 Found 71 hits with Last Name = 'kuramoto' and Initial = 'k'
Found 71 hits with Last Name = 'kuramoto' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244234
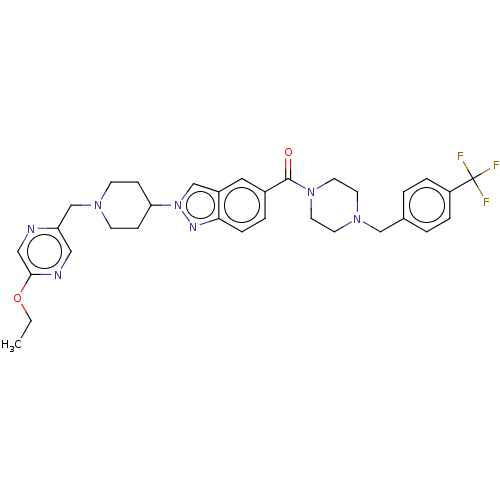
(US9428501, 58)Show SMILES CCOc1cnc(CN2CCC(CC2)n2cc3cc(ccc3n2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H36F3N7O2/c1-2-44-30-19-36-27(18-37-30)22-39-11-9-28(10-12-39)42-21-25-17-24(5-8-29(25)38-42)31(43)41-15-13-40(14-16-41)20-23-3-6-26(7-4-23)32(33,34)35/h3-8,17-19,21,28H,2,9-16,20,22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244233
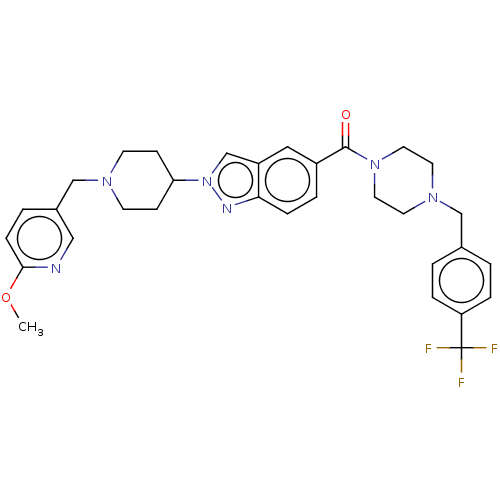
(US9428501, 57)Show SMILES COc1ccc(CN2CCC(CC2)n2cc3cc(ccc3n2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-30-9-4-24(19-36-30)21-38-12-10-28(11-13-38)41-22-26-18-25(5-8-29(26)37-41)31(42)40-16-14-39(15-17-40)20-23-2-6-27(7-3-23)32(33,34)35/h2-9,18-19,22,28H,10-17,20-21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244226
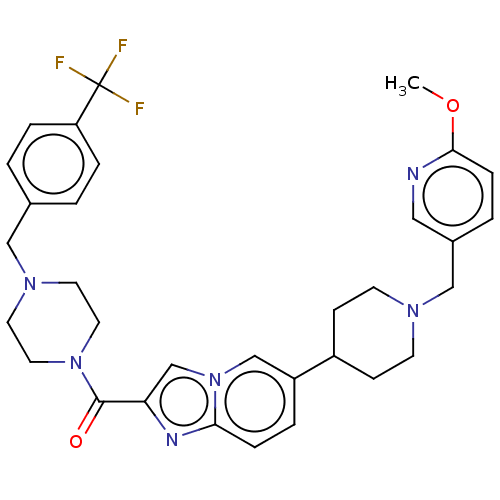
(US9428501, 35)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc(cn3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-30-9-4-24(18-36-30)20-38-12-10-25(11-13-38)26-5-8-29-37-28(22-41(29)21-26)31(42)40-16-14-39(15-17-40)19-23-2-6-27(7-3-23)32(33,34)35/h2-9,18,21-22,25H,10-17,19-20H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244223
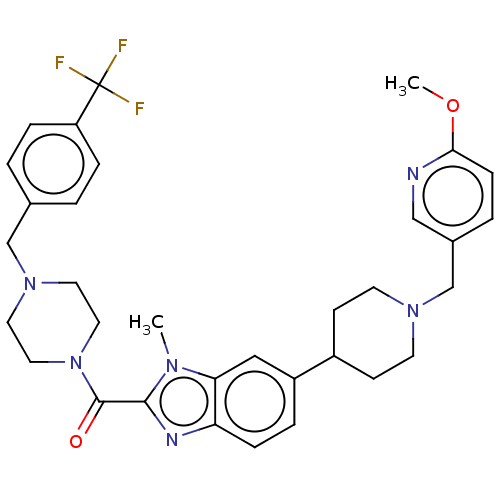
(US9428501, 18)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc(C(=O)N4CCN(Cc5ccc(cc5)C(F)(F)F)CC4)n(C)c3c2)cn1 Show InChI InChI=1S/C33H37F3N6O2/c1-39-29-19-26(25-11-13-40(14-12-25)22-24-5-10-30(44-2)37-20-24)6-9-28(29)38-31(39)32(43)42-17-15-41(16-18-42)21-23-3-7-27(8-4-23)33(34,35)36/h3-10,19-20,25H,11-18,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244231

