 Found 33 hits with Last Name = 'jamie' and Initial = 'jf'
Found 33 hits with Last Name = 'jamie' and Initial = 'jf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300296
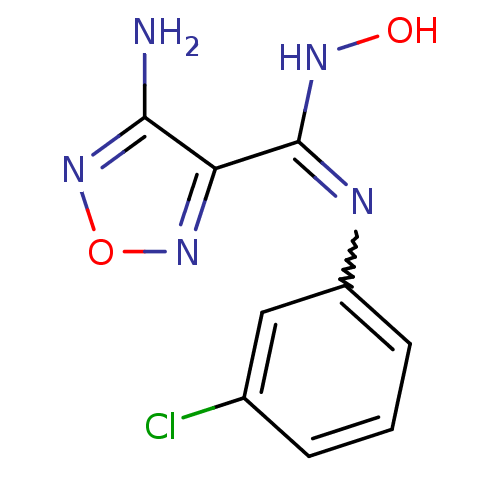
(4-Amino-N-(3-chlorophenyl)-N'-hydroxy-1,2,5-oxadia...)Show InChI InChI=1S/C9H8ClN5O2/c10-5-2-1-3-6(4-5)12-9(13-16)7-8(11)15-17-14-7/h1-4,16H,(H2,11,15)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 by magnetic circular dichroism spectroscopic analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50325571
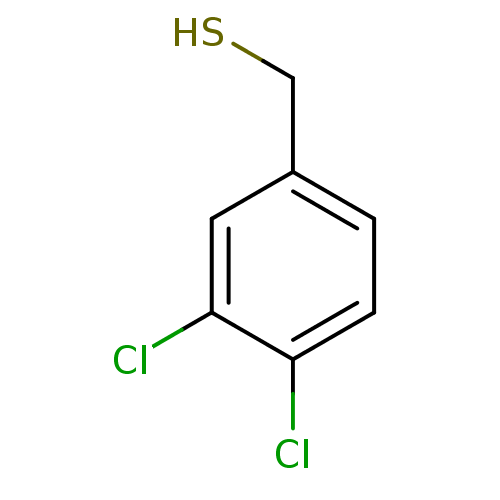
(3,4-Dichlorobenzenemethanethiol | CHEMBL1224626)Show InChI InChI=1S/C7H6Cl2S/c8-6-2-1-5(4-10)3-7(6)9/h1-3,10H,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50444455
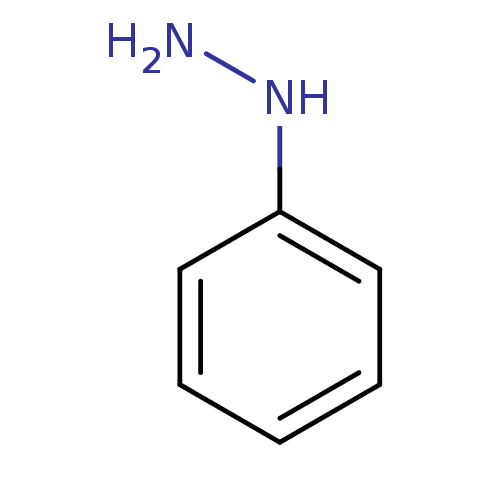
(CHEBI:27924 | Phenylhydrazine)Show InChI InChI=1S/C6H8N2/c7-8-6-4-2-1-3-5-6/h1-5,8H,7H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444455

(CHEBI:27924 | Phenylhydrazine)Show InChI InChI=1S/C6H8N2/c7-8-6-4-2-1-3-5-6/h1-5,8H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50325541
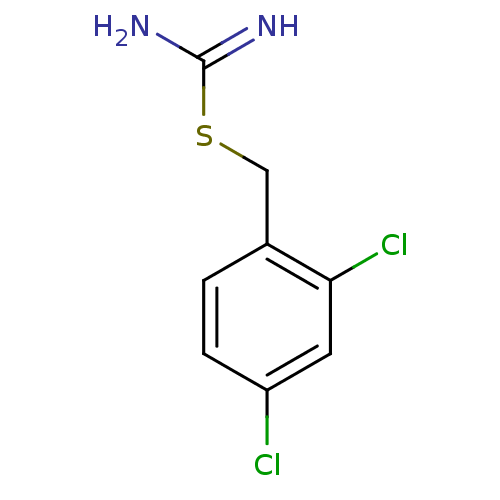
(CHEMBL1224483 | CHEMBL1229058 | S-(2,4-Dichloroben...)Show InChI InChI=1S/C8H8Cl2N2S/c9-6-2-1-5(7(10)3-6)4-13-8(11)12/h1-3H,4H2,(H3,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300312
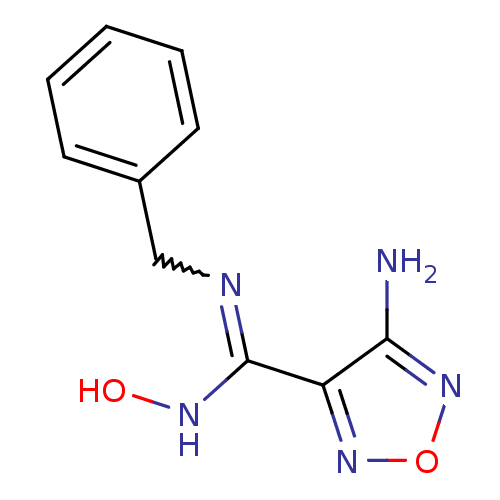
(4-amino-1,2,5-oxadiazole-3-carboximidamide | CHEMB...)Show InChI InChI=1S/C10H11N5O2/c11-9-8(14-17-15-9)10(13-16)12-6-7-4-2-1-3-5-7/h1-5,16H,6H2,(H2,11,15)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 by magnetic circular dichroism spectroscopic analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444455

(CHEBI:27924 | Phenylhydrazine)Show InChI InChI=1S/C6H8N2/c7-8-6-4-2-1-3-5-6/h1-5,8H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300308
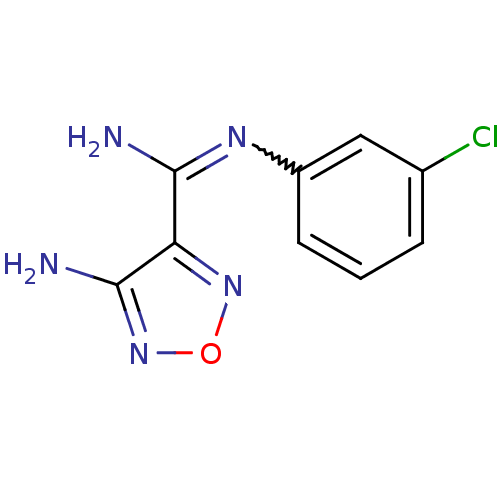
(4-Amino-N-(3-chlorophenyl)-1,2,5-oxadiazole-3-carb...)Show InChI InChI=1S/C9H8ClN5O/c10-5-2-1-3-6(4-5)13-8(11)7-9(12)15-16-14-7/h1-4H,(H2,11,13)(H2,12,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 by magnetic circular dichroism spectroscopic analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444457

(CHEMBL3092383)Show InChI InChI=1S/C7H6ClN3S/c8-4-1-2-6-5(3-4)10-7(11-9)12-6/h1-3H,9H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300302
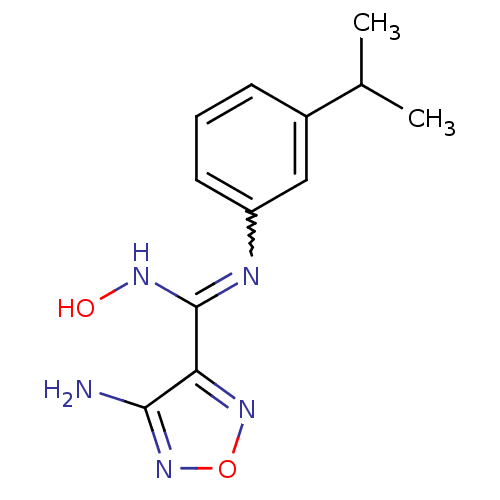
(4-Amino-N'-hydroxy-N-(3-isopropylphenyl)-1,2,5-oxa...)Show InChI InChI=1S/C12H15N5O2/c1-7(2)8-4-3-5-9(6-8)14-12(15-18)10-11(13)17-19-16-10/h3-7,18H,1-2H3,(H2,13,17)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 by magnetic circular dichroism spectroscopic analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50325570
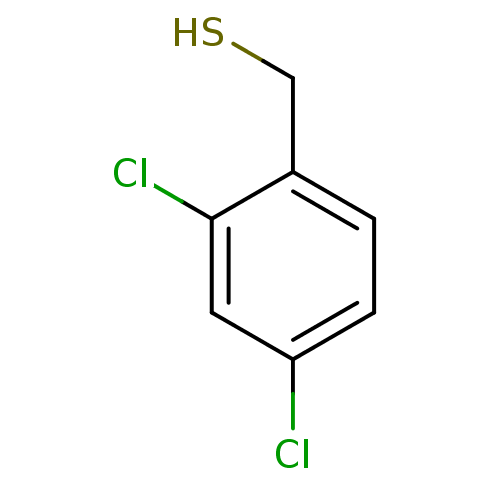
(2,4-Dichlorobenzenemethanethiol | CHEMBL1224561)Show InChI InChI=1S/C7H6Cl2S/c8-6-2-1-5(4-10)7(9)3-6/h1-3,10H,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300297

