 Found 124 hits with Last Name = 'yamagishi' and Initial = 'j'
Found 124 hits with Last Name = 'yamagishi' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP-9 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.422 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP-2 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549085
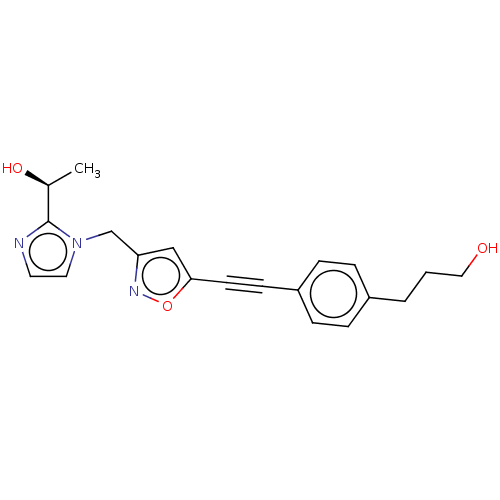
(CHEMBL4752810)Show SMILES C[C@H](O)c1nccn1Cc1cc(on1)C#Cc1ccc(CCCO)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549082
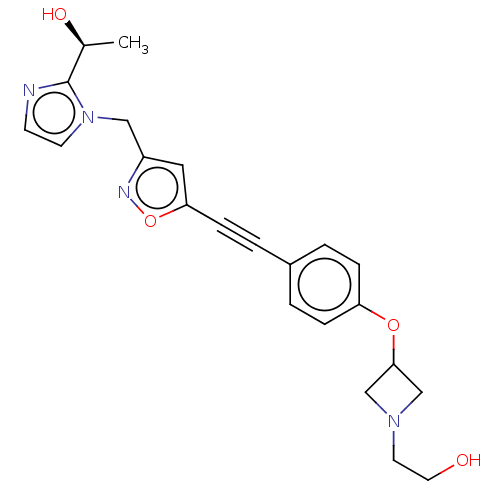
(CHEMBL4763699)Show SMILES C[C@H](O)c1nccn1Cc1cc(on1)C#Cc1ccc(OC2CN(CCO)C2)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549081
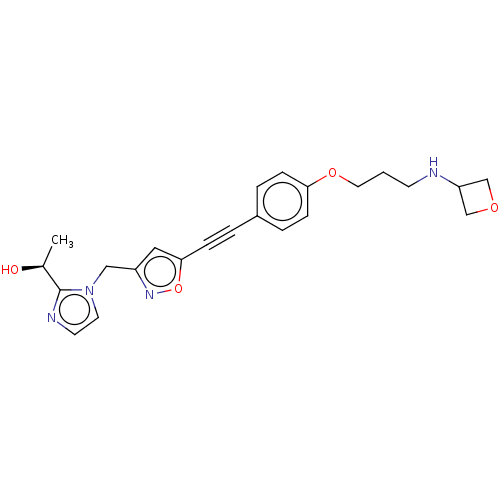
(CHEMBL4779477)Show SMILES C[C@H](O)c1nccn1Cc1cc(on1)C#Cc1ccc(OCCCNC2COC2)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549083