(US9428501, 49)Show SMILES COc1ccc(CN2CCC(CC2)c2ccn3nc(cc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-30-7-4-24(20-36-30)22-38-11-8-25(9-12-38)26-10-13-41-28(18-26)19-29(37-41)31(42)40-16-14-39(15-17-40)21-23-2-5-27(6-3-23)32(33,34)35/h2-7,10,13,18-20,25H,8-9,11-12,14-17,21-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244236
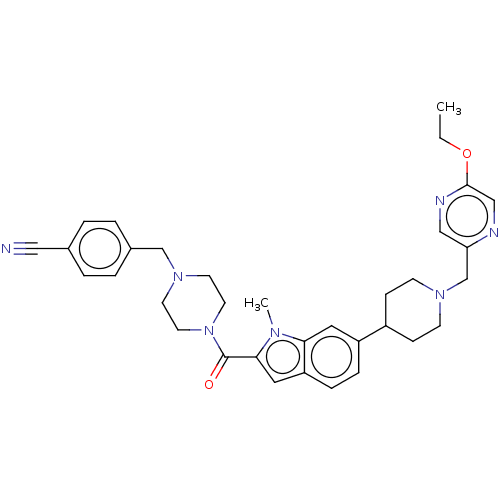
(US9428501, 69)Show SMILES CCOc1cnc(CN2CCC(CC2)c2ccc3cc(C(=O)N4CCN(Cc5ccc(cc5)C#N)CC4)n(C)c3c2)cn1 Show InChI InChI=1S/C34H39N7O2/c1-3-43-33-22-36-30(21-37-33)24-39-12-10-27(11-13-39)28-8-9-29-19-32(38(2)31(29)18-28)34(42)41-16-14-40(15-17-41)23-26-6-4-25(20-35)5-7-26/h4-9,18-19,21-22,27H,3,10-17,23-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244232
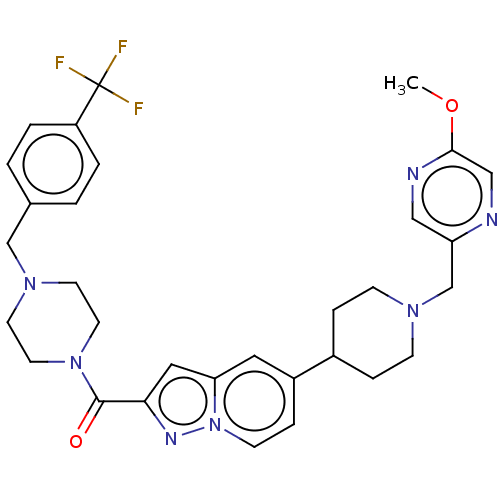
(US9428501, 50)Show SMILES COc1cnc(CN2CCC(CC2)c2ccn3nc(cc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C31H34F3N7O2/c1-43-29-19-35-26(18-36-29)21-38-9-6-23(7-10-38)24-8-11-41-27(16-24)17-28(37-41)30(42)40-14-12-39(13-15-40)20-22-2-4-25(5-3-22)31(32,33)34/h2-5,8,11,16-19,23H,6-7,9-10,12-15,20-21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244230

(US9428501, 44)Show SMILES COc1ccc(CN2CCC(CC2)c2cc3cc(C(=O)N4CCN(Cc5ccc(cc5)C(F)(F)F)CC4)n(C)c3cn2)cn1 Show InChI InChI=1S/C33H37F3N6O2/c1-39-29(32(43)42-15-13-41(14-16-42)21-23-3-6-27(7-4-23)33(34,35)36)18-26-17-28(37-20-30(26)39)25-9-11-40(12-10-25)22-24-5-8-31(44-2)38-19-24/h3-8,17-20,25H,9-16,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244226

(US9428501, 35)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc(cn3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-30-9-4-24(18-36-30)20-38-12-10-25(11-13-38)26-5-8-29-37-28(22-41(29)21-26)31(42)40-16-14-39(15-17-40)19-23-2-6-27(7-3-23)32(33,34)35/h2-9,18,21-22,25H,10-17,19-20H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244235

(US9428501, 66)Show SMILES CCOc1cnc(CN2CCC(CC2)c2ccc3n(C)c(cc3c2)C(=O)N2CCN(Cc3ccc(cc3)C#N)CC2)cn1 Show InChI InChI=1S/C34H39N7O2/c1-3-43-33-22-36-30(21-37-33)24-39-12-10-27(11-13-39)28-8-9-31-29(18-28)19-32(38(31)2)34(42)41-16-14-40(15-17-41)23-26-6-4-25(20-35)5-7-26/h4-9,18-19,21-22,27H,3,10-17,23-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244223