(4-Amino-N-(4-chlorophenyl)-N'-hydroxy-1,2,5-oxadia...)Show InChI InChI=1S/C9H8ClN5O2/c10-5-1-3-6(4-2-5)12-9(13-16)7-8(11)15-17-14-7/h1-4,16H,(H2,11,15)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 by magnetic circular dichroism spectroscopic analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444456

(2-Hydrazinylbenzo[D]Thiazole | CHEMBL1933308)Show InChI InChI=1S/C7H7N3S/c8-10-7-9-5-3-1-2-4-6(5)11-7/h1-4H,8H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50241727

((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by spectrophotometric analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50325537
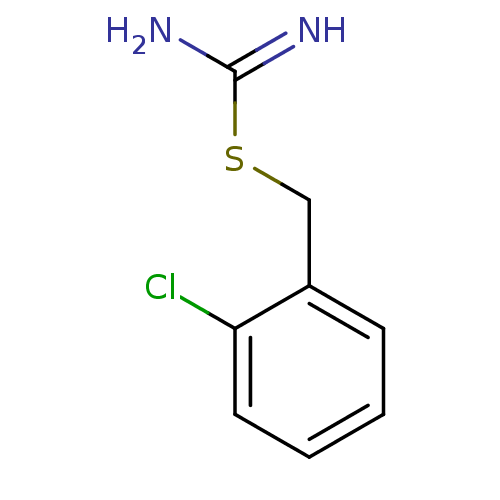
(CHEMBL1224310 | CHEMBL1229095 | S-(2-Chlorobenzyl)...)Show InChI InChI=1S/C8H9ClN2S/c9-7-4-2-1-3-6(7)5-12-8(10)11/h1-4H,5H2,(H3,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50360482

(CHEMBL1935084)Show InChI InChI=1S/C11H9NO2/c12-10-6-8-4-2-1-3-7(8)5-9(10)11(13)14/h1-6H,12H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by spectrophotometric analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50360489

(CHEMBL1935087)Show SMILES [O-][N+](=O)c1cc(ccc1Oc1ccc(cc1)C(=[NH2+])[N-]OC(=O)c1cc2ccccc2oc1=O)C(F)(F)F Show InChI InChI=1S/C24H13F3N3O7/c25-24(26,27)15-7-10-20(18(12-15)30(33)34)35-16-8-5-13(6-9-16)21(28)29-37-23(32)17-11-14-3-1-2-4-19(14)36-22(17)31/h1-12H,(H-,28,29)/q-1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by Ehrlich's method |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50360491
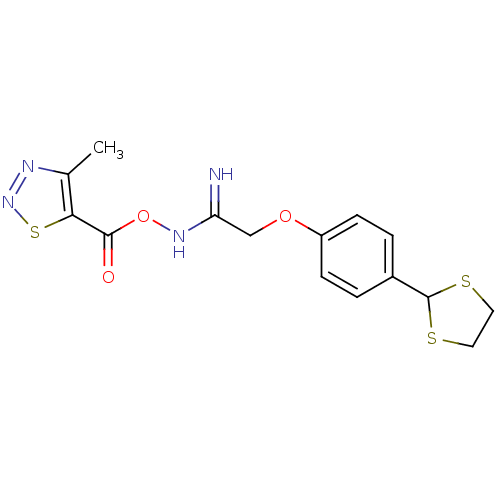
(CHEMBL1935098)Show InChI InChI=1S/C15H16N4O3S3/c1-9-13(25-19-17-9)14(20)22-18-12(16)8-21-11-4-2-10(3-5-11)15-23-6-7-24-15/h2-5,15H,6-8H2,1H3,(H2,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by Ehrlich's method |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50360488

(CHEMBL1414365)Show InChI InChI=1S/C9H9N3S/c10-6-1-2-8-7(5-6)12-4-3-11-9(12)13-8/h1-2,5H,3-4,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by HPLC analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50444456

(2-Hydrazinylbenzo[D]Thiazole | CHEMBL1933308)Show InChI InChI=1S/C7H7N3S/c8-10-7-9-5-3-1-2-4-6(5)11-7/h1-4H,8H2,(H,9,10) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50325556

(CHEMBL1224487 | CHEMBL1229062 | S-(4-Chloropheneth...)Show InChI InChI=1S/C9H11ClN2S/c10-8-3-1-7(2-4-8)5-6-13-9(11)12/h1-4H,5-6H2,(H3,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50360490

(CHEMBL1935094)Show SMILES NC(=NOC(=O)c1ccccc1Cl)c1cccc(CS(=O)(=O)c2ccc(Cl)cc2)c1 |w:2.2| Show InChI InChI=1S/C21H16Cl2N2O4S/c22-16-8-10-17(11-9-16)30(27,28)13-14-4-3-5-15(12-14)20(24)25-29-21(26)18-6-1-2-7-19(18)23/h1-12H,13H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by Ehrlich's method |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50271830
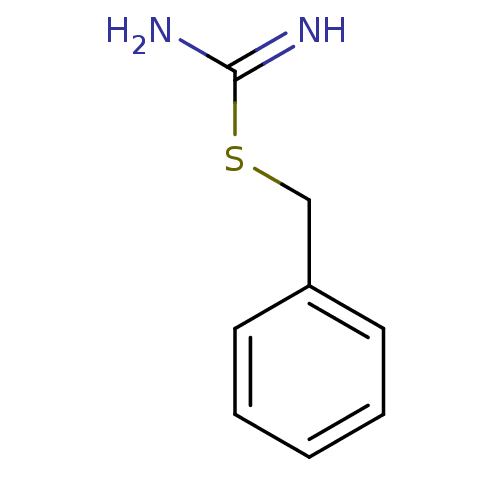
(2-Benzyl-isothiourea | CHEMBL1224309 | CHEMBL50566...)Show InChI InChI=1S/C8H10N2S/c9-8(10)11-6-7-4-2-1-3-5-7/h1-5H,6H2,(H3,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444456

(2-Hydrazinylbenzo[D]Thiazole | CHEMBL1933308)Show InChI InChI=1S/C7H7N3S/c8-10-7-9-5-3-1-2-4-6(5)11-7/h1-4H,8H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50013811
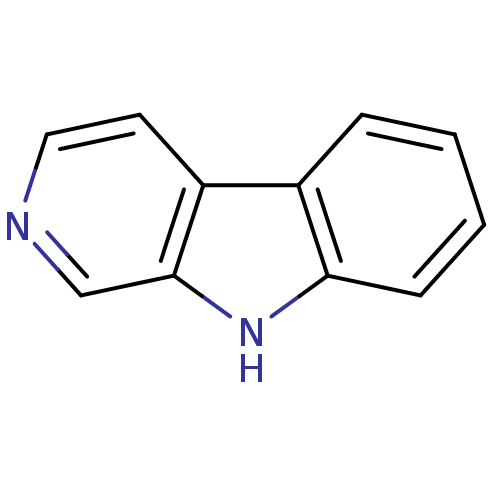
(9H-beta-Carboline | 9H-pyrido[3,4-b]indole | CHEMB...)Show InChI InChI=1S/C11H8N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-7,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by spectrophotometric analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444459
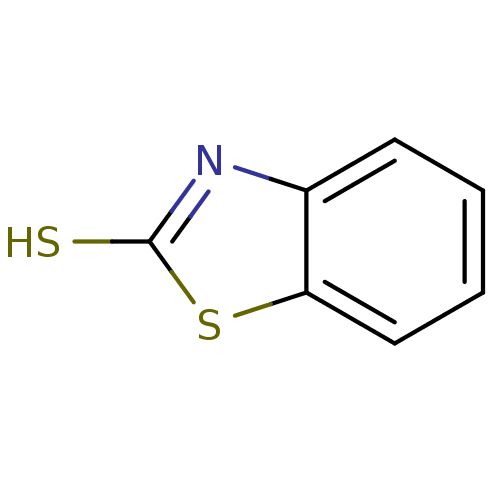
(2-Mercaptobenzothiazole | 2-Mercaptobenzothioazole...)Show InChI InChI=1S/C7H5NS2/c9-7-8-5-3-1-2-4-6(5)10-7/h1-4H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50360483