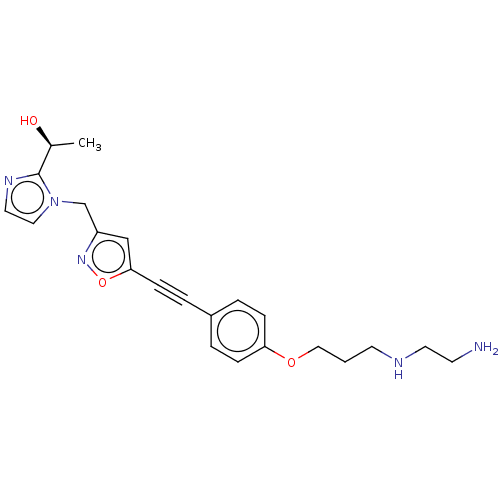
(CHEMBL4798463)Show SMILES C[C@H](O)c1nccn1Cc1cc(on1)C#Cc1ccc(OCCCNCCN)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549086
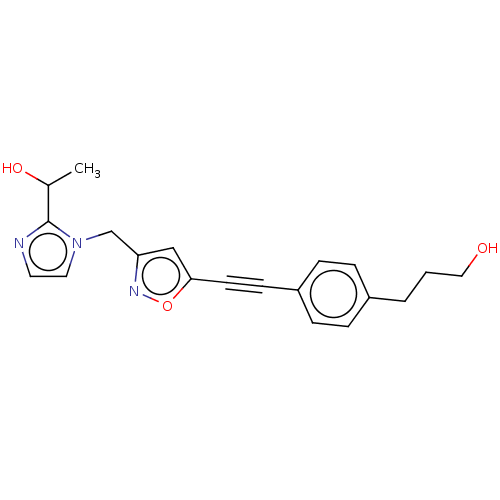
(CHEMBL4795002) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP-3 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP-2 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549079
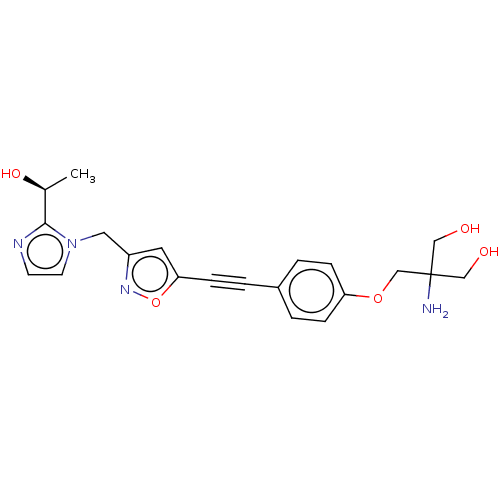
(CHEMBL4747965)Show SMILES C[C@H](O)c1nccn1Cc1cc(on1)C#Cc1ccc(OCC(N)(CO)CO)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549087
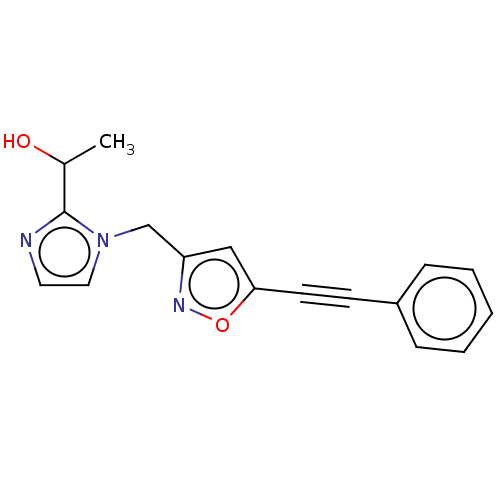
(CHEMBL4750316) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549102
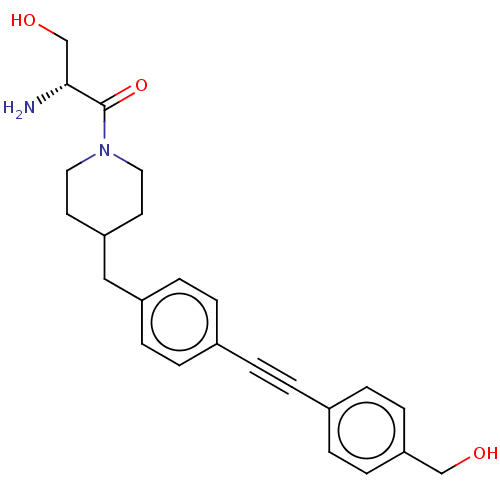
(CHEMBL4743193)Show SMILES N[C@H](CO)C(=O)N1CCC(Cc2ccc(cc2)C#Cc2ccc(CO)cc2)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549092

(CHEMBL4793599) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549103
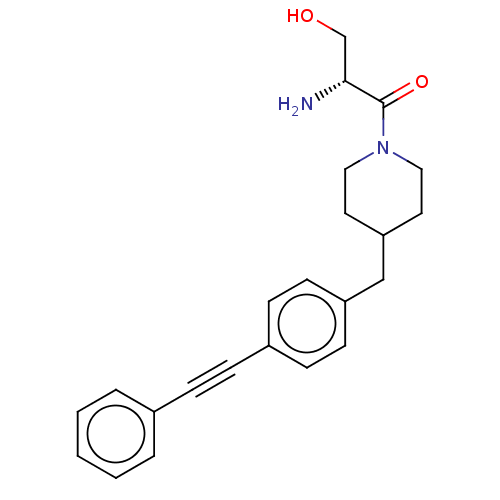
(CHEMBL4786856)Show SMILES N[C@H](CO)C(=O)N1CCC(Cc2ccc(cc2)C#Cc2ccccc2)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549087

(CHEMBL4750316) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549089
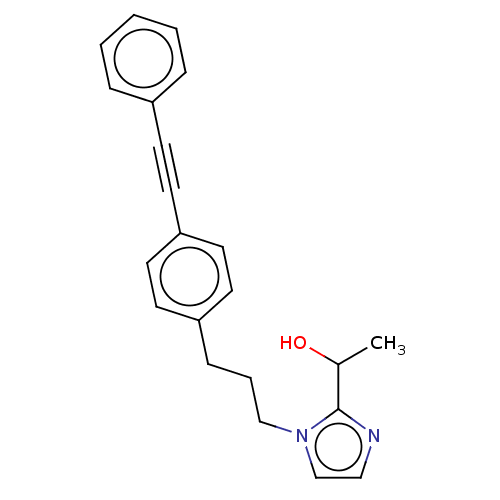
(CHEMBL4794374) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549091
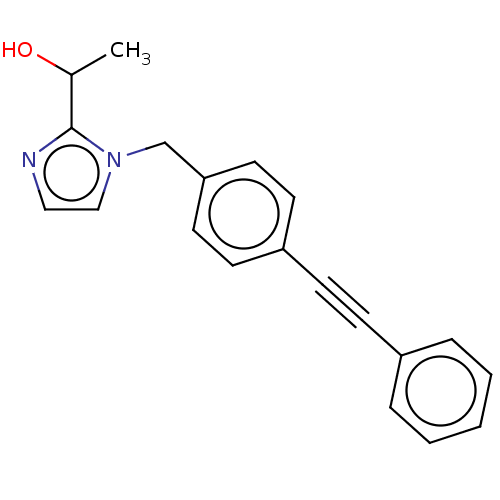
(CHEMBL4791421) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP-9 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549088
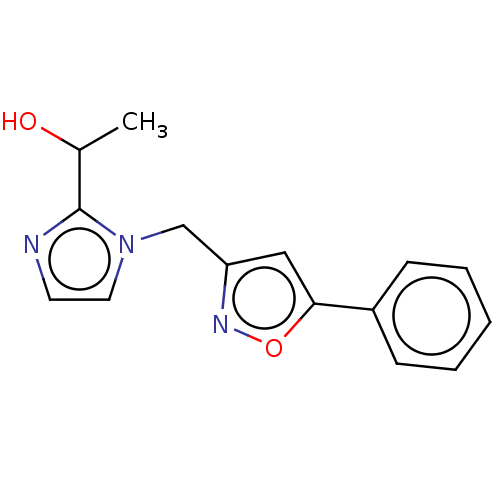
(CHEMBL4795750) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549092

(CHEMBL4793599) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549089

(CHEMBL4794374) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50226181
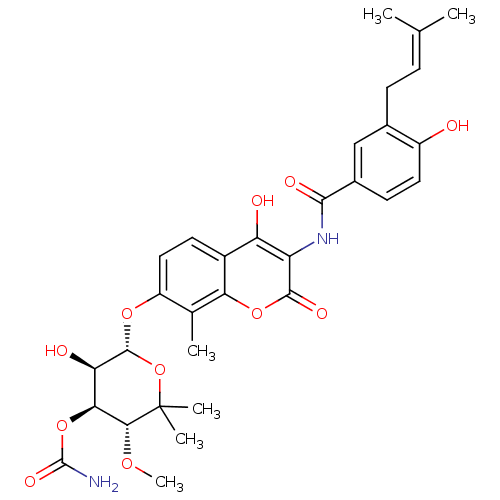
((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#8]-[#6](-[#7])=O)-[#6@@H](-[#8])-[#6@H](-[#8]-c2ccc3c(-[#8])c(-[#7]-[#6](=O)-c4ccc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c4)c(=O)oc3c2-[#6])-[#8]C1([#6])[#6] |r| Show InChI InChI=1S/C31H36N2O11/c1-14(2)7-8-16-13-17(9-11-19(16)34)27(37)33-21-22(35)18-10-12-20(15(3)24(18)42-28(21)38)41-29-23(36)25(43-30(32)39)26(40-6)31(4,5)44-29/h7,9-13,23,25-26,29,34-36H,8H2,1-6H3,(H2,32,39)(H,33,37)/t23-,25+,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Iinhibitory activity against Escherichia coli DNA gyrase |
J Med Chem 47: 3693-6 (2004)
Article DOI: 10.1021/jm030394f
BindingDB Entry DOI: 10.7270/Q2CN76N7 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50226181

((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#8]-[#6](-[#7])=O)-[#6@@H](-[#8])-[#6@H](-[#8]-c2ccc3c(-[#8])c(-[#7]-[#6](=O)-c4ccc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c4)c(=O)oc3c2-[#6])-[#8]C1([#6])[#6] |r| Show InChI InChI=1S/C31H36N2O11/c1-14(2)7-8-16-13-17(9-11-19(16)34)27(37)33-21-22(35)18-10-12-20(15(3)24(18)42-28(21)38)41-29-23(36)25(43-30(32)39)26(40-6)31(4,5)44-29/h7,9-13,23,25-26,29,34-36H,8H2,1-6H3,(H2,32,39)(H,33,37)/t23-,25+,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PubMed
| n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 416 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MMP-3 by fluorometric assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223579

(CHEMBL442194)Show SMILES Cc1cc(=O)oc2cc(CCc3n[nH]c4cccc(OCc5ccc(cc5)C(C)(C)C)c34)ccc12 Show InChI InChI=1S/C30H30N2O3/c1-19-16-28(33)35-27-17-20(10-14-23(19)27)11-15-25-29-24(31-32-25)6-5-7-26(29)34-18-21-8-12-22(13-9-21)30(2,3)4/h5-10,12-14,16-17H,11,15,18H2,1-4H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 535 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549094