(US9428501, 18)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc(C(=O)N4CCN(Cc5ccc(cc5)C(F)(F)F)CC4)n(C)c3c2)cn1 Show InChI InChI=1S/C33H37F3N6O2/c1-39-29-19-26(25-11-13-40(14-12-25)22-24-5-10-30(44-2)37-20-24)6-9-28(29)38-31(39)32(43)42-17-15-41(16-18-42)21-23-3-7-27(8-4-23)33(34,35)36/h3-10,19-20,25H,11-18,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244222
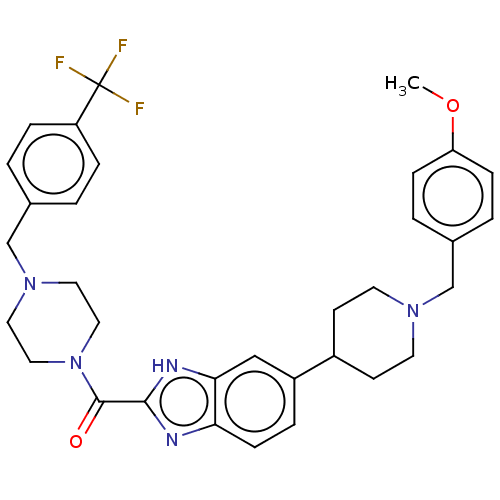
(US9428501, 14)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cc1 Show InChI InChI=1S/C33H36F3N5O2/c1-43-28-9-4-24(5-10-28)21-39-14-12-25(13-15-39)26-6-11-29-30(20-26)38-31(37-29)32(42)41-18-16-40(17-19-41)22-23-2-7-27(8-3-23)33(34,35)36/h2-11,20,25H,12-19,21-22H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244237
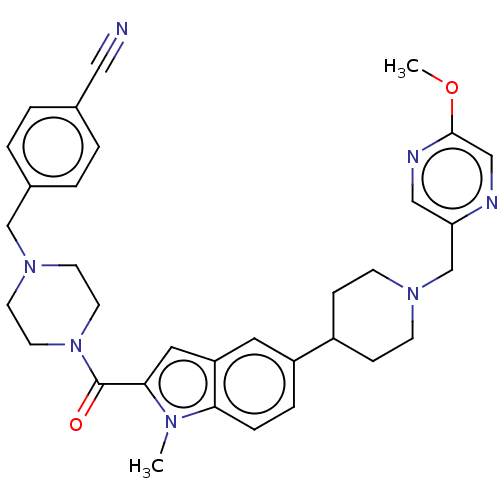
(US9428501, 79)Show SMILES COc1cnc(CN2CCC(CC2)c2ccc3n(C)c(cc3c2)C(=O)N2CCN(Cc3ccc(cc3)C#N)CC2)cn1 Show InChI InChI=1S/C33H37N7O2/c1-37-30-8-7-27(26-9-11-38(12-10-26)23-29-20-36-32(42-2)21-35-29)17-28(30)18-31(37)33(41)40-15-13-39(14-16-40)22-25-5-3-24(19-34)4-6-25/h3-8,17-18,20-21,26H,9-16,22-23H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244229
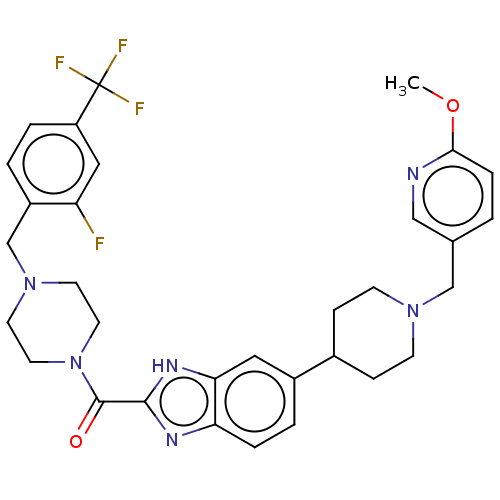
(US9428501, 38)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc(cc3F)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H34F4N6O2/c1-44-29-7-2-21(18-37-29)19-40-10-8-22(9-11-40)23-4-6-27-28(16-23)39-30(38-27)31(43)42-14-12-41(13-15-42)20-24-3-5-25(17-26(24)33)32(34,35)36/h2-7,16-18,22H,8-15,19-20H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244224
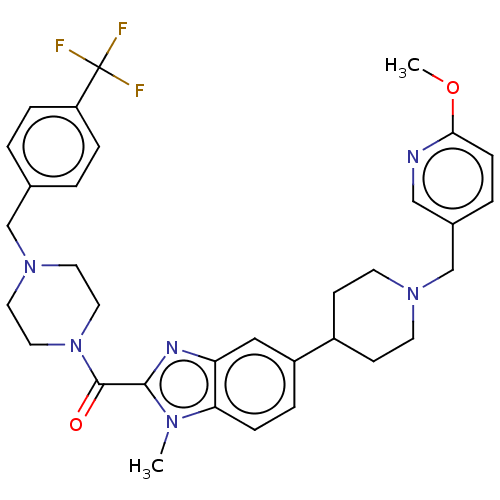
(US9428501, 19)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3n(C)c(nc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C33H37F3N6O2/c1-39-29-9-6-26(25-11-13-40(14-12-25)22-24-5-10-30(44-2)37-20-24)19-28(29)38-31(39)32(43)42-17-15-41(16-18-42)21-23-3-7-27(8-4-23)33(34,35)36/h3-10,19-20,25H,11-18,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244228

(US9428501, 37)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc4OC(F)(F)Oc4c3)CC2)cn1 Show InChI InChI=1S/C32H34F2N6O4/c1-42-29-7-3-22(18-35-29)20-38-10-8-23(9-11-38)24-4-5-25-26(17-24)37-30(36-25)31(41)40-14-12-39(13-15-40)19-21-2-6-27-28(16-21)44-32(33,34)43-27/h2-7,16-18,23H,8-15,19-20H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244227
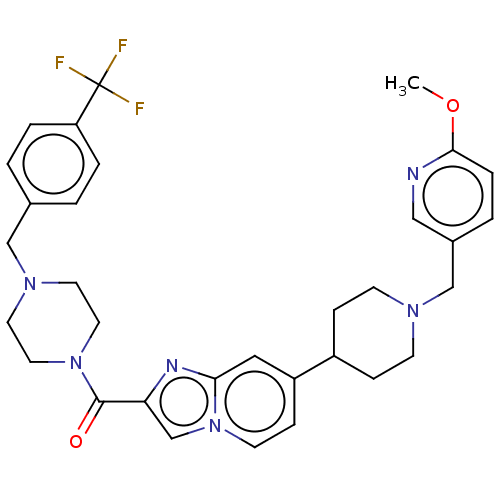
(US9428501, 36)Show SMILES COc1ccc(CN2CCC(CC2)c2ccn3cc(nc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-30-7-4-24(19-36-30)21-38-11-8-25(9-12-38)26-10-13-41-22-28(37-29(41)18-26)31(42)40-16-14-39(15-17-40)20-23-2-5-27(6-3-23)32(33,34)35/h2-7,10,13,18-19,22,25H,8-9,11-12,14-17,20-21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244220

(US9428501, 7)Show SMILES COc1cnc(CN2CCC(CC2)c2ccc3[nH]c(nc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C31H34F3N7O2/c1-43-28-18-35-25(17-36-28)20-39-10-8-22(9-11-39)23-4-7-26-27(16-23)38-29(37-26)30(42)41-14-12-40(13-15-41)19-21-2-5-24(6-3-21)31(32,33)34/h2-7,16-18,22H,8-15,19-20H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244219

(US9428501, 1)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3[nH]c(nc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-29-9-4-23(19-36-29)21-39-12-10-24(11-13-39)25-5-8-27-28(18-25)38-30(37-27)31(42)41-16-14-40(15-17-41)20-22-2-6-26(7-3-22)32(33,34)35/h2-9,18-19,24H,10-17,20-21H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244233