(CHEMBL1935085)Show InChI InChI=1S/C11H11NO/c12-11-6-9-4-2-1-3-8(9)5-10(11)7-13/h1-6,13H,7,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by spectrophotometric analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50360484

(CHEMBL145337)Show InChI InChI=1S/C12H11NO2/c1-15-12(14)10-6-8-4-2-3-5-9(8)7-11(10)13/h2-7H,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by spectrophotometric analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444458
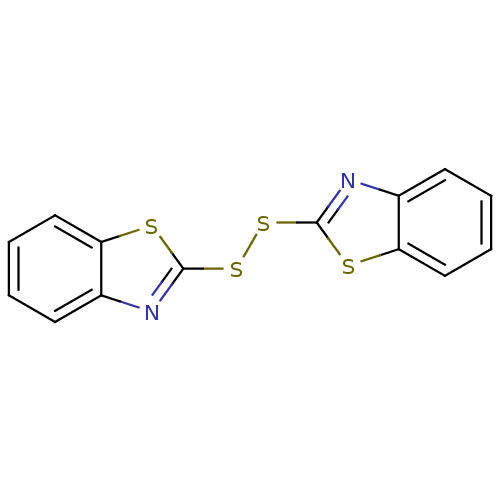
(BENZOTHIAZYL DISULFIDE | BI-87F4 | CHEBI:53239)Show InChI InChI=1S/C14H8N2S4/c1-3-7-11-9(5-1)15-13(17-11)19-20-14-16-10-6-2-4-8-12(10)18-14/h1-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50335098

(6-Nitro-benzothiazol-2-ylamine | 6-nitrobenzo[d]th...)Show InChI InChI=1S/C7H5N3O2S/c8-7-9-5-2-1-4(10(11)12)3-6(5)13-7/h1-3H,(H2,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444460
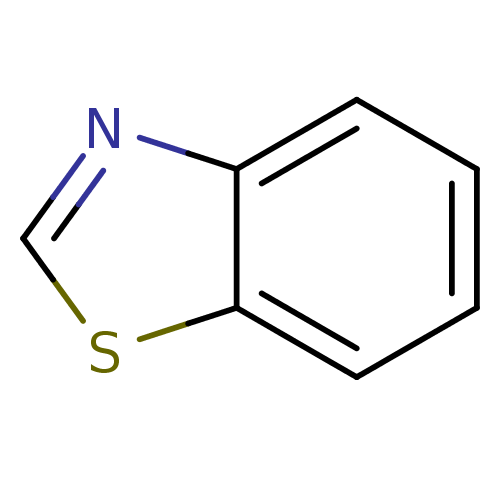
(Benzo[D]Thiazole | Benzothiazole | CHEBI:45993)Show InChI InChI=1S/C7H5NS/c1-2-4-7-6(3-1)8-5-9-7/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50219485

(3-hydroxy-2-naphthoic acid | CHEMBL122279 | CHEMBL...)Show InChI InChI=1S/C11H8O3/c12-10-6-8-4-2-1-3-7(8)5-9(10)11(13)14/h1-6,12H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged recombinant human IDO1 expressed in Escherichia coli using tryptophan as substrate by spectrophotometric analysis |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50047009
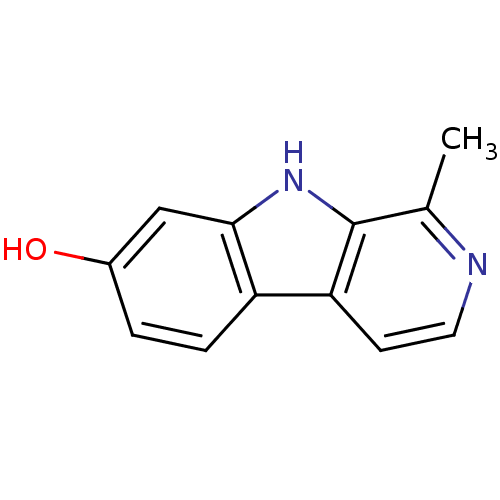
(1-Methyl-9H-beta-carbolin-7-ol | 1-Methyl-9H-beta-...)Show InChI InChI=1S/C12H10N2O/c1-7-12-10(4-5-13-7)9-3-2-8(15)6-11(9)14-12/h2-6,14-15H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Macquarie University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 |
Bioorg Med Chem 20: 1354-63 (2012)
Article DOI: 10.1016/j.bmc.2011.10.068
BindingDB Entry DOI: 10.7270/Q28P60ZT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data