

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368077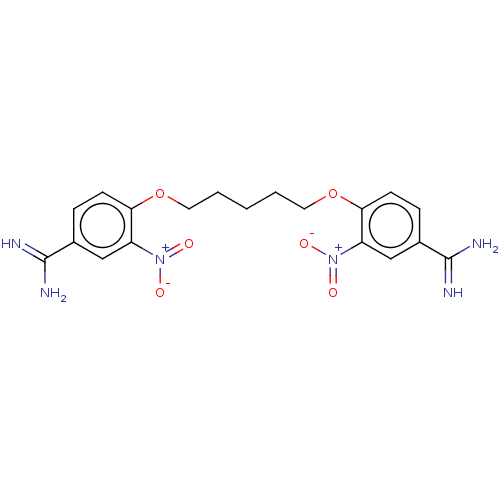 (CHEMBL3216901 | CHEMBL493336) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368081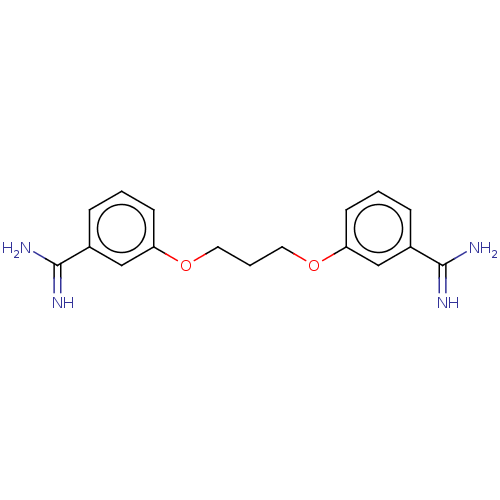 (CHEMBL3217116 | CHEMBL522538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368096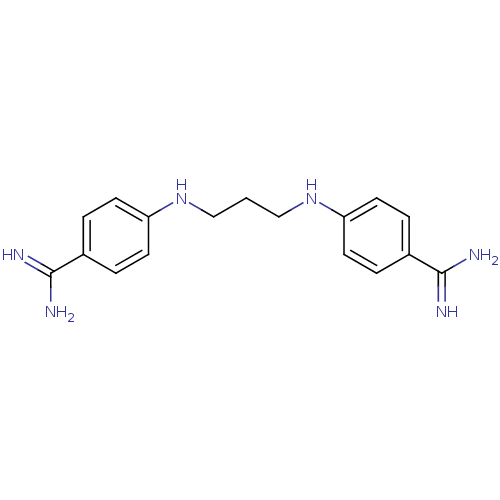 (CHEMBL1204157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368094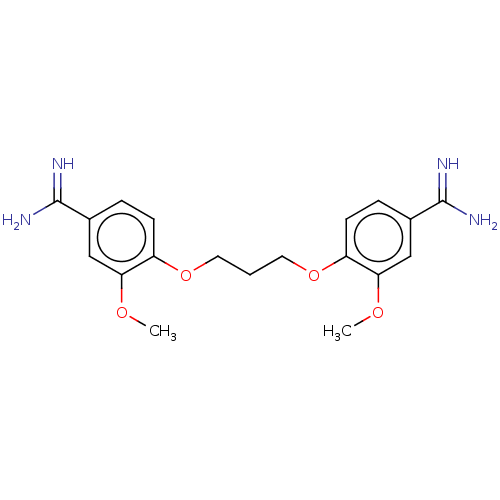 (CHEMBL3216660 | CHEMBL494405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368082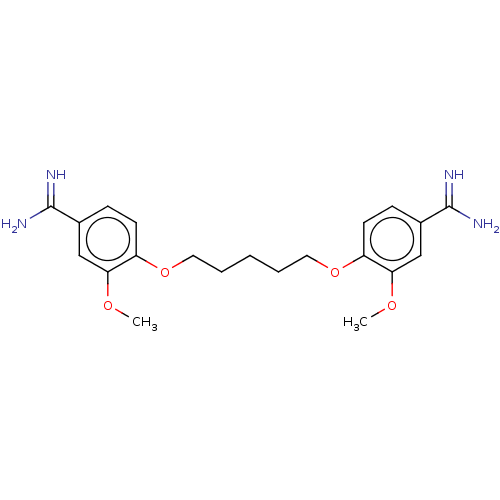 (CHEMBL3216435 | CHEMBL523180) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368098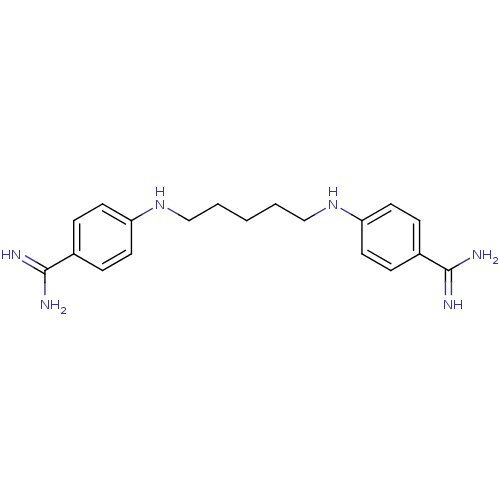 (CHEMBL492579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368102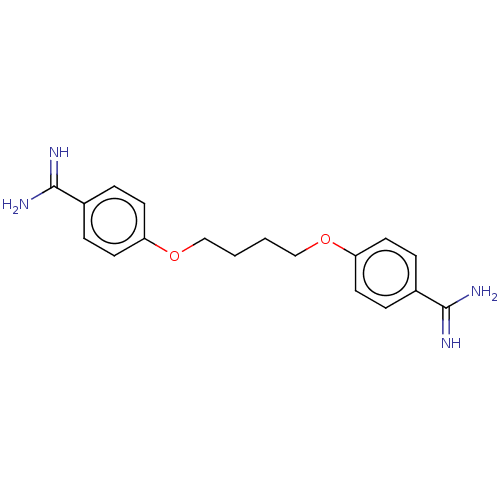 (CHEMBL3216029 | CHEMBL494850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368093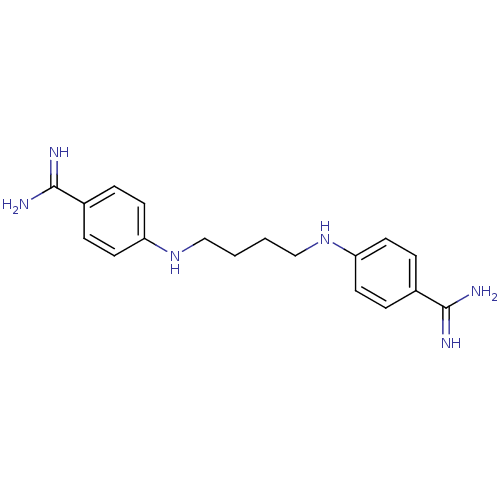 (CHEMBL1204156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368083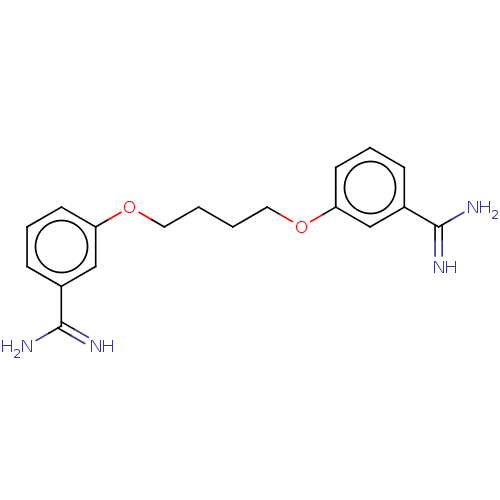 (CHEMBL3216032 | CHEMBL495206) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368100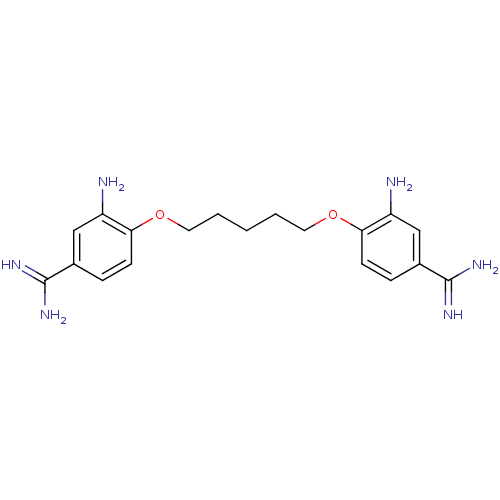 (CHEMBL1204159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM45440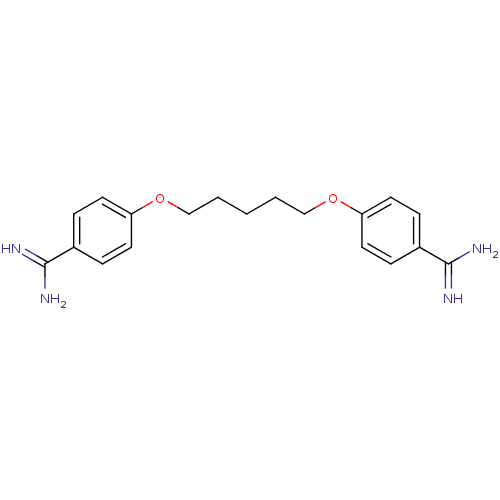 (4-[5-(4-amidinophenoxy)pentoxy]benzamidine;2-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50015234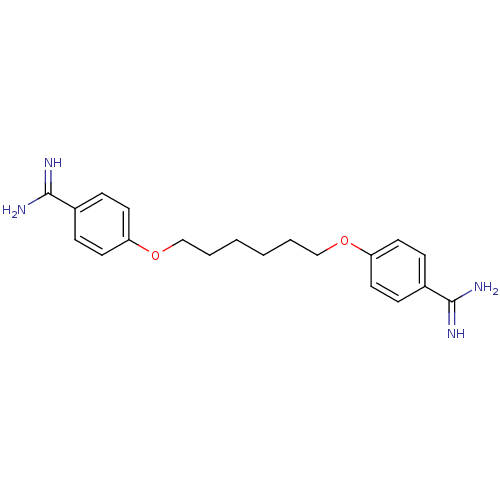 (4,4''[1,6-HEXANEDIYLBIS(OXY)]BISBENZENECARBOXIMIDA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368092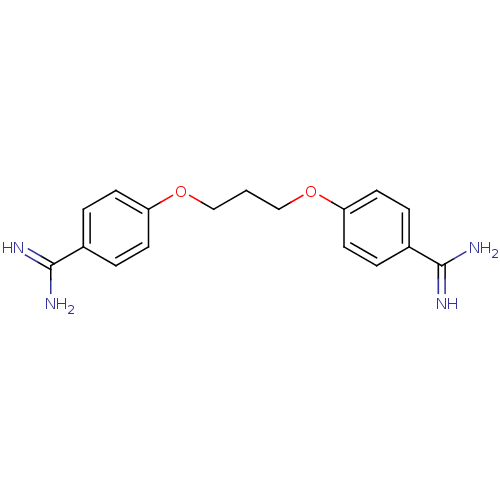 (GNF-PF-3839 | PROPAMIDINE CHLORIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368087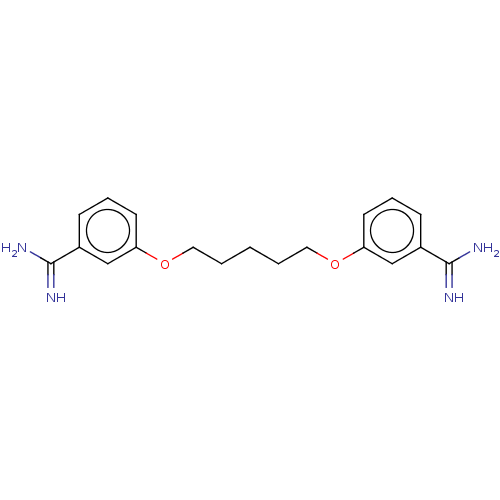 (CHEMBL3215566 | CHEMBL493170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368085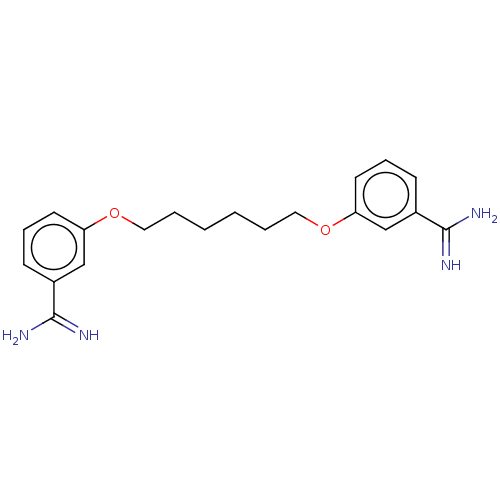 (CHEMBL3216215 | CHEMBL492970) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368074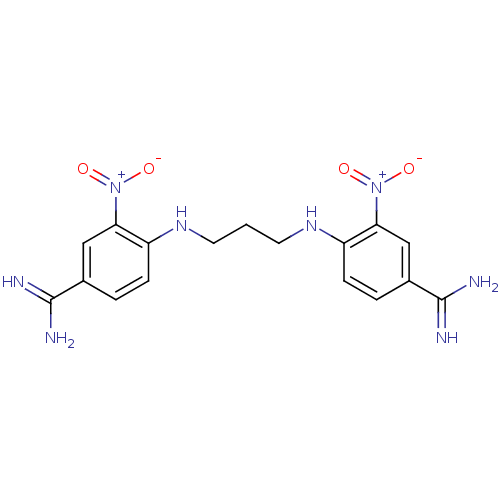 (CHEMBL1204155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368075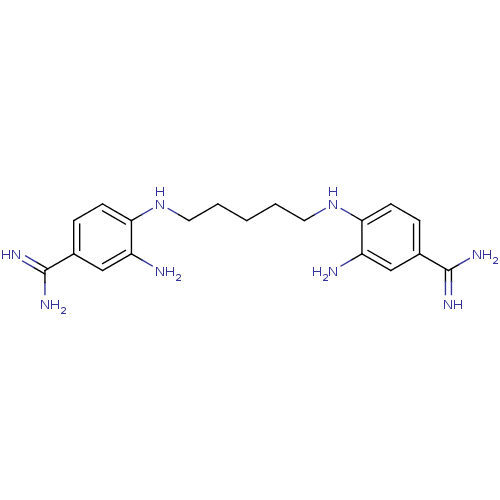 (CHEMBL1202472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368095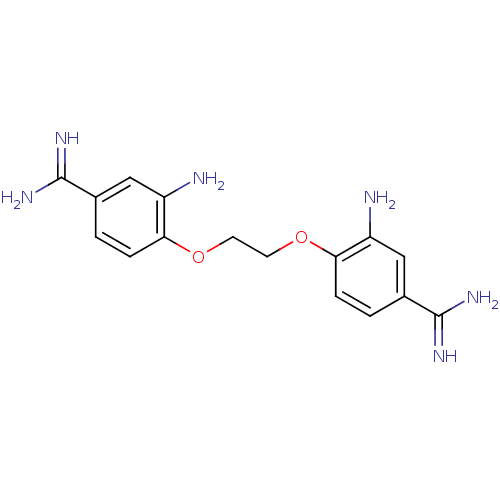 (CHEMBL1204150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368078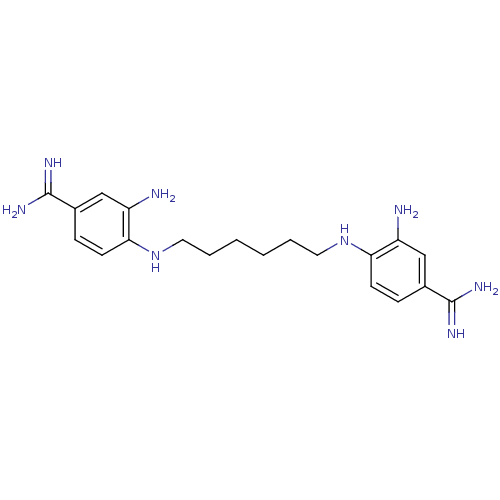 (CHEMBL1204161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368079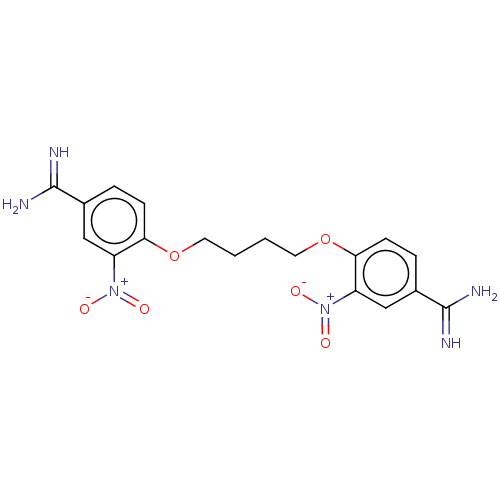 (CHEMBL3216662 | CHEMBL492360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368089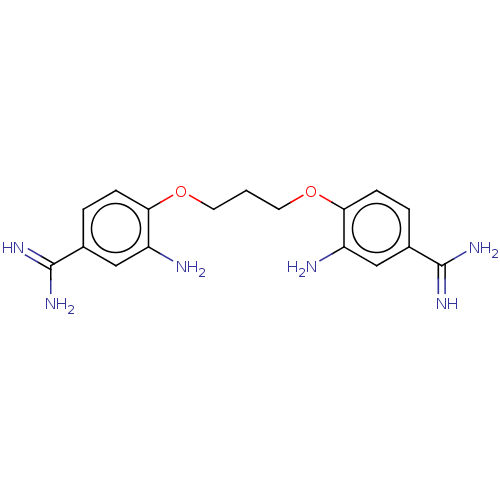 (CHEMBL3215570 | CHEMBL522667) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368097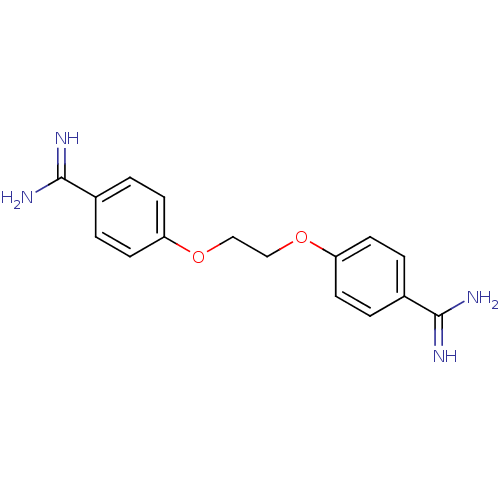 (CHEMBL1204158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368084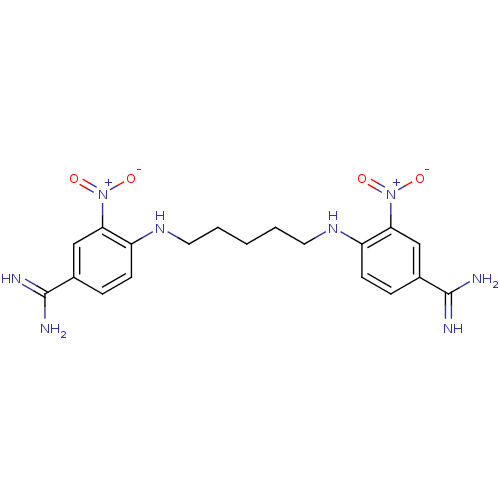 (CHEMBL1204154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368076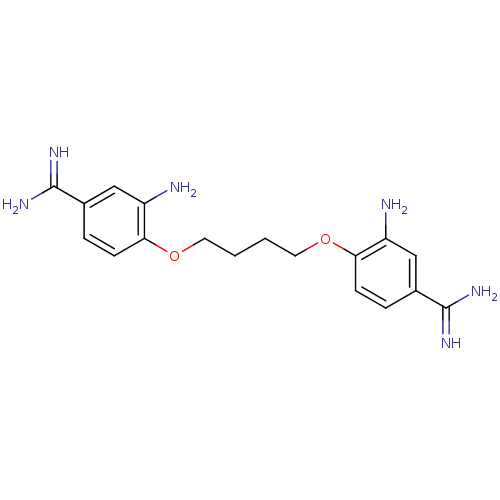 (CHEMBL1204149) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368086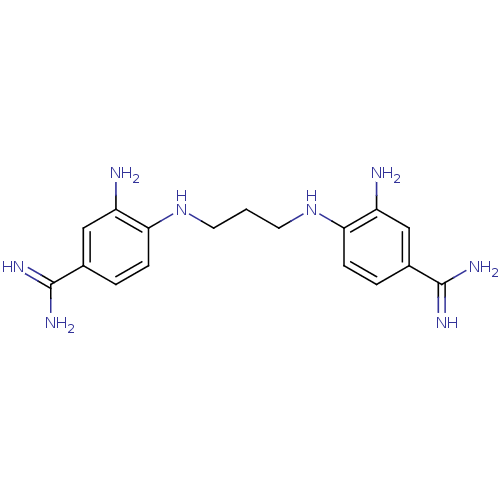 (CHEMBL1204153) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368080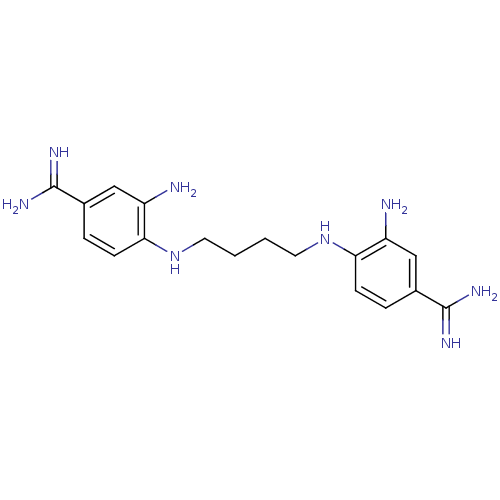 (CHEMBL1204152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368090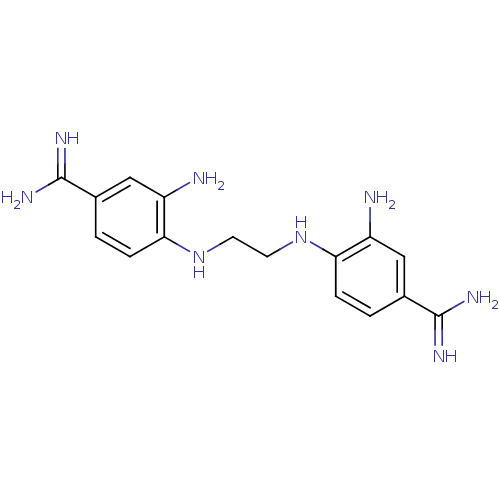 (CHEMBL1204160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368088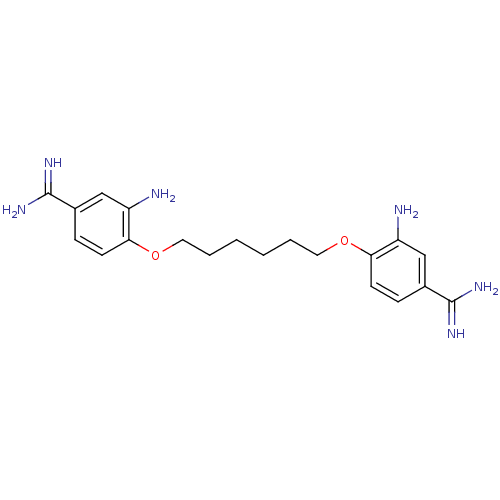 (CHEMBL1204151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50368099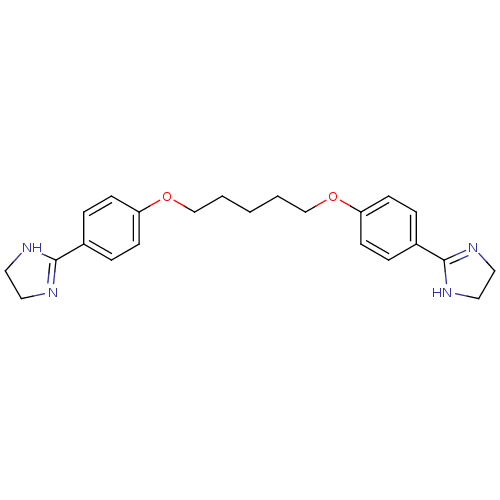 (CHEMBL494956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of trypsin by amidase assay. | J Med Chem 33: 1252-7 (1990) BindingDB Entry DOI: 10.7270/Q2154HNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037791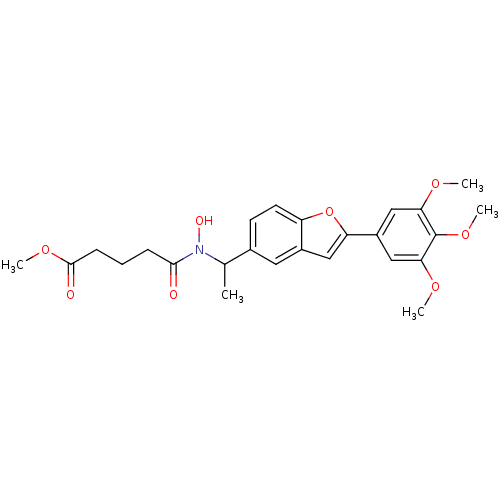 (4-(Hydroxy-{1-[2-(3,4,5-trimethoxy-phenyl)-benzofu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037795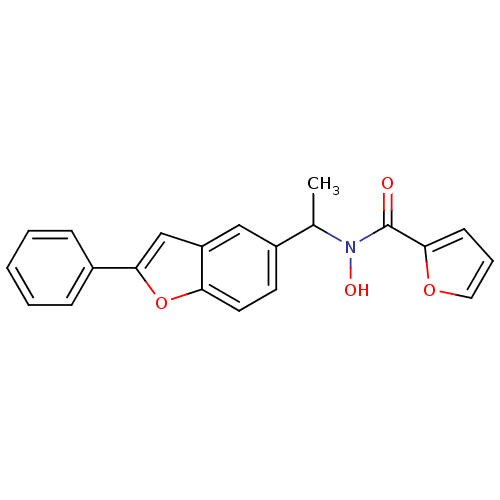 (CHEMBL120885 | Furan-2-carboxylic acid hydroxy-[1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037807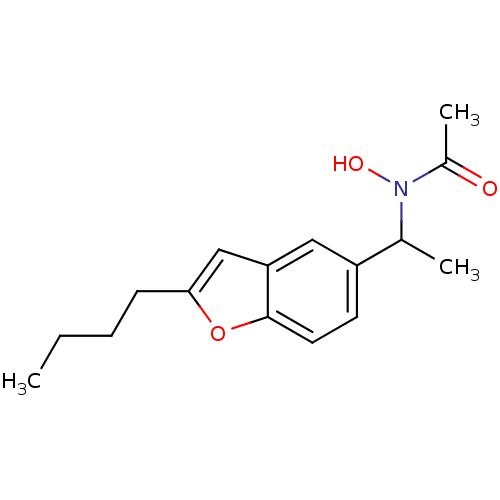 (CHEMBL331626 | N-[1-(2-Butyl-benzofuran-5-yl)-ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037805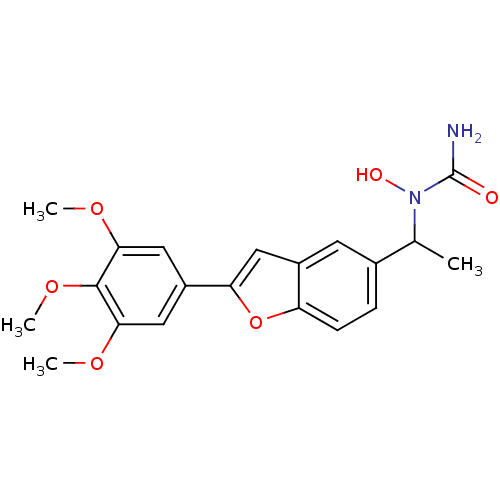 (CHEMBL121025 | N-hydroxy-N-[1-[2-(3,4,5-trimethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037792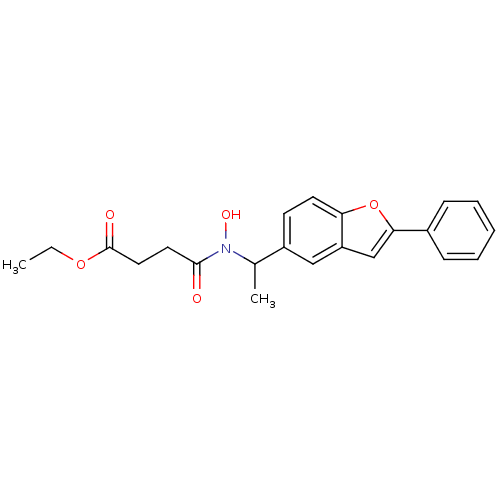 (CHEMBL122955 | N-Hydroxy-N-[1-(2-phenyl-benzofuran...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037803 (4-{[1-(2-Butyl-benzofuran-5-yl)-ethyl]-hydroxy-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037793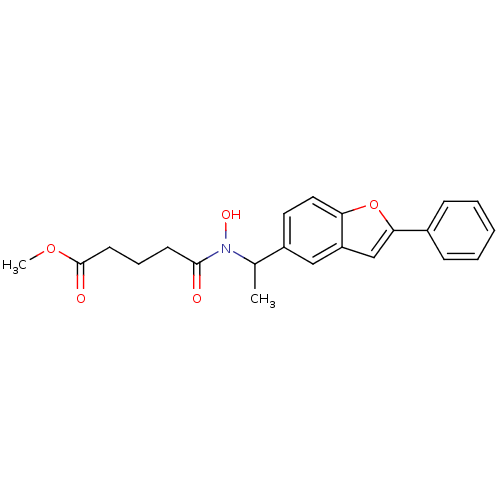 (4-{Hydroxy-[1-(2-phenyl-benzofuran-5-yl)-ethyl]-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037788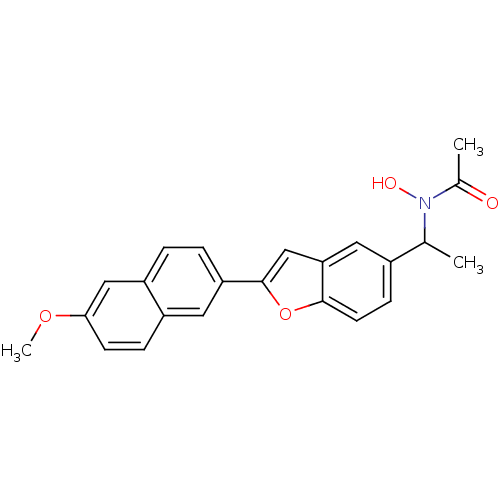 (CHEMBL421255 | N-Hydroxy-N-{1-[2-(6-methoxy-naphth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037801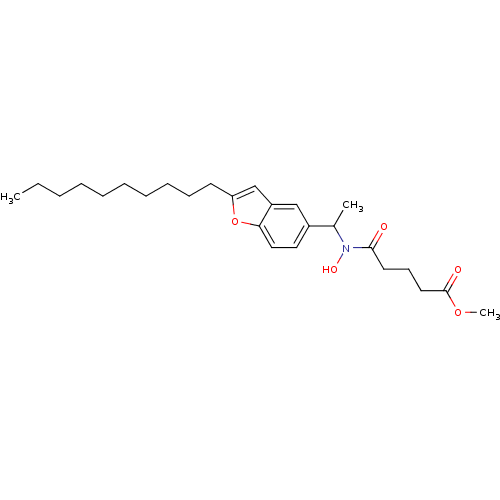 (4-{[1-(2-Decyl-benzofuran-5-yl)-ethyl]-hydroxy-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037796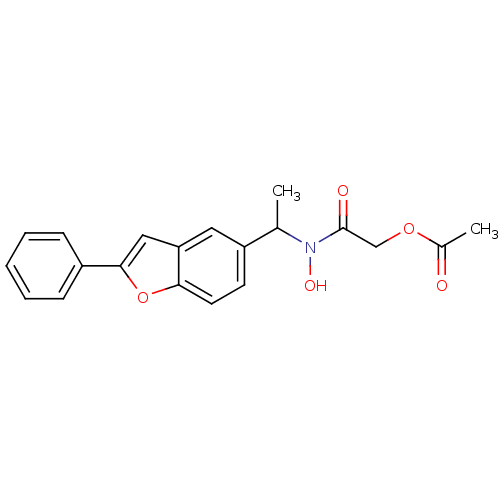 (Acetic acid {hydroxy-[1-(2-phenyl-benzofuran-5-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037789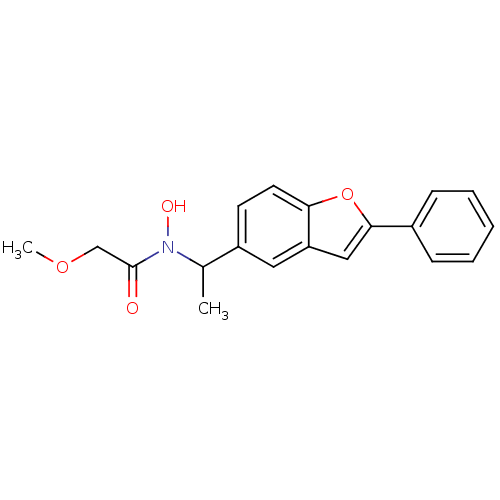 (CHEMBL332395 | N-Hydroxy-2-methoxy-N-[1-(2-phenyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037797 (CHEMBL123396 | N-Hydroxy-3,4-dimethoxy-N-[1-(2-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037806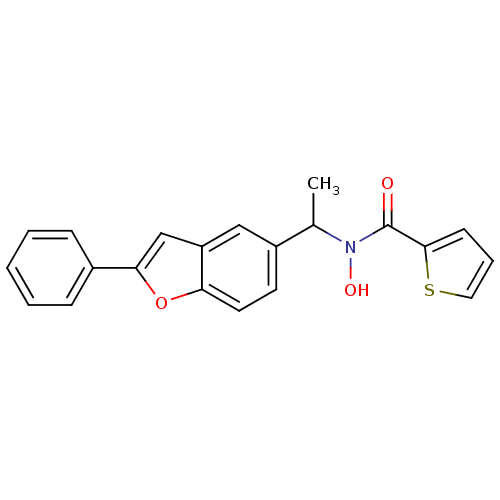 (CHEMBL123177 | Thiophene-2-carboxylic acid hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037794 (CHEMBL331060 | N-Hydroxy-N-{1-[2-(3,4,5-trimethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50022683 (CHEMBL418304 | N-[1-(4-Benzyloxy-phenyl)-ethyl]-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037799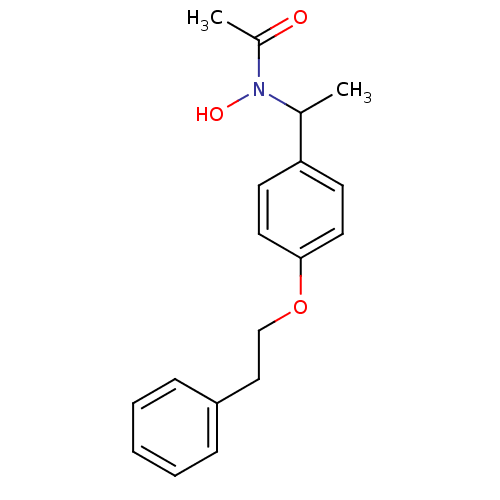 (CHEMBL123649 | N-Hydroxy-N-[1-(4-phenethyloxy-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037798 (CHEMBL121390 | N-hydroxy-N-[1-(2-phinyl-5-benzofur...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037790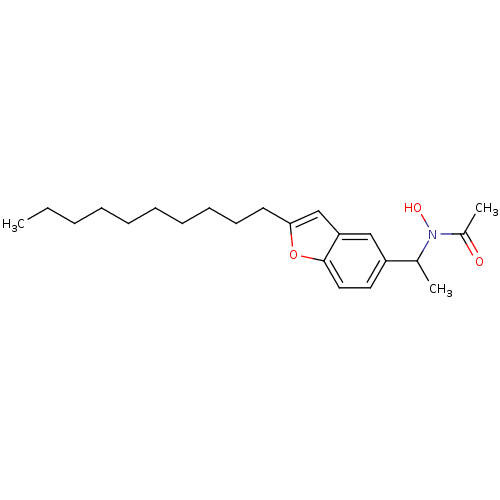 (CHEMBL331881 | N-[1-(2-Decyl-benzofuran-5-yl)-ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50037787 (CHEMBL332814 | N-Hydroxy-N-[1-(2-phenyl-benzofuran...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50022697 (CHEMBL62114 | N-Hydroxy-N-{1-[4-(1-phenyl-ethoxy)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of 5-lipoxygenase (5-HETE) derived from the 9000xg supernatant of RBL broken cell assay | J Med Chem 37: 3663-7 (1994) BindingDB Entry DOI: 10.7270/Q27943Q2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |