15-16,22-23,26,32-33H,2,5,8,11,14,17-21,24-25H2,1H3,(H,31,34)/b4-3-,7-6-,10-9-,13-12-,16-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS
Curated by ChEMBL
| Assay Description
Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... |
Bioorg Med Chem Lett 11: 447-9 (2001)
BindingDB Entry DOI: 10.7270/Q2VT1RBM |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50096882
((5Z,9Z,12Z)-Octadeca-5,9,12-trienoic acid [2-(3,4-...)Show SMILES CCCCC\C=C/C\C=C/CC\C=C/CCCC(=O)NCCc1ccc(O)c(O)c1 Show InChI InChI=1S/C26H39NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26(30)27-21-20-23-18-19-24(28)25(29)22-23/h6-7,9-10,13-14,18-19,22,28-29H,2-5,8,11-12,15-17,20-21H2,1H3,(H,27,30)/b7-6-,10-9-,14-13- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS
Curated by ChEMBL
| Assay Description
Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... |
Bioorg Med Chem Lett 11: 447-9 (2001)
BindingDB Entry DOI: 10.7270/Q2VT1RBM |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594017
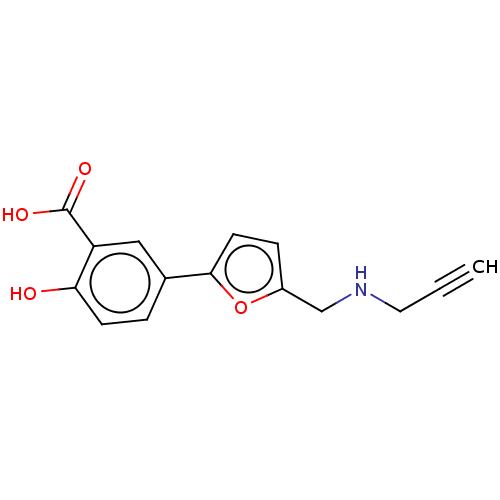
(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50096885
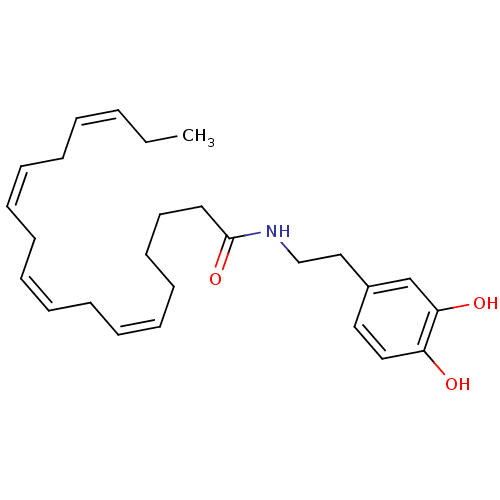
((6Z,9Z,12Z,15Z)-Octadeca-6,9,12,15-tetraenoic acid...)Show SMILES CC\C=C/C\C=C/C\C=C/C\C=C/CCCCC(=O)NCCc1ccc(O)c(O)c1 Show InChI InChI=1S/C26H37NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26(30)27-21-20-23-18-19-24(28)25(29)22-23/h3-4,6-7,9-10,12-13,18-19,22,28-29H,2,5,8,11,14-17,20-21H2,1H3,(H,27,30)/b4-3-,7-6-,10-9-,13-12- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS
Curated by ChEMBL
| Assay Description
Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... |
Bioorg Med Chem Lett 11: 447-9 (2001)
BindingDB Entry DOI: 10.7270/Q2VT1RBM |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430966