(US9428501, 57)Show SMILES COc1ccc(CN2CCC(CC2)n2cc3cc(ccc3n2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-30-9-4-24(19-36-30)21-38-12-10-28(11-13-38)41-22-26-18-25(5-8-29(26)37-41)31(42)40-16-14-39(15-17-40)20-23-2-6-27(7-3-23)32(33,34)35/h2-9,18-19,22,28H,10-17,20-21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244231

(US9428501, 49)Show SMILES COc1ccc(CN2CCC(CC2)c2ccn3nc(cc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-30-7-4-24(20-36-30)22-38-11-8-25(9-12-38)26-10-13-41-28(18-26)19-29(37-41)31(42)40-16-14-39(15-17-40)21-23-2-5-27(6-3-23)32(33,34)35/h2-7,10,13,18-20,25H,8-9,11-12,14-17,21-22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244236

(US9428501, 69)Show SMILES CCOc1cnc(CN2CCC(CC2)c2ccc3cc(C(=O)N4CCN(Cc5ccc(cc5)C#N)CC4)n(C)c3c2)cn1 Show InChI InChI=1S/C34H39N7O2/c1-3-43-33-22-36-30(21-37-33)24-39-12-10-27(11-13-39)28-8-9-29-19-32(38(2)31(29)18-28)34(42)41-16-14-40(15-17-41)23-26-6-4-25(20-35)5-7-26/h4-9,18-19,21-22,27H,3,10-17,23-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244221

(US9428501, 8)Show SMILES COc1cnc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C31H34F3N7O2/c1-43-28-18-35-25(17-36-28)20-39-10-8-22(9-11-39)23-4-7-26-27(16-23)38-29(37-26)30(42)41-14-12-40(13-15-41)19-21-2-5-24(6-3-21)31(32,33)34/h2-7,16-18,22H,8-15,19-20H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244228

(US9428501, 37)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc4OC(F)(F)Oc4c3)CC2)cn1 Show InChI InChI=1S/C32H34F2N6O4/c1-42-29-7-3-22(18-35-29)20-38-10-8-23(9-11-38)24-4-5-25-26(17-24)37-30(36-25)31(41)40-14-12-39(13-15-40)19-21-2-6-27-28(16-21)44-32(33,34)43-27/h2-7,16-18,23H,8-15,19-20H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244225

(US9428501, 23)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc(cc3)C#N)CC2)cn1 Show InChI InChI=1S/C32H35N7O2/c1-41-30-9-6-25(20-34-30)22-37-12-10-26(11-13-37)27-7-8-28-29(18-27)36-31(35-28)32(40)39-16-14-38(15-17-39)21-24-4-2-23(19-33)3-5-24/h2-9,18,20,26H,10-17,21-22H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
In order to make human PIK3 CA mutation-positive breast cancer cell line MDA-MB-453 cells become 500 cells per well in a 384 well non-adhesive plate ... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244219

(US9428501, 1)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3[nH]c(nc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-29-9-4-23(19-36-29)21-39-12-10-24(11-13-39)25-5-8-27-28(18-25)38-30(37-27)31(42)41-16-14-40(15-17-41)20-22-2-6-26(7-3-22)32(33,34)35/h2-9,18-19,24H,10-17,20-21H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244227

(US9428501, 36)Show SMILES COc1ccc(CN2CCC(CC2)c2ccn3cc(nc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H35F3N6O2/c1-43-30-7-4-24(19-36-30)21-38-11-8-25(9-12-38)26-10-13-41-22-28(37-29(41)18-26)31(42)40-16-14-39(15-17-40)20-23-2-5-27(6-3-23)32(33,34)35/h2-7,10,13,18-19,22,25H,8-9,11-12,14-17,20-21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244229

(US9428501, 38)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc(cc3F)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C32H34F4N6O2/c1-44-29-7-2-21(18-37-29)19-40-10-8-22(9-11-40)23-4-6-27-28(16-23)39-30(38-27)31(43)42-14-12-41(13-15-42)20-24-3-5-25(17-26(24)33)32(34,35)36/h2-7,16-18,22H,8-15,19-20H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590536
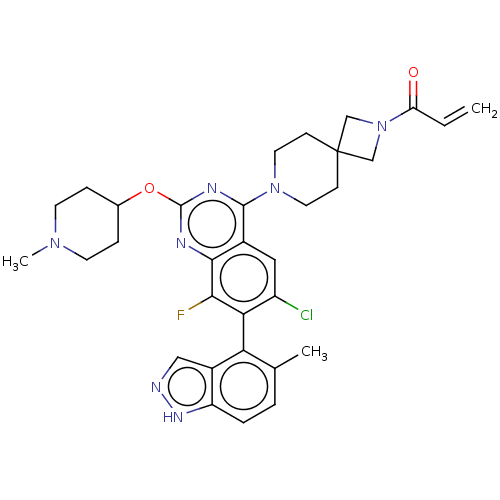
(CHEMBL5209329)Show SMILES CN1CCC(CC1)Oc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-2.62,;7.23,-3.39,;5.9,-2.62,;4.56,-3.39,;4.56,-4.93,;5.9,-5.7,;7.23,-4.93,;3.23,-5.7,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.9,1.21,;1.9,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.43,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.72,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244220

(US9428501, 7)Show SMILES COc1cnc(CN2CCC(CC2)c2ccc3[nH]c(nc3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C31H34F3N7O2/c1-43-28-18-35-25(17-36-28)20-39-10-8-22(9-11-39)23-4-7-26-27(16-23)38-29(37-26)30(42)41-14-12-40(13-15-41)19-21-2-5-24(6-3-21)31(32,33)34/h2-7,16-18,22H,8-15,19-20H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590537
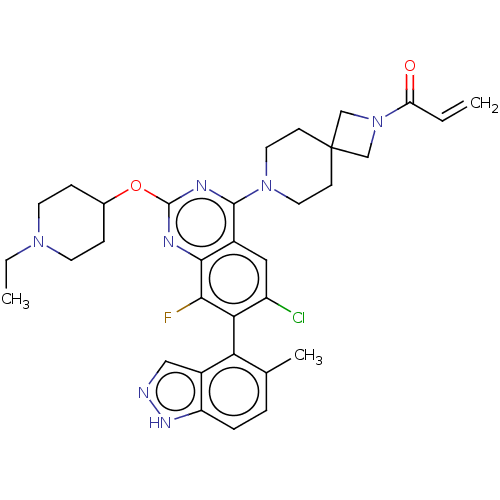
(CHEMBL5205152)Show SMILES CCN1CCC(CC1)Oc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-1.08,;8.57,-2.62,;7.23,-3.39,;5.9,-2.62,;4.56,-3.39,;4.56,-4.93,;5.9,-5.7,;7.23,-4.93,;3.23,-5.7,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.9,1.21,;1.9,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.43,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.72,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244221

