

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173356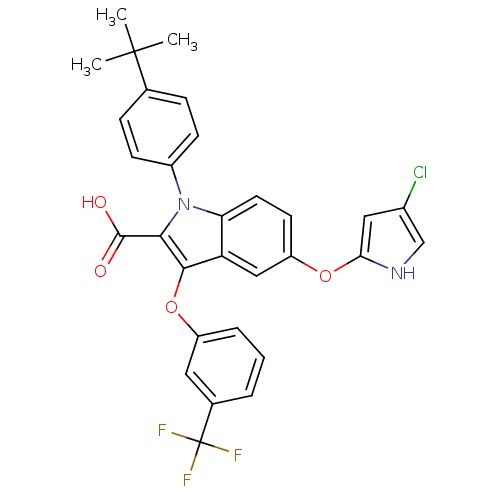 (1-(4-tert-Butyl-phenyl)-5-(4-chloro-1H-pyrrol-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234607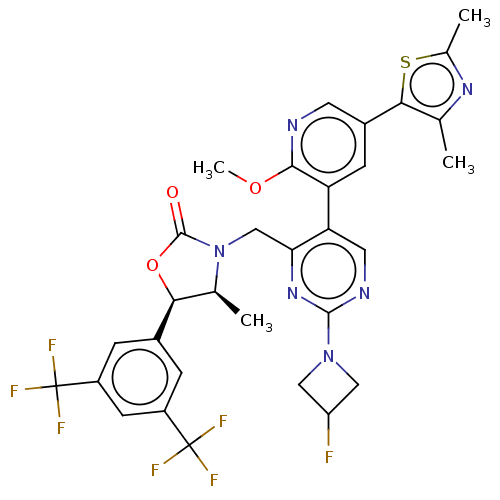 (US9353101, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234611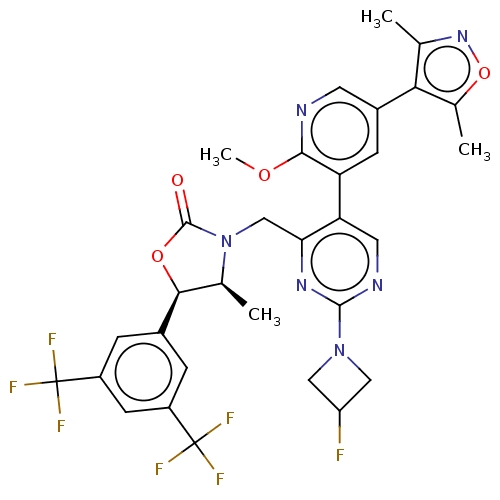 (US9353101, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234610 (US9353101, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173344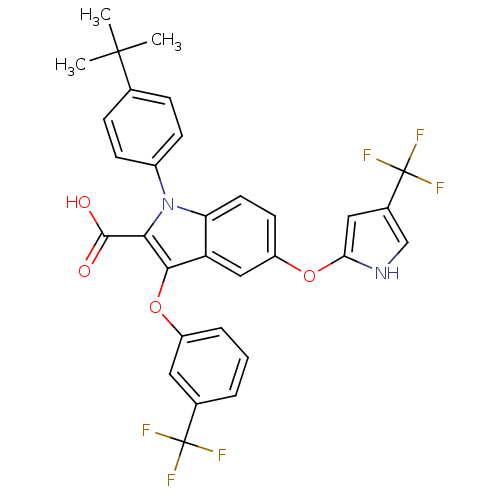 (1-(4-tert-Butyl-phenyl)-3-(3-trifluoromethyl-pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234621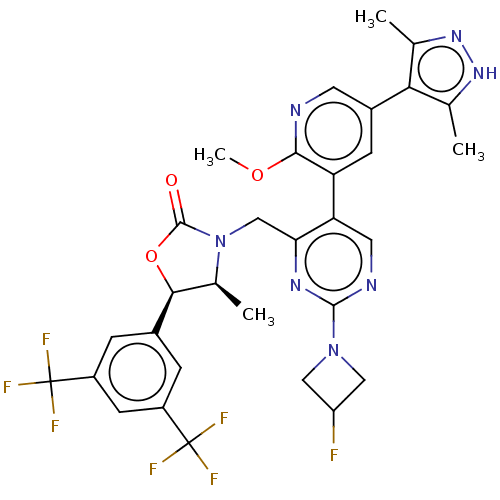 (US9353101, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234664 (US9353101, 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234635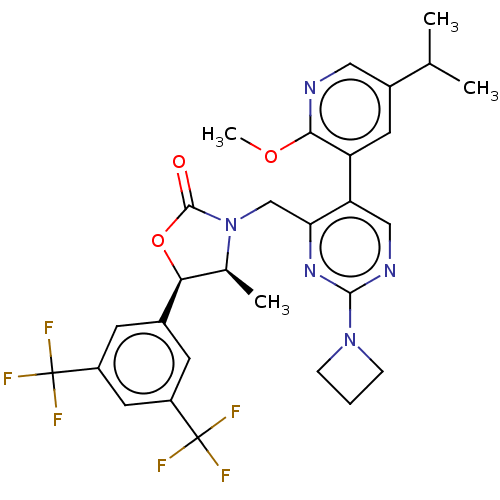 (US9353101, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234661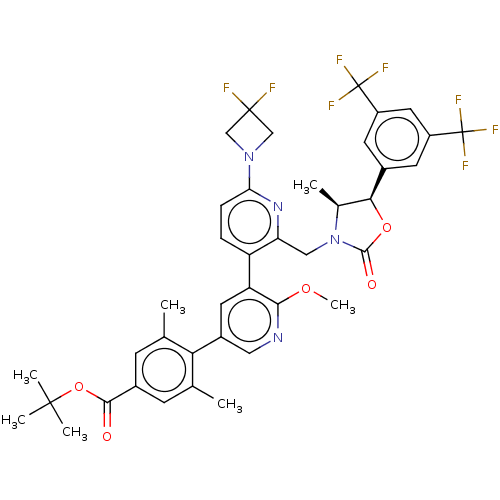 (US9353101, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234632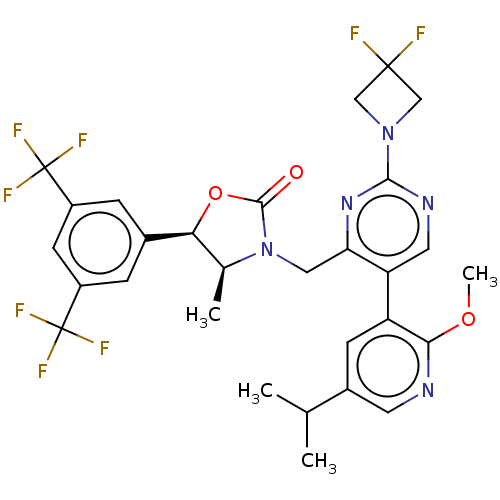 (US9353101, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234666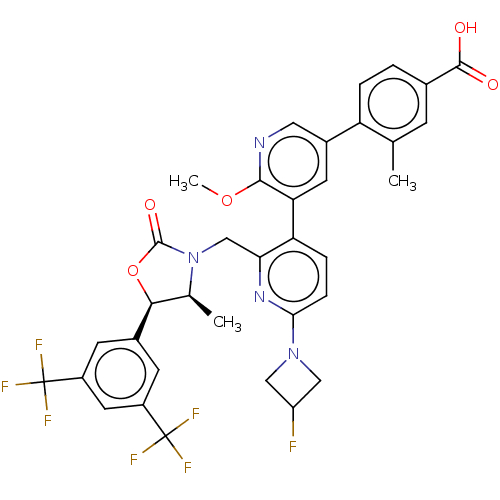 (US9353101, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234662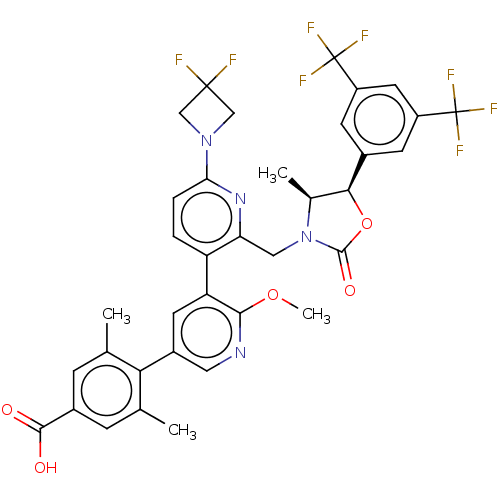 (US9353101, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234636 (US9353101, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234637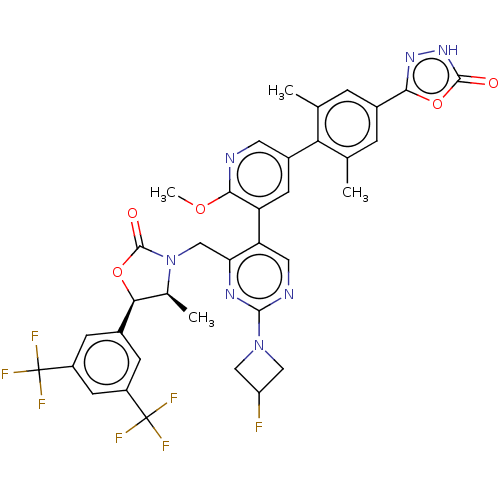 (US9353101, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234623 (US9353101, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234660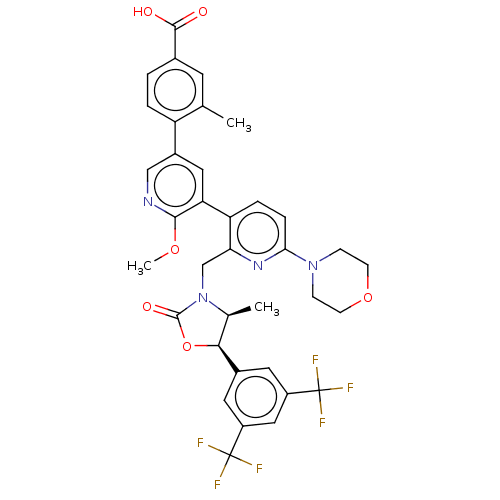 (US9353101, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234651 (US9353101, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234646 (US9353101, 42) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234638 (US9353101, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234641 (US9353101, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234639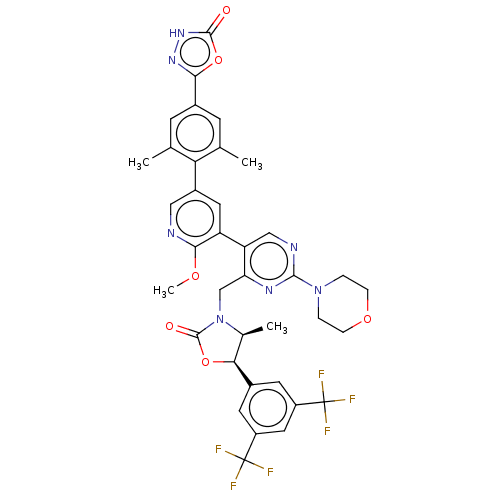 (US9353101, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173351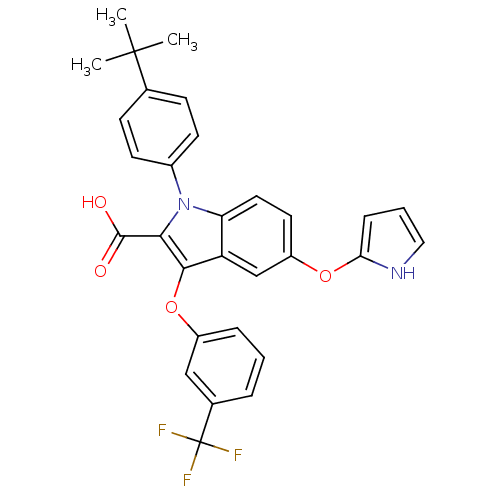 (1-(4-tert-Butyl-phenyl)-5-(1H-pyrrol-2-yloxy)-3-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234645 (US9353101, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234606 (US9353101, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description First, low density lipoprotein (LDL) (Meridian) was biotinylated by incubating LDL with biotin for 1 hour on ice, after which it was dialyzed to remo... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173359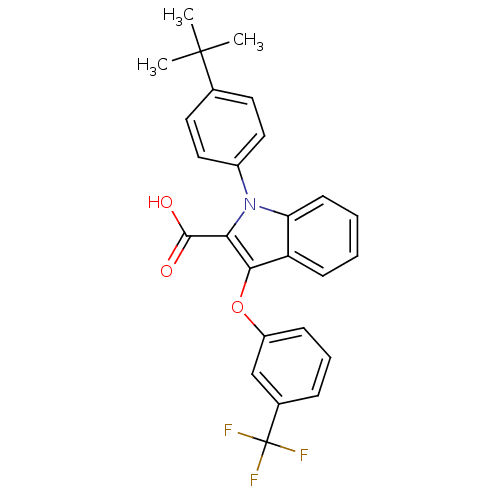 (1-(4-tert-Butyl-phenyl)-3-(3-trifluoromethyl-pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234614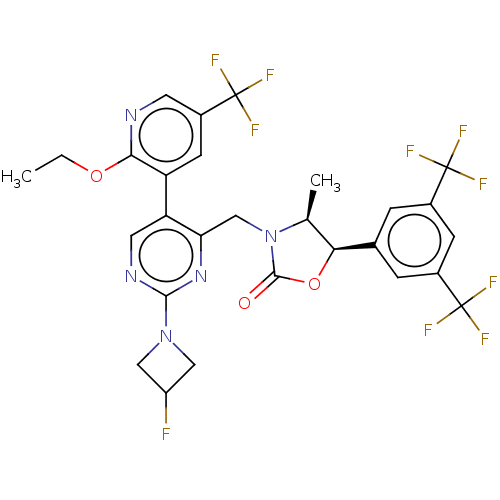 (US9353101, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234650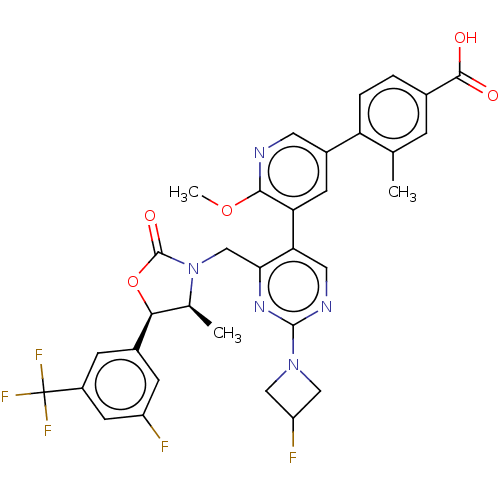 (US9353101, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234640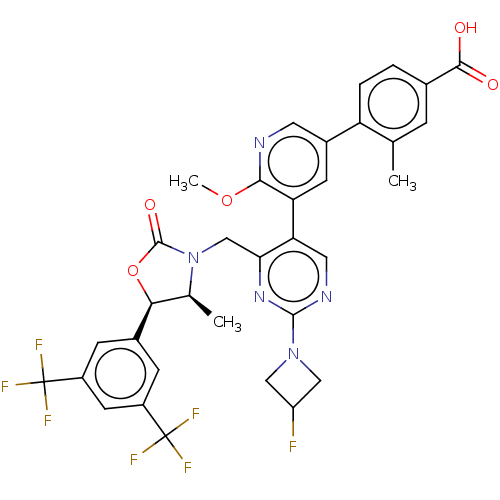 (US9353101, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234642 (US9353101, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234622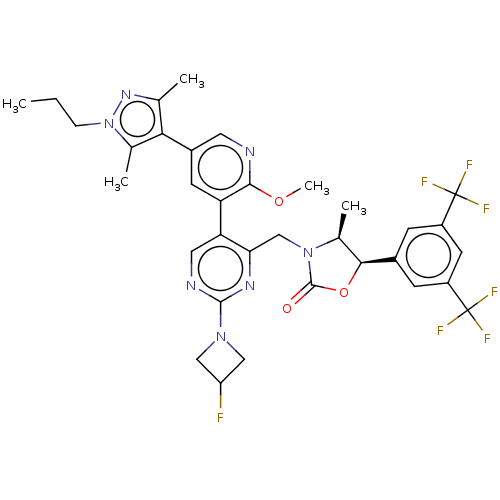 (US9353101, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234643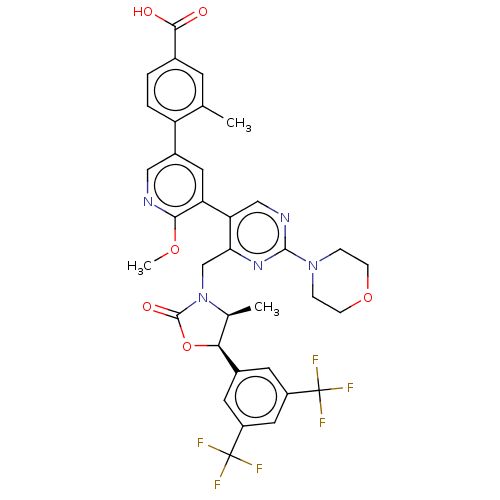 (US9353101, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173348 (1-(4-tert-Butyl-phenyl)-5-chloro-3-(4-chloro-3-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234620 (US9353101, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234613 (US9353101, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234627 (US9353101, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234665 (US9353101, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234663 (US9353101, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234652 (US9353101, 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234618 (US9353101, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173366 (1-(4-tert-Butyl-phenyl)-5-chloro-3-(3-trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173357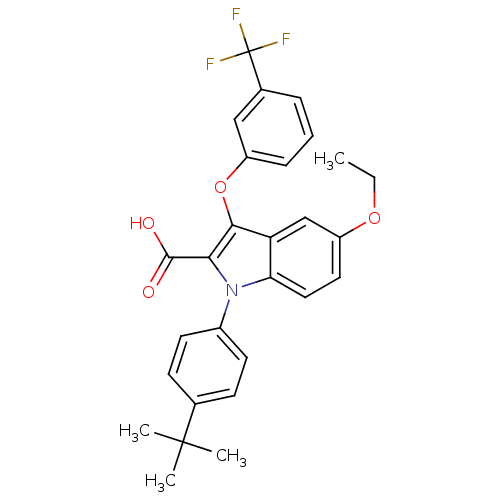 (1-(4-tert-Butyl-phenyl)-5-ethoxy-3-(3-trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234634 (US9353101, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234625 (US9353101, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173354 (1-(4-tert-Butyl-phenyl)-5-isobutoxy-3-(3-trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234631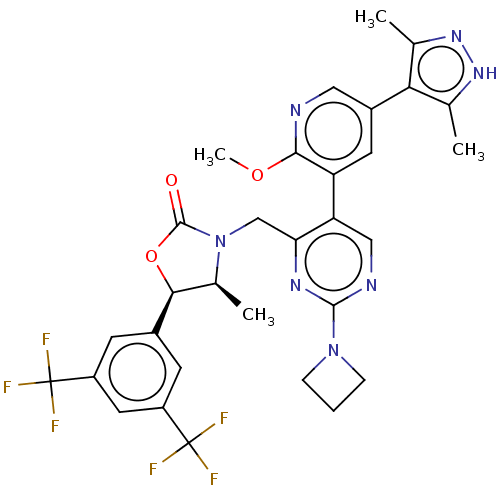 (US9353101, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173343 (1-(4-tert-Butyl-phenyl)-6-chloro-3-(3-isopropyl-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234629 (US9353101, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50173362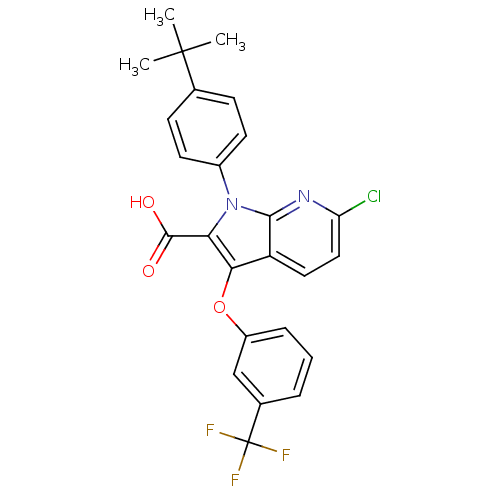 (1-(4-tert-Butyl-phenyl)-6-chloro-3-(3-trifluoromet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human peroxisome proliferator activated receptor gamma in SPA assay | Bioorg Med Chem Lett 15: 5035-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.002 BindingDB Entry DOI: 10.7270/Q27H1J56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234659 (US9353101, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234626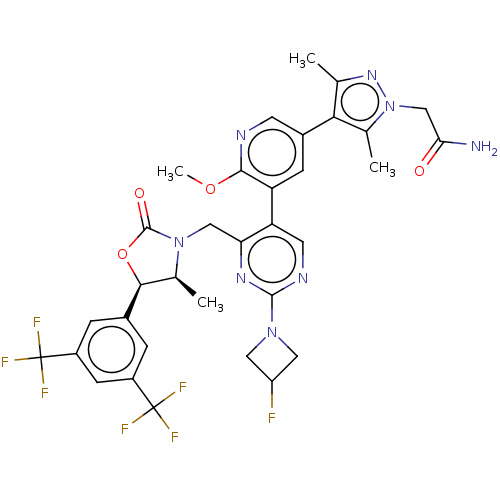 (US9353101, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 356 total ) | Next | Last >> |