(CHEMBL2337849)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C42H47N5O8/c1-27(36(48)45-35(37(49)43-25-29-13-7-5-8-14-29)24-42(53)32-17-11-12-18-33(32)46-39(42)51)44-38(50)34(47-40(52)55-41(2,3)4)23-28-19-21-31(22-20-28)54-26-30-15-9-6-10-16-30/h5-22,27,34-35,53H,23-26H2,1-4H3,(H,43,49)(H,44,50)(H,45,48)(H,46,51)(H,47,52)/t27-,34-,35-,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50096884
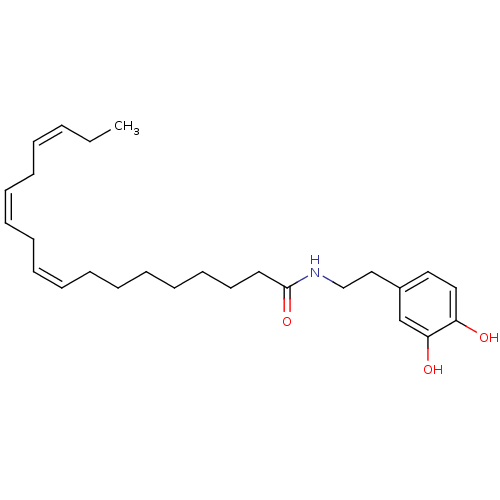
((9Z,12Z,15Z)-Octadeca-9,12,15-trienoic acid [2-(3,...)Show SMILES CC\C=C/C\C=C/C\C=C/CCCCCCCC(=O)NCCc1ccc(O)c(O)c1 Show InChI InChI=1S/C26H39NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26(30)27-21-20-23-18-19-24(28)25(29)22-23/h3-4,6-7,9-10,18-19,22,28-29H,2,5,8,11-17,20-21H2,1H3,(H,27,30)/b4-3-,7-6-,10-9- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Bioorganic Chemistry RAS
Curated by ChEMBL
| Assay Description
Concentration required to displace 0.4 nM [3H]-SR-141,716A from CB1 receptor in rat brain preparations in the presence of 0.1 mM phenylmethyl sulphon... |
Bioorg Med Chem Lett 11: 447-9 (2001)
BindingDB Entry DOI: 10.7270/Q2VT1RBM |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial [N368D]
(Bos taurus (bovine)) | BDBM21960

(3-[(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl]-1-n...)Show SMILES NCCNC[C@@H](N)CCCN=C(N)N[N+]([O-])=O |w:10.9| Show InChI InChI=1S/C8H21N7O2/c9-3-5-12-6-7(10)2-1-4-13-8(11)14-15(16)17/h7,12H,1-6,9-10H2,(H3,11,13,14)/t7-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60E+3 | -31.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Struct Mol Biol 11: 54-9 (2004)
Article DOI: 10.1038/nsmb704
BindingDB Entry DOI: 10.7270/Q27942ZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50430966

(CHEMBL2337849)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(OCc2ccccc2)cc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](C[C@]1(O)C(=O)Nc2ccccc12)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C42H47N5O8/c1-27(36(48)45-35(37(49)43-25-29-13-7-5-8-14-29)24-42(53)32-17-11-12-18-33(32)46-39(42)51)44-38(50)34(47-40(52)55-41(2,3)4)23-28-19-21-31(22-20-28)54-26-30-15-9-6-10-16-30/h5-22,27,34-35,53H,23-26H2,1-4H3,(H,43,49)(H,44,50)(H,45,48)(H,46,51)(H,47,52)/t27-,34-,35-,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6
Curated by ChEMBL
| Assay Description
Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... |
J Med Chem 56: 3367-78 (2013)
Article DOI: 10.1021/jm4002007
BindingDB Entry DOI: 10.7270/Q2F19135 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial [N368D]
(Bos taurus (bovine)) | BDBM21961

((2S,4R)-4-[(2S)-2-amino-5-(1-nitrocarbamimidamido)...)Show SMILES N[C@@H](CCCN=C(N)N[N+]([O-])=O)C(=O)N[C@H]1CN[C@@H](C1)C(N)=O |w:5.4| Show InChI InChI=1S/C11H22N8O4/c12-7(2-1-3-15-11(14)18-19(22)23)10(21)17-6-4-8(9(13)20)16-5-6/h6-8,16H,1-5,12H2,(H2,13,20)(H,17,21)(H3,14,15,18)/t6-,7+,8+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 5.10E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Struct Mol Biol 11: 54-9 (2004)
Article DOI: 10.1038/nsmb704
BindingDB Entry DOI: 10.7270/Q27942ZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50196043

(3-N-(L-ArgNO2)-trans-3-amino-L-proline-NH2 ditrifl...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(=O)N[C@H]1CCN[C@@H]1C(N)=O |w:8.8| Show InChI InChI=1S/C11H22N8O4/c12-6(2-1-4-16-11(14)18-19(22)23)10(21)17-7-3-5-15-8(7)9(13)20/h6-8,15H,1-5,12H2,(H2,13,20)(H,17,21)(H3,14,16,18)/t6-,7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of murine macrophage iNOS |
J Med Chem 49: 6254-63 (2006)
Article DOI: 10.1021/jm0604124
BindingDB Entry DOI: 10.7270/Q2W37VZ8 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50196044
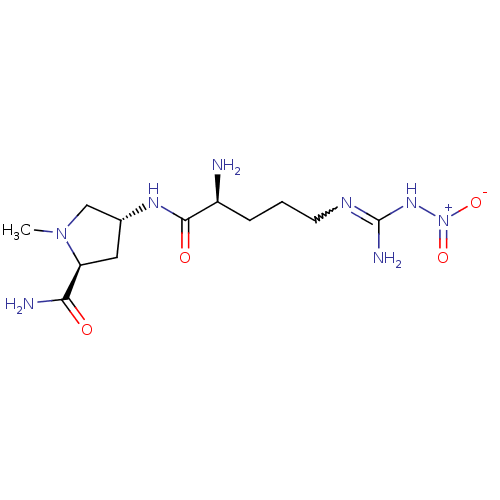
(4-N-(L-ArgNO2)-Nalpha-methyl-trans-4-amino-L-proli...)Show SMILES CN1C[C@@H](C[C@H]1C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N[N+]([O-])=O |w:20.21| Show InChI InChI=1S/C12H24N8O4/c1-19-6-7(5-9(19)10(14)21)17-11(22)8(13)3-2-4-16-12(15)18-20(23)24/h7-9H,2-6,13H2,1H3,(H2,14,21)(H,17,22)(H3,15,16,18)/t7-,8+,9+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS |
J Med Chem 49: 6254-63 (2006)
Article DOI: 10.1021/jm0604124
BindingDB Entry DOI: 10.7270/Q2W37VZ8 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial [N368D]
(Bos taurus (bovine)) | BDBM22030

((2S)-2-amino-N-[(1S)-3-amino-1-(aminocarbonyl)prop...)Show SMILES NCC[C@H](NC(=O)[C@@H](N)CCCN=C(N)N[N+]([O-])=O)C(N)=O |r,w:12.11| Show InChI InChI=1S/C10H22N8O4/c11-4-3-7(8(13)19)16-9(20)6(12)2-1-5-15-10(14)17-18(21)22/h6-7H,1-5,11-12H2,(H2,13,19)(H,16,20)(H3,14,15,17)/t6-,7-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 9.50E+3 | -29.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Struct Mol Biol 11: 54-9 (2004)
Article DOI: 10.1038/nsmb704
BindingDB Entry DOI: 10.7270/Q27942ZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50594017

(CHEMBL5190607) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Homo sapiens (Human)) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
2-Hydroxyacid oxidase 1
(Mus musculus) | BDBM50463047

(CHEMBL1794748)Show InChI InChI=1S/C9H5ClN2O2S2/c10-5-1-3-6(4-2-5)15-9-7(8(13)14)11-12-16-9/h1-4H,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada
Curated by ChEMBL
| Assay Description
Inhibition of mouse N-terminal His-tagged glycolate oxidase expressed in BL21(DE3) Escherichia coli using glycolate as substrate by Cornish-Bowden pl... |
J Med Chem 61: 7144-7167 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00399
BindingDB Entry DOI: 10.7270/Q23B62SD |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain [D597N]
(Rattus norvegicus (rat)) | BDBM21961

((2S,4R)-4-[(2S)-2-amino-5-(1-nitrocarbamimidamido)...)Show SMILES N[C@@H](CCCN=C(N)N[N+]([O-])=O)C(=O)N[C@H]1CN[C@@H](C1)C(N)=O |w:5.4| Show InChI InChI=1S/C11H22N8O4/c12-7(2-1-3-15-11(14)18-19(22)23)10(21)17-6-4-8(9(13)20)16-5-6/h6-8,16H,1-5,12H2,(H2,13,20)(H,17,21)(H3,14,15,18)/t6-,7+,8+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.10E+4 | -27.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
University of California at Irvine
| Assay Description
Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... |
Nat Struct Mol Biol 11: 54-9 (2004)
Article DOI: 10.1038/nsmb704
BindingDB Entry DOI: 10.7270/Q27942ZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data