(US9428501, 8)Show SMILES COc1cnc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cn1 Show InChI InChI=1S/C31H34F3N7O2/c1-43-28-18-35-25(17-36-28)20-39-10-8-22(9-11-39)23-4-7-26-27(16-23)38-29(37-26)30(42)41-14-12-40(13-15-41)19-21-2-5-24(6-3-21)31(32,33)34/h2-7,16-18,22H,8-15,19-20H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244230

(US9428501, 44)Show SMILES COc1ccc(CN2CCC(CC2)c2cc3cc(C(=O)N4CCN(Cc5ccc(cc5)C(F)(F)F)CC4)n(C)c3cn2)cn1 Show InChI InChI=1S/C33H37F3N6O2/c1-39-29(32(43)42-15-13-41(14-16-42)21-23-3-6-27(7-4-23)33(34,35)36)18-26-17-28(37-20-30(26)39)25-9-11-40(12-10-25)22-24-5-8-31(44-2)38-19-24/h3-8,17-20,25H,9-16,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244235

(US9428501, 66)Show SMILES CCOc1cnc(CN2CCC(CC2)c2ccc3n(C)c(cc3c2)C(=O)N2CCN(Cc3ccc(cc3)C#N)CC2)cn1 Show InChI InChI=1S/C34H39N7O2/c1-3-43-33-22-36-30(21-37-33)24-39-12-10-27(11-13-39)28-8-9-31-29(18-28)19-32(38(31)2)34(42)41-16-14-40(15-17-41)23-26-6-4-25(20-35)5-7-26/h4-9,18-19,21-22,27H,3,10-17,23-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244237

(US9428501, 79)Show SMILES COc1cnc(CN2CCC(CC2)c2ccc3n(C)c(cc3c2)C(=O)N2CCN(Cc3ccc(cc3)C#N)CC2)cn1 Show InChI InChI=1S/C33H37N7O2/c1-37-30-8-7-27(26-9-11-38(12-10-26)23-29-20-36-32(42-2)21-35-29)17-28(30)18-31(37)33(41)40-15-13-39(14-16-40)22-25-5-3-24(19-34)4-6-25/h3-8,17-18,20-21,26H,9-16,22-23H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590541
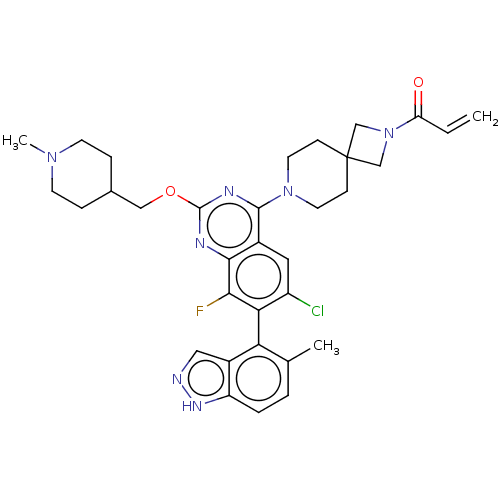
(CHEMBL5181467)Show SMILES CN1CCC(COc2nc(N3CCC4(CN(C4)C(=O)C=C)CC3)c3cc(Cl)c(c(F)c3n2)-c2c(C)ccc3[nH]ncc23)CC1 |(9.23,-8.01,;7.9,-7.24,;7.9,-5.7,;6.56,-4.93,;5.23,-5.7,;3.9,-4.93,;2.56,-5.7,;1.23,-4.93,;1.22,-3.41,;-.11,-2.64,;-.11,-1.1,;-1.44,-.33,;-1.44,1.21,;-.11,1.98,;-1.21,3.09,;-.12,4.17,;.98,3.07,;-.12,5.71,;-1.46,6.48,;1.21,6.48,;1.21,8.02,;1.23,1.21,;1.23,-.33,;-1.43,-3.41,;-2.76,-2.64,;-4.09,-3.4,;-5.43,-2.63,;-4.09,-4.94,;-2.75,-5.72,;-2.75,-7.26,;-1.42,-4.94,;-.09,-5.71,;-5.43,-5.71,;-5.43,-7.25,;-4.09,-8.02,;-6.75,-8.02,;-8.09,-7.26,;-8.09,-5.71,;-9.23,-4.68,;-8.61,-3.28,;-7.07,-3.44,;-6.75,-4.95,;5.23,-7.24,;6.56,-8.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM244225

