 Found 639 hits with Last Name = 'brown' and Initial = 'ka'
Found 639 hits with Last Name = 'brown' and Initial = 'ka' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50512416

(CHEMBL4447162)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCCN4CCC(CC4)c4ccc(cc4)-c4cc(cc5cc(ccc45)-c4ccc(cc4)C(F)(F)F)C(O)=O)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(41.72,-13.14,;41.86,-14.67,;40.6,-15.57,;40.74,-17.1,;42.13,-17.74,;42.28,-19.27,;41.03,-20.17,;41.18,-21.7,;39.93,-22.59,;38.53,-21.95,;38.38,-20.42,;39.63,-19.53,;39.47,-17.99,;37.94,-17.87,;39.87,-16.5,;37.28,-22.84,;37.42,-24.37,;35.88,-22.2,;34.62,-23.09,;33.22,-22.45,;31.97,-23.35,;30.57,-22.71,;29.31,-23.6,;27.91,-22.96,;26.66,-23.85,;25.2,-23.36,;24.28,-24.6,;22.74,-24.59,;21.98,-23.25,;20.44,-23.23,;19.69,-21.89,;18.15,-21.87,;17.36,-23.2,;15.83,-23.18,;15.07,-21.84,;15.84,-20.51,;17.39,-20.53,;13.54,-21.84,;12.76,-23.17,;11.22,-23.15,;10.46,-21.81,;11.23,-20.49,;12.77,-20.49,;8.92,-21.8,;8.16,-20.45,;6.61,-20.45,;5.83,-21.78,;6.59,-23.12,;5.82,-24.44,;6.58,-25.78,;8.12,-25.8,;8.9,-24.47,;8.14,-23.13,;5.79,-27.1,;4.25,-27.09,;3.47,-28.41,;4.23,-29.76,;5.78,-29.76,;6.55,-28.44,;3.45,-31.08,;1.91,-31.07,;4.21,-32.42,;2.67,-32.41,;5.84,-19.11,;4.3,-19.1,;6.62,-17.78,;25.17,-25.85,;26.64,-25.39,;43.68,-19.91,;43.82,-21.43,;45.21,-22.07,;46.46,-21.19,;47.86,-21.84,;46.33,-19.66,;44.93,-19.02,;44.8,-17.49,;43.39,-16.85,;43.26,-15.32,;44.51,-14.44,;45.91,-15.09,;43.74,-13.1,;45.28,-13.09,;47.58,-18.77,;48.98,-19.41,;46.8,-17.43,;48.35,-17.43,)| Show InChI InChI=1S/C62H58F3N7O12S2/c63-62(64,65)44-16-12-37(13-17-44)40-14-18-46-42(31-40)32-43(60(74)75)34-50(46)39-10-8-36(9-11-39)38-24-29-71(30-25-38)27-6-3-7-45-35-72(70-69-45)28-5-2-1-4-26-68-59(73)41-15-19-47(51(33-41)61(76)77)54-48-20-22-52(66)57(85(78,79)80)55(48)84-56-49(54)21-23-53(67)58(56)86(81,82)83/h8-23,31-35,38,66H,1-7,24-30,67H2,(H,68,73)(H,74,75)(H,76,77)(H,78,79,80)(H,81,82,83) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50532693
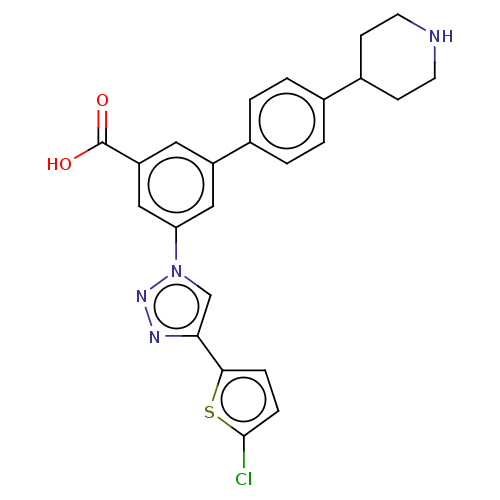
(CHEMBL4544251)Show SMILES OC(=O)c1cc(cc(c1)-n1cc(nn1)-c1ccc(Cl)s1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C24H21ClN4O2S/c25-23-6-5-22(32-23)21-14-29(28-27-21)20-12-18(11-19(13-20)24(30)31)16-3-1-15(2-4-16)17-7-9-26-10-8-17/h1-6,11-14,17,26H,7-10H2,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in HEK cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50532693

