

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50539387 (CHEMBL4648474) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126892 BindingDB Entry DOI: 10.7270/Q2G73J8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50585028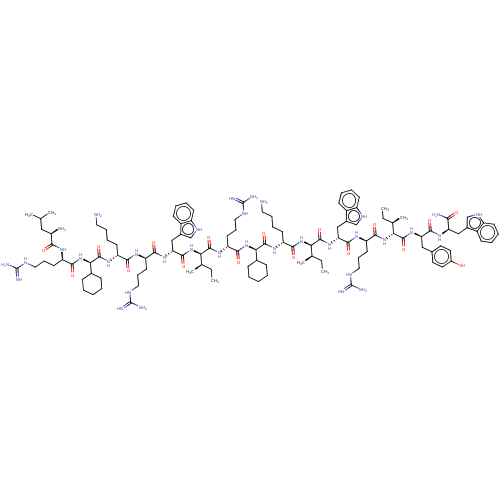 (CHEMBL5078185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50585029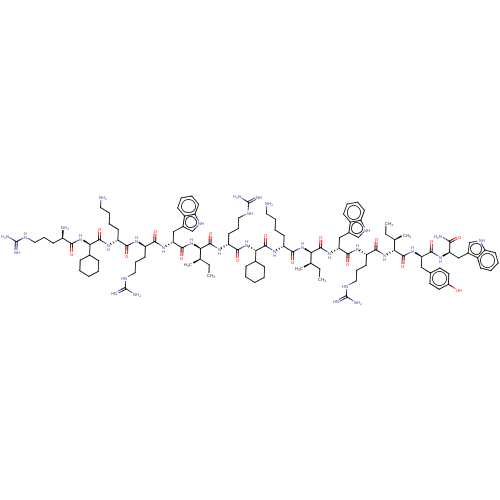 (CHEMBL5092917) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transforming growth factor beta-1 proprotein (Homo sapiens (Human)) | BDBM50585028 (CHEMBL5078185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGF beta 1 (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126892 BindingDB Entry DOI: 10.7270/Q2G73J8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180049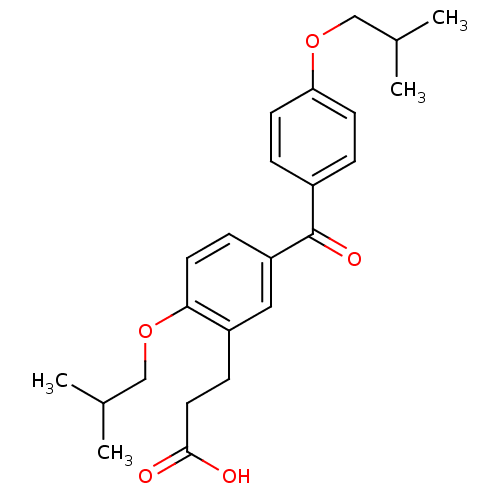 (3-[2-isobutoxy-5-(4-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of the expression of AP1-luciferase by TPA-stimulated NIH3T3 cells | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180047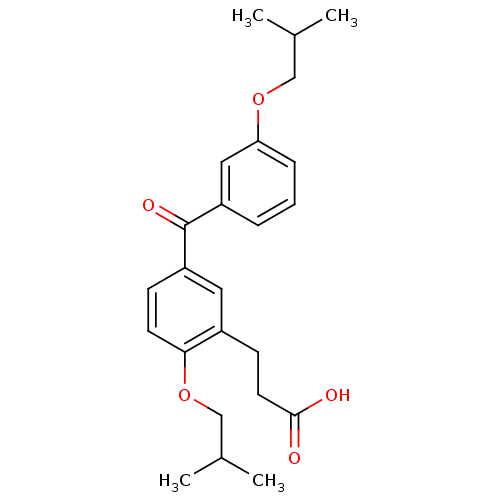 (3-[2-isobutoxy-5-(3-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of the expression of AP1-luciferase by TPA-stimulated NIH3T3 cells | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50539386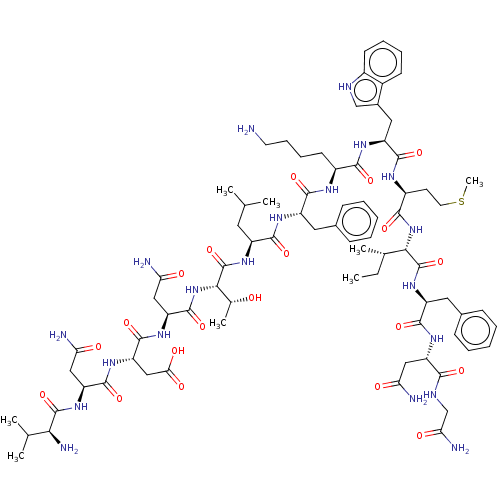 (CHEMBL4646947) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy and Life Sciences Curated by ChEMBL | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells incubated for 4 hrs by dual luciferase reporter gene assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126892 BindingDB Entry DOI: 10.7270/Q2G73J8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transforming growth factor beta-1 proprotein (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGF beta 1 (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151079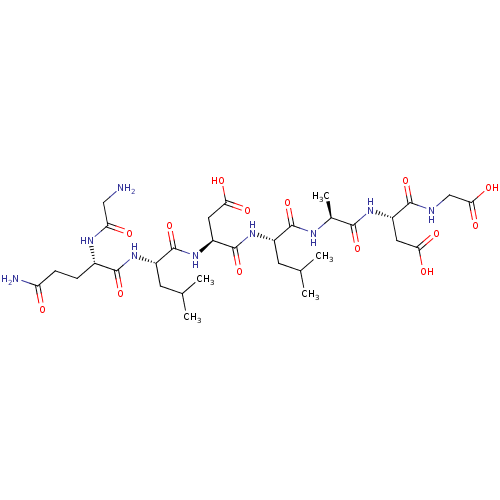 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180051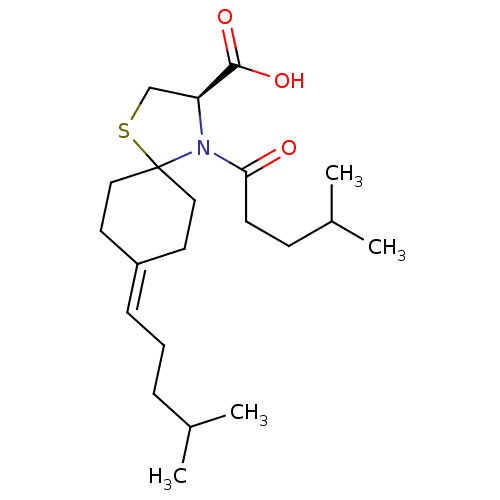 ((R)-4-(4-methylpentanoyl)-8-(4-methylpentylidene)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of the expression of AP1-luciferase by TPA-stimulated NIH3T3 cells | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180048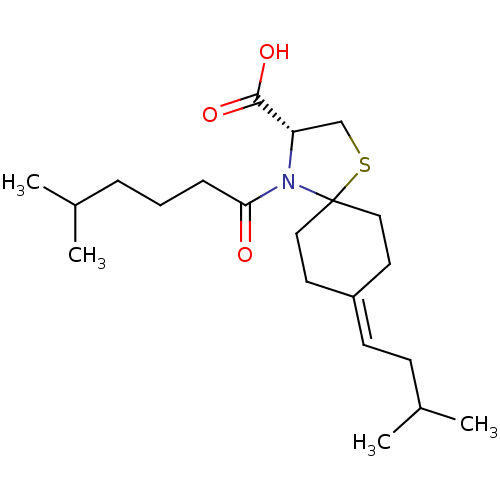 ((R)-8-(3-methylbutylidene)-4-(5-methylhexanoyl)-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of the expression of AP1-luciferase by TPA-stimulated NIH3T3 cells | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151090 ((S)-3-{(S)-2-[(S)-2-(2-Amino-acetylamino)-4-carbam...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151095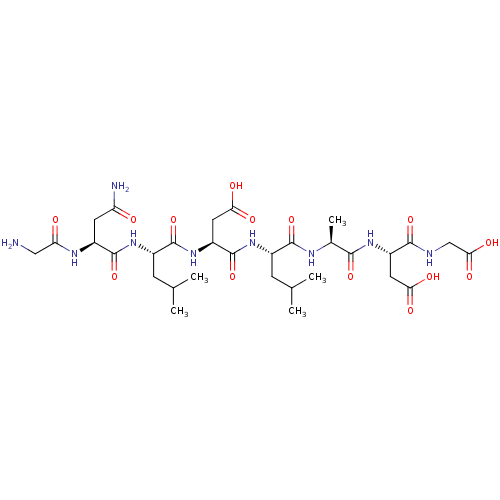 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151089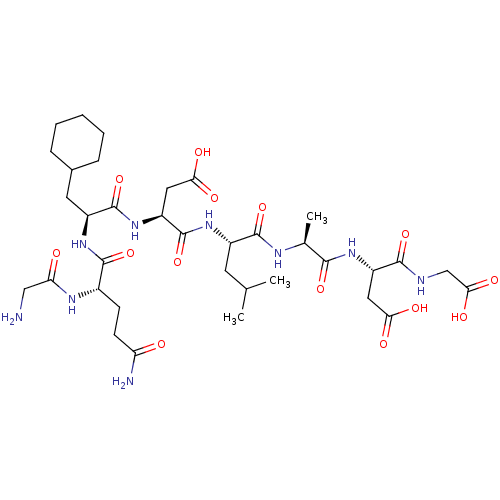 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151091 ((S)-3-{(S)-2-[(S)-2-(2-Amino-acetylamino)-4-carbam...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151088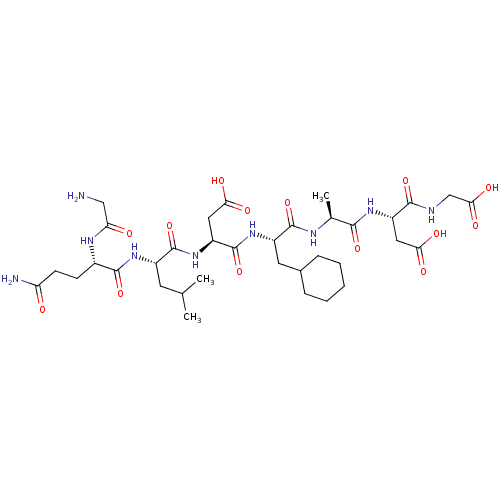 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151087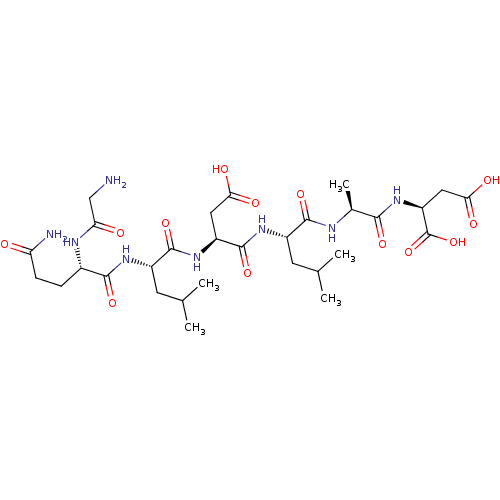 ((S)-2-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151086 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151085 ((S)-3-[(S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-Amino-4-c...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151084 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151083 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151081 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151080 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-((S)-2-Am...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151082 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-6-Amino-2-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151094 ((S)-3-{2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-acet...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151093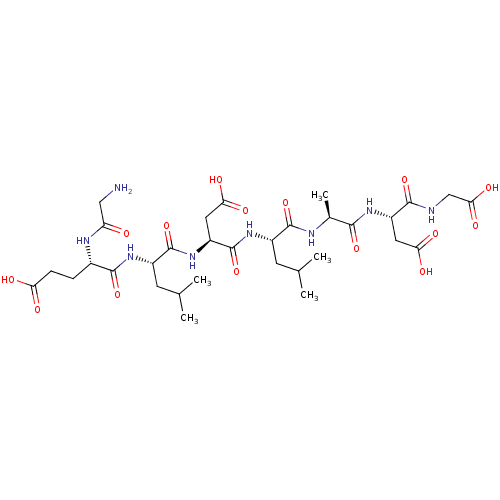 ((S)-4-(2-Amino-acetylamino)-4-{(S)-1-[(S)-2-carbox...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50151092 ((S)-3-{(S)-2-[(S)-2-((S)-2-{(S)-2-[(S)-2-(2-Amino-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against transcription activator protein-1 (AP-1) | J Med Chem 47: 4239-46 (2004) Article DOI: 10.1021/jm049890+ BindingDB Entry DOI: 10.7270/Q2RX9BJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180050 (3-[2-benzyloxy-5-(4-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180048 ((R)-8-(3-methylbutylidene)-4-(5-methylhexanoyl)-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180047 (3-[2-isobutoxy-5-(3-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180049 (3-[2-isobutoxy-5-(4-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180051 ((R)-4-(4-methylpentanoyl)-8-(4-methylpentylidene)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180052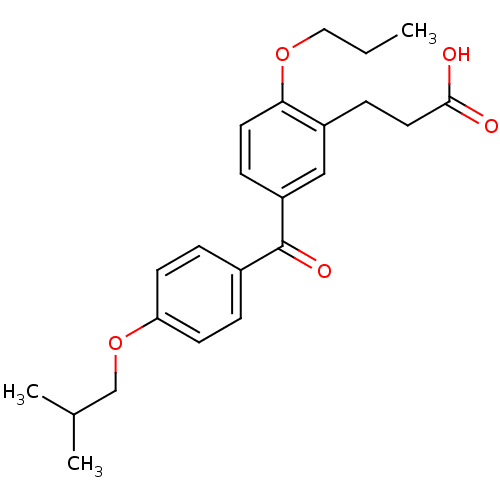 (3-[5-(4-isobutoxybenzoyl)-2-propoxyphenyl]propioni...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180046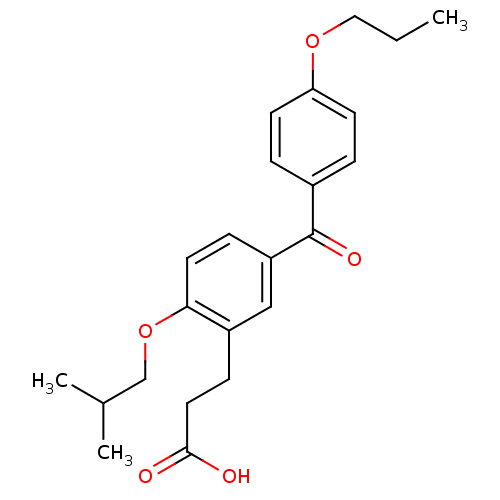 (3-[2-isobutoxy-5-(4-propoxybenzoyl)phenyl]propioni...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180053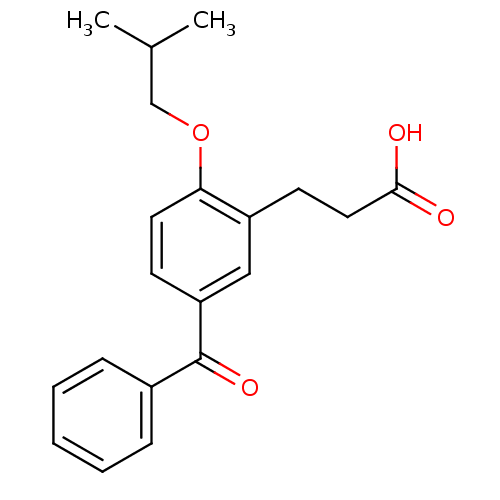 (3-(5-benzoyl-2-isobutoxyphenyl)propionic acid | CH...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180045 (3-[3-(4-isobutoxybenzoyl)phenyl]propionic acid | C...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||