(US9428501, 23)Show SMILES COc1ccc(CN2CCC(CC2)c2ccc3nc([nH]c3c2)C(=O)N2CCN(Cc3ccc(cc3)C#N)CC2)cn1 Show InChI InChI=1S/C32H35N7O2/c1-41-30-9-6-25(20-34-30)22-37-12-10-26(11-13-37)27-7-8-28-29(18-27)36-31(35-28)32(40)39-16-14-38(15-17-39)21-24-4-2-23(19-33)3-5-24/h2-9,18,20,26H,10-17,21-22H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astellas Pharma Inc.
US Patent
| Assay Description
Human PIK3CA mutation-positive breast cancer cell line MDA-MB-453 cells were subcutaneously implanted into nude mice, MDA-MB-453 tumor was excised, a... |
US Patent US9428501 (2016)
BindingDB Entry DOI: 10.7270/Q28051JK |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590532
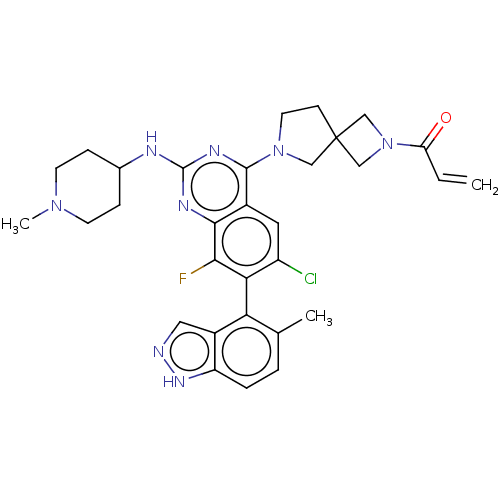
(CHEMBL5208546)Show SMILES CN1CCC(CC1)Nc1nc(N2CCC3(CN(C3)C(=O)C=C)C2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-1.53,;7.23,-2.3,;5.9,-1.53,;4.56,-2.3,;4.56,-3.84,;5.9,-4.61,;7.23,-3.84,;3.23,-4.61,;1.9,-3.84,;1.89,-2.32,;.56,-1.55,;.56,-.01,;-.68,.89,;-.21,2.36,;1.33,2.36,;.93,3.87,;2.41,4.27,;2.82,2.75,;3.18,5.6,;2.41,6.94,;4.72,5.6,;5.49,6.94,;1.81,.89,;-.76,-2.32,;-2.09,-1.55,;-3.43,-2.31,;-4.76,-1.54,;-3.43,-3.86,;-2.09,-4.63,;-2.09,-6.17,;-.76,-3.85,;.58,-4.62,;-4.76,-4.62,;-4.76,-6.17,;-3.43,-6.94,;-6.08,-6.93,;-7.42,-6.17,;-7.42,-4.63,;-8.57,-3.6,;-7.94,-2.19,;-6.41,-2.35,;-6.09,-3.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590543
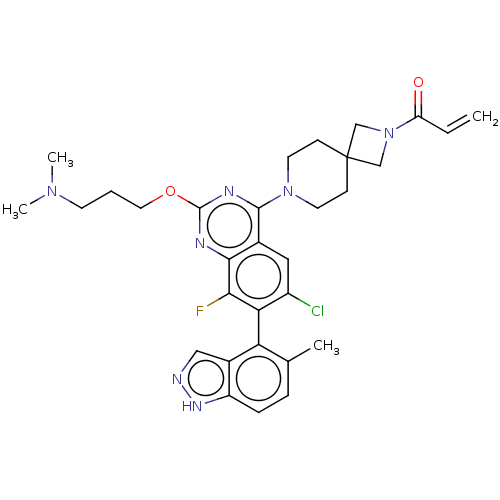
(CHEMBL5186010)Show SMILES CN(C)CCCOc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(9.23,-4.93,;7.9,-5.7,;7.9,-7.24,;6.57,-4.93,;5.23,-5.7,;3.9,-4.93,;2.56,-5.7,;1.23,-4.93,;1.22,-3.41,;-.11,-2.64,;-.11,-1.1,;-1.44,-.33,;-1.44,1.21,;-.11,1.98,;-1.21,3.09,;-.12,4.17,;.98,3.07,;-.12,5.71,;-1.46,6.48,;1.21,6.48,;1.21,8.02,;1.23,1.21,;1.23,-.33,;-1.43,-3.41,;-2.76,-2.64,;-4.09,-3.4,;-5.43,-2.63,;-4.09,-4.94,;-2.75,-5.72,;-2.75,-7.26,;-1.42,-4.94,;-.09,-5.71,;-5.43,-5.71,;-5.43,-7.25,;-4.09,-8.02,;-6.75,-8.02,;-8.09,-7.26,;-8.09,-5.72,;-9.23,-4.68,;-8.61,-3.28,;-7.07,-3.44,;-6.76,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590542

(CHEMBL5191000)Show SMILES CN(C)CCOc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-5.7,;7.23,-4.93,;7.23,-3.39,;5.9,-5.7,;4.56,-4.93,;3.23,-5.7,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.9,1.21,;1.9,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.43,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.72,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590533
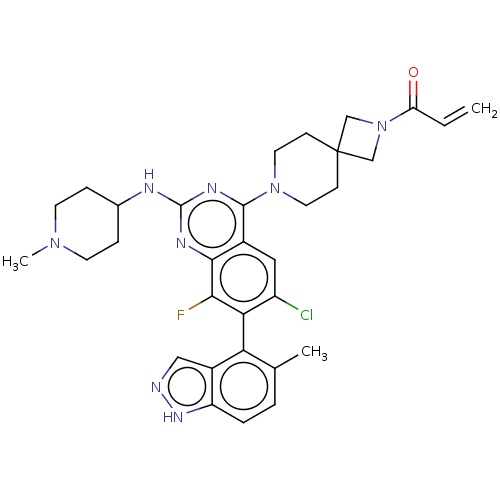
(CHEMBL5204061)Show SMILES CN1CCC(CC1)Nc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-2.62,;7.23,-3.39,;5.9,-2.62,;4.56,-3.39,;4.56,-4.93,;5.9,-5.7,;7.23,-4.93,;3.23,-5.7,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.9,1.21,;1.9,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.43,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.72,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590545