(CHEMBL4751265) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549091

(CHEMBL4791421) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50366822
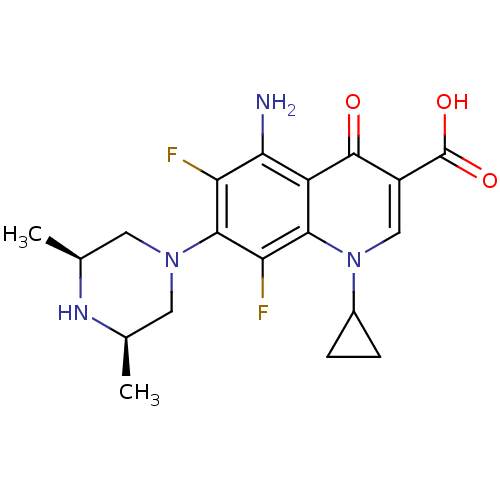
(AT-4140 | CI-978 | SPARFLOXACIN | Spara)Show SMILES C[C@H]1CN(C[C@@H](C)N1)c1c(F)c(N)c2c(c1F)n(cc(C(O)=O)c2=O)C1CC1 |r| Show InChI InChI=1S/C19H22F2N4O3/c1-8-5-24(6-9(2)23-8)17-13(20)15(22)12-16(14(17)21)25(10-3-4-10)7-11(18(12)26)19(27)28/h7-10,23H,3-6,22H2,1-2H3,(H,27,28)/t8-,9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 637 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Iinhibitory activity against Escherichia coli DNA gyrase |
J Med Chem 47: 3693-6 (2004)
Article DOI: 10.1021/jm030394f
BindingDB Entry DOI: 10.7270/Q2CN76N7 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549105
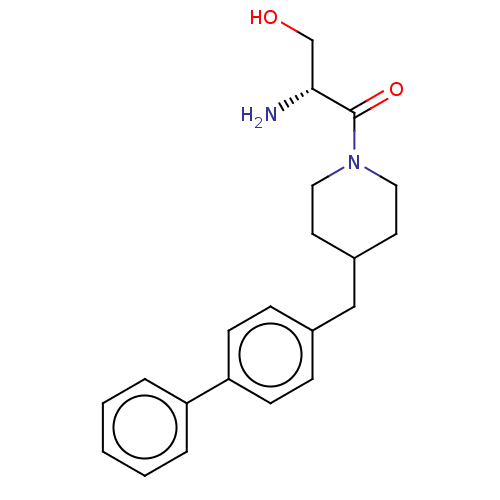
(CHEMBL4751118)Show SMILES N[C@H](CO)C(=O)N1CCC(Cc2ccc(cc2)-c2ccccc2)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549097
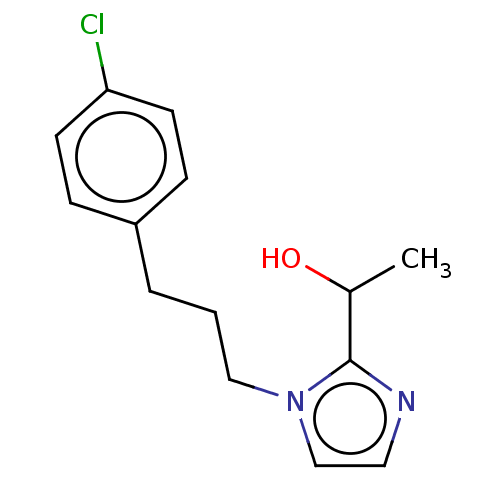
(CHEMBL4794206) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 809 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549088

(CHEMBL4795750) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 884 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223575
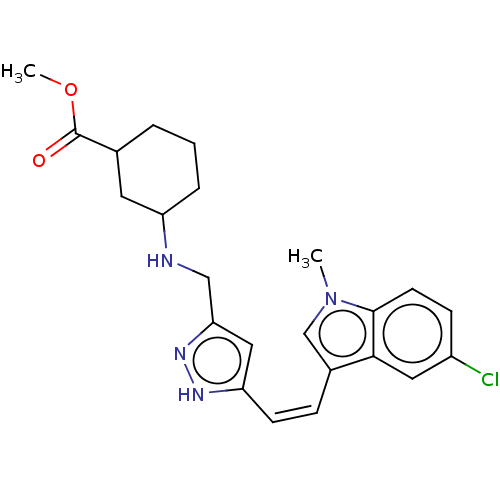
(CHEMBL318448)Show SMILES COC(=O)C1CCCC(C1)NCc1cc(\C=C/c2cn(C)c3ccc(Cl)cc23)[nH]n1 Show InChI InChI=1S/C23H27ClN4O2/c1-28-14-16(21-11-17(24)7-9-22(21)28)6-8-19-12-20(27-26-19)13-25-18-5-3-4-15(10-18)23(29)30-2/h6-9,11-12,14-15,18,25H,3-5,10,13H2,1-2H3,(H,26,27)/b8-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549084

(CHEMBL4757624)Show SMILES C[C@@H](O)c1nccn1Cc1cc(on1)C#Cc1ccc(CCCO)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549090

(CHEMBL4759498) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223589
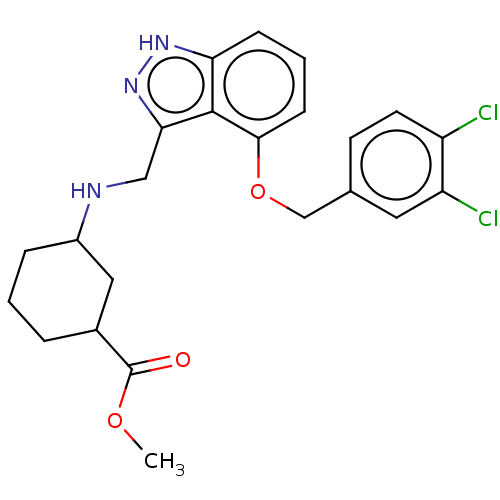
(CHEMBL97620)Show SMILES COC(=O)C1CCCC(C1)NCc1n[nH]c2cccc(OCc3ccc(Cl)c(Cl)c3)c12 Show InChI InChI=1S/C23H25Cl2N3O3/c1-30-23(29)15-4-2-5-16(11-15)26-12-20-22-19(27-28-20)6-3-7-21(22)31-13-14-8-9-17(24)18(25)10-14/h3,6-10,15-16,26H,2,4-5,11-13H2,1H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223614
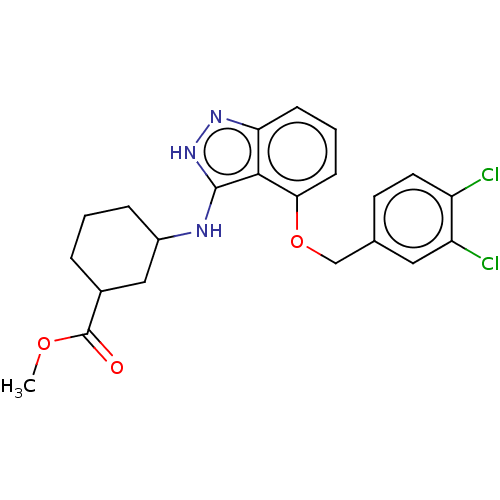
(CHEMBL97490)Show SMILES COC(=O)C1CCCC(C1)Nc1[nH]nc2cccc(OCc3ccc(Cl)c(Cl)c3)c12 Show InChI InChI=1S/C22H23Cl2N3O3/c1-29-22(28)14-4-2-5-15(11-14)25-21-20-18(26-27-21)6-3-7-19(20)30-12-13-8-9-16(23)17(24)10-13/h3,6-10,14-15H,2,4-5,11-12H2,1H3,(H2,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223600

(CHEMBL316656)Show SMILES COC(=O)C1CCCC(C1)NCc1cc(\C=C\c2cn(C)c3ccc(Cl)cc23)[nH]n1 Show InChI InChI=1S/C23H27ClN4O2/c1-28-14-16(21-11-17(24)7-9-22(21)28)6-8-19-12-20(27-26-19)13-25-18-5-3-4-15(10-18)23(29)30-2/h6-9,11-12,14-15,18,25H,3-5,10,13H2,1-2H3,(H,26,27)/b8-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223575

(CHEMBL318448)Show SMILES COC(=O)C1CCCC(C1)NCc1cc(\C=C/c2cn(C)c3ccc(Cl)cc23)[nH]n1 Show InChI InChI=1S/C23H27ClN4O2/c1-28-14-16(21-11-17(24)7-9-22(21)28)6-8-19-12-20(27-26-19)13-25-18-5-3-4-15(10-18)23(29)30-2/h6-9,11-12,14-15,18,25H,3-5,10,13H2,1-2H3,(H,26,27)/b8-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223600

(CHEMBL316656)Show SMILES COC(=O)C1CCCC(C1)NCc1cc(\C=C\c2cn(C)c3ccc(Cl)cc23)[nH]n1 Show InChI InChI=1S/C23H27ClN4O2/c1-28-14-16(21-11-17(24)7-9-22(21)28)6-8-19-12-20(27-26-19)13-25-18-5-3-4-15(10-18)23(29)30-2/h6-9,11-12,14-15,18,25H,3-5,10,13H2,1-2H3,(H,26,27)/b8-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549090

(CHEMBL4759498) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549094

(CHEMBL4751265) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549104

(CHEMBL4750137)Show SMILES N[C@H](CO)C(=O)N1CCC(Cc2ccc(CCc3ccccc3)cc2)CC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549107
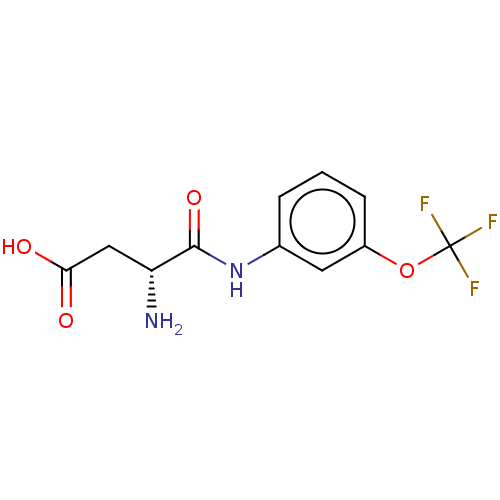
(CHEMBL4755686)Show SMILES N[C@H](CC(O)=O)C(=O)Nc1cccc(OC(F)(F)F)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549093

(CHEMBL4747127) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223603

(CHEMBL95741)Show SMILES COC(=O)C1CCCC(C1)NCc1n[nH]c2cccc(OCc3ccc(cc3)C(C)(C)C)c12 Show InChI InChI=1S/C27H35N3O3/c1-27(2,3)20-13-11-18(12-14-20)17-33-24-10-6-9-22-25(24)23(30-29-22)16-28-21-8-5-7-19(15-21)26(31)32-4/h6,9-14,19,21,28H,5,7-8,15-17H2,1-4H3,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223585
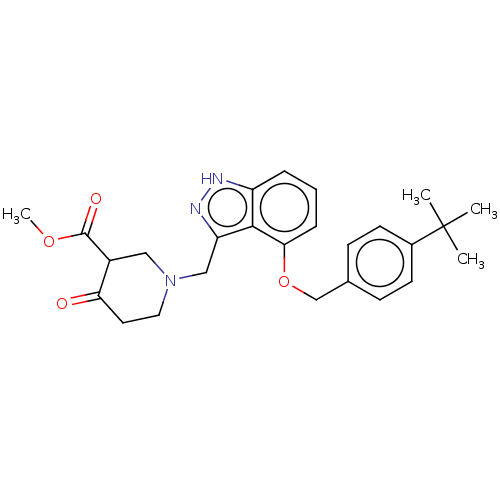
(CHEMBL95667)Show SMILES COC(=O)C1CN(Cc2n[nH]c3cccc(OCc4ccc(cc4)C(C)(C)C)c23)CCC1=O Show InChI InChI=1S/C26H31N3O4/c1-26(2,3)18-10-8-17(9-11-18)16-33-23-7-5-6-20-24(23)21(28-27-20)15-29-13-12-22(30)19(14-29)25(31)32-4/h5-11,19H,12-16H2,1-4H3,(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549109

(CHEMBL4744809)Show SMILES CS(=O)(=O)CC[C@@H](N)C(=O)Nc1cccc(OC(F)(F)F)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of (S)-N-(2-(N-(3-biphenyl-4-ylcarboxamido-4-(hydroxyamino)-4-oxobutyl)sulfamoyl)ethyl)-3',6'-dihydroxy-3-oxo-3H-spiro[isobenzofuran-1,9... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549113

(CHEMBL4747065) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50223613

(CHEMBL96995)Show SMILES COC(=O)C1CCCC(C1)Nc1[nH]nc2cccc(OCc3ccc(cc3)C(C)(C)C)c12 Show InChI InChI=1S/C26H33N3O3/c1-26(2,3)19-13-11-17(12-14-19)16-32-22-10-6-9-21-23(22)24(29-28-21)27-20-8-5-7-18(15-20)25(30)31-4/h6,9-14,18,20H,5,7-8,15-16H2,1-4H3,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli DNA gyrase |
Bioorg Med Chem Lett 14: 2857-62 (2004)
BindingDB Entry DOI: 10.7270/Q2HH6N85 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50549109

(CHEMBL4744809)Show SMILES CS(=O)(=O)CC[C@@H](N)C(=O)Nc1cccc(OC(F)(F)F)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histidine-tagged Pseudomonas aeruginosa LpxC (1-303) expressed in Escherichia coli BL21 (DE3) using UDP-3-O-(R-3-hydroxydecanoyl)-N-ace... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01215
BindingDB Entry DOI: 10.7270/Q2Q81HPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data