(CHEMBL5206689)Show SMILES Cc1ccc2[nH]ncc2c1-c1c(Cl)cc2c(nc(OCCCN3CCCCC3)nc2c1F)N1CCC2(CN(C2)C(=O)C=C)CC1 |(-4.76,-8.02,;-6.09,-7.25,;-7.42,-8.02,;-8.76,-7.26,;-8.76,-5.72,;-9.9,-4.68,;-9.27,-3.28,;-7.74,-3.44,;-7.42,-4.95,;-6.09,-5.71,;-4.76,-4.94,;-4.76,-3.4,;-6.09,-2.63,;-3.43,-2.64,;-2.09,-3.41,;-.77,-2.64,;.56,-3.41,;.56,-4.93,;1.9,-5.7,;3.23,-4.93,;4.56,-5.7,;5.9,-4.93,;7.23,-5.7,;8.57,-4.93,;9.9,-5.7,;9.9,-7.24,;8.57,-8.01,;7.23,-7.24,;-.76,-5.71,;-2.09,-4.94,;-3.42,-5.72,;-3.42,-7.26,;-.77,-1.1,;-2.11,-.33,;-2.11,1.21,;-.77,1.98,;-1.88,3.09,;-.79,4.17,;.32,3.07,;-.79,5.71,;-2.12,6.48,;.54,6.48,;.54,8.02,;.56,1.21,;.56,-.33,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590530
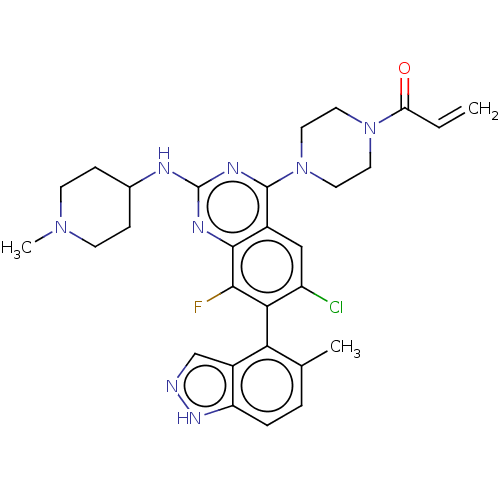
(CHEMBL5196734)Show SMILES CN1CCC(CC1)Nc1nc(N2CCN(CC2)C(=O)C=C)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-1.52,;7.23,-2.29,;5.9,-1.52,;4.56,-2.29,;4.56,-3.83,;5.9,-4.6,;7.23,-3.83,;3.23,-4.6,;1.9,-3.83,;1.89,-2.31,;.56,-1.54,;.56,-0,;-.77,.77,;-.77,2.31,;.56,3.08,;1.9,2.31,;1.9,.77,;.56,4.62,;-.77,5.39,;1.9,5.39,;1.9,6.93,;-.76,-2.31,;-2.09,-1.54,;-3.43,-2.31,;-4.76,-1.54,;-3.43,-3.85,;-2.09,-4.62,;-2.09,-6.16,;-.76,-3.84,;.58,-4.61,;-4.76,-4.62,;-4.76,-6.16,;-3.43,-6.93,;-6.08,-6.92,;-7.42,-6.16,;-7.42,-4.62,;-8.57,-3.59,;-7.94,-2.18,;-6.41,-2.34,;-6.09,-3.85,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590546
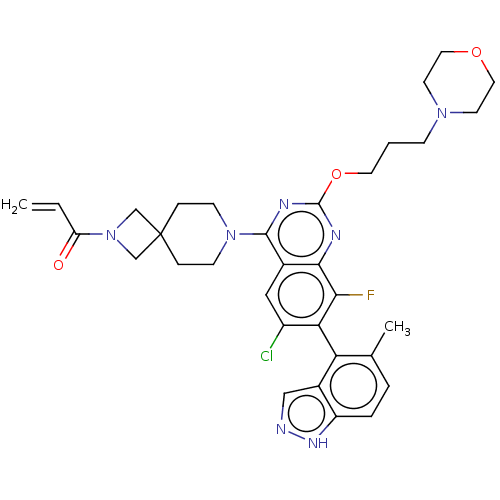
(CHEMBL5177793)Show SMILES Cc1ccc2[nH]ncc2c1-c1c(Cl)cc2c(nc(OCCCN3CCOCC3)nc2c1F)N1CCC2(CN(C2)C(=O)C=C)CC1 |(-4.76,-8.02,;-6.09,-7.25,;-7.42,-8.02,;-8.76,-7.26,;-8.76,-5.72,;-9.9,-4.68,;-9.27,-3.28,;-7.74,-3.44,;-7.42,-4.95,;-6.09,-5.71,;-4.76,-4.94,;-4.76,-3.4,;-6.09,-2.63,;-3.43,-2.64,;-2.09,-3.41,;-.77,-2.64,;.56,-3.41,;.56,-4.93,;1.9,-5.7,;3.23,-4.93,;4.56,-5.7,;5.9,-4.93,;7.23,-5.7,;8.57,-4.93,;9.9,-5.7,;9.9,-7.24,;8.57,-8.01,;7.23,-7.24,;-.76,-5.71,;-2.09,-4.94,;-3.42,-5.72,;-3.42,-7.26,;-.77,-1.1,;-2.11,-.33,;-2.11,1.21,;-.77,1.98,;-1.88,3.09,;-.79,4.17,;.32,3.07,;-.79,5.71,;-2.12,6.48,;.54,6.48,;.54,8.02,;.56,1.21,;.56,-.33,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590544
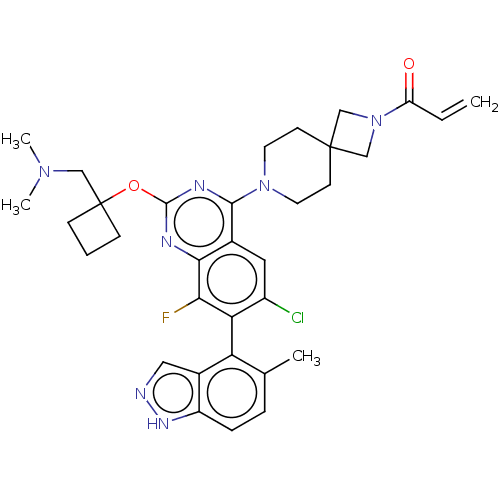
(CHEMBL5196761)Show SMILES CN(C)CC1(CCC1)Oc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-5.7,;7.23,-4.93,;7.23,-3.39,;5.9,-5.7,;4.56,-4.93,;3.46,-3.82,;4.55,-2.73,;5.65,-3.84,;3.23,-5.7,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.9,1.21,;1.9,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.43,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.72,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590538

(CHEMBL5181293)Show SMILES CCN1CCC(CC1)Sc1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-1.08,;8.57,-2.62,;7.23,-3.39,;5.9,-2.62,;4.56,-3.39,;4.56,-4.93,;5.9,-5.7,;7.23,-4.93,;3.23,-5.7,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.9,1.21,;1.9,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.43,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.72,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590547

(CHEMBL5202742)Show SMILES Cc1ccc2[nH]ncc2c1-c1c(Cl)cc2c(nc(OC3CCOCC3)nc2c1F)N1CCC2(CN(C2)C(=O)C=C)CC1 |(-2.76,-8.02,;-4.09,-7.25,;-5.42,-8.02,;-6.75,-7.26,;-6.75,-5.72,;-7.9,-4.68,;-7.27,-3.28,;-5.74,-3.44,;-5.42,-4.95,;-4.09,-5.71,;-2.76,-4.94,;-2.76,-3.4,;-4.09,-2.63,;-1.43,-2.64,;-.09,-3.41,;1.23,-2.64,;2.56,-3.41,;2.56,-4.93,;3.9,-5.7,;5.23,-4.93,;5.23,-3.39,;6.56,-2.62,;7.9,-3.39,;7.9,-4.93,;6.56,-5.7,;1.24,-5.71,;-.09,-4.94,;-1.42,-5.72,;-1.42,-7.26,;1.23,-1.1,;-.11,-.33,;-.11,1.21,;1.23,1.98,;.12,3.09,;1.21,4.17,;2.32,3.07,;1.21,5.71,;-.12,6.48,;2.54,6.48,;2.54,8.02,;2.56,1.21,;2.56,-.33,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590531

(CHEMBL5199429)Show SMILES CN1CCC(CC1)Nc1nc(N2CC3(CN(C3)C(=O)C=C)C2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-2.18,;7.23,-2.95,;5.9,-2.18,;4.56,-2.95,;4.56,-4.49,;5.9,-5.26,;7.23,-4.49,;3.23,-5.26,;1.9,-4.49,;1.89,-2.96,;.56,-2.2,;.56,-.66,;-.55,.45,;.54,1.54,;-.56,2.64,;.52,3.73,;1.63,2.63,;.52,5.27,;-.81,6.04,;1.86,6.04,;1.86,7.58,;1.65,.43,;-.76,-2.97,;-2.09,-2.2,;-3.43,-2.96,;-4.76,-2.19,;-3.43,-4.5,;-2.09,-5.28,;-2.09,-6.82,;-.76,-4.5,;.58,-5.27,;-4.76,-5.27,;-4.76,-6.81,;-3.43,-7.58,;-6.08,-7.58,;-7.42,-6.82,;-7.42,-5.27,;-8.57,-4.24,;-7.94,-2.84,;-6.41,-3,;-6.09,-4.5,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590539

(CHEMBL5187482)Show SMILES CCN1CCC(CC1)N(C)c1nc(N2CCC3(CN(C3)C(=O)C=C)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-1.08,;8.57,-2.62,;7.23,-3.39,;5.9,-2.62,;4.56,-3.39,;4.56,-4.93,;5.9,-5.7,;7.23,-4.93,;3.23,-5.7,;3.23,-7.24,;1.9,-4.93,;1.89,-3.41,;.56,-2.64,;.56,-1.1,;-.77,-.33,;-.77,1.21,;.56,1.98,;-.55,3.09,;.54,4.17,;1.65,3.07,;.54,5.71,;-.79,6.48,;1.88,6.48,;1.88,8.02,;1.89,1.21,;1.89,-.33,;-.76,-3.41,;-2.09,-2.64,;-3.42,-3.4,;-4.76,-2.63,;-3.43,-4.94,;-2.09,-5.72,;-2.09,-7.26,;-.76,-4.94,;.58,-5.71,;-4.76,-5.71,;-4.76,-7.25,;-3.43,-8.02,;-6.08,-8.02,;-7.42,-7.26,;-7.42,-5.71,;-8.57,-4.68,;-7.94,-3.28,;-6.41,-3.44,;-6.09,-4.95,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |
GTPase KRas
(Homo sapiens (Human)) | BDBM50590540

(CHEMBL5193630)Show SMILES CCN1CCC(CC1)Oc1nc(N2CCC3(CN(C3)C(=O)\C=C\CN3CCOCC3)CC2)c2cc(Cl)c(c(F)c2n1)-c1c(C)ccc2[nH]ncc12 |(8.57,-3.78,;8.57,-5.32,;7.23,-6.09,;5.9,-5.32,;4.56,-6.09,;4.56,-7.63,;5.9,-8.4,;7.23,-7.63,;3.23,-8.4,;1.9,-7.63,;1.89,-6.1,;.56,-5.34,;.56,-3.8,;-.77,-3.03,;-.77,-1.49,;.56,-.72,;-.55,.39,;.54,1.48,;1.65,.37,;.54,3.02,;-.79,3.79,;1.88,3.79,;1.88,5.33,;3.21,6.1,;3.21,7.64,;1.88,8.41,;1.88,9.95,;3.21,10.72,;4.54,9.95,;4.54,8.41,;1.89,-1.49,;1.89,-3.03,;-.76,-6.1,;-2.09,-5.33,;-3.42,-6.1,;-4.76,-5.33,;-3.43,-7.64,;-2.09,-8.41,;-2.09,-9.95,;-.76,-7.64,;.58,-8.4,;-4.76,-8.41,;-4.76,-9.95,;-3.43,-10.72,;-6.08,-10.72,;-7.42,-9.96,;-7.42,-8.41,;-8.57,-7.38,;-7.94,-5.97,;-6.41,-6.13,;-6.09,-7.64,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116949
BindingDB Entry DOI: 10.7270/Q23T9N5B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data