(CHEMBL4544251)Show SMILES OC(=O)c1cc(cc(c1)-n1cc(nn1)-c1ccc(Cl)s1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C24H21ClN4O2S/c25-23-6-5-22(32-23)21-14-29(28-27-21)20-12-18(11-19(13-20)24(30)31)16-3-1-15(2-4-16)17-7-9-26-10-8-17/h1-6,11-14,17,26H,7-10H2,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in HEK cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50532693

(CHEMBL4544251)Show SMILES OC(=O)c1cc(cc(c1)-n1cc(nn1)-c1ccc(Cl)s1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C24H21ClN4O2S/c25-23-6-5-22(32-23)21-14-29(28-27-21)20-12-18(11-19(13-20)24(30)31)16-3-1-15(2-4-16)17-7-9-26-10-8-17/h1-6,11-14,17,26H,7-10H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha2C receptor expressed in MDCK cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50532693

(CHEMBL4544251)Show SMILES OC(=O)c1cc(cc(c1)-n1cc(nn1)-c1ccc(Cl)s1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C24H21ClN4O2S/c25-23-6-5-22(32-23)21-14-29(28-27-21)20-12-18(11-19(13-20)24(30)31)16-3-1-15(2-4-16)17-7-9-26-10-8-17/h1-6,11-14,17,26H,7-10H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha2C receptor expressed in MDCK cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50532691
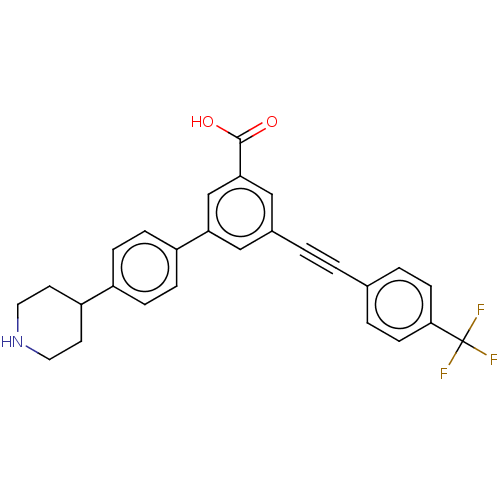
(CHEMBL4455037)Show SMILES OC(=O)c1cc(cc(c1)-c1ccc(cc1)C1CCNCC1)C#Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H22F3NO2/c28-27(29,30)25-9-3-18(4-10-25)1-2-19-15-23(17-24(16-19)26(32)33)21-7-5-20(6-8-21)22-11-13-31-14-12-22/h3-10,15-17,22,31H,11-14H2,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to guinea pig sigma1 receptor by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50532691

(CHEMBL4455037)Show SMILES OC(=O)c1cc(cc(c1)-c1ccc(cc1)C1CCNCC1)C#Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H22F3NO2/c28-27(29,30)25-9-3-18(4-10-25)1-2-19-15-23(17-24(16-19)26(32)33)21-7-5-20(6-8-21)22-11-13-31-14-12-22/h3-10,15-17,22,31H,11-14H2,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to guinea pig sigma1 receptor by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50532693

(CHEMBL4544251)Show SMILES OC(=O)c1cc(cc(c1)-n1cc(nn1)-c1ccc(Cl)s1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C24H21ClN4O2S/c25-23-6-5-22(32-23)21-14-29(28-27-21)20-12-18(11-19(13-20)24(30)31)16-3-1-15(2-4-16)17-7-9-26-10-8-17/h1-6,11-14,17,26H,7-10H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha2A receptor expressed in MDCK cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50532693

(CHEMBL4544251)Show SMILES OC(=O)c1cc(cc(c1)-n1cc(nn1)-c1ccc(Cl)s1)-c1ccc(cc1)C1CCNCC1 Show InChI InChI=1S/C24H21ClN4O2S/c25-23-6-5-22(32-23)21-14-29(28-27-21)20-12-18(11-19(13-20)24(30)31)16-3-1-15(2-4-16)17-7-9-26-10-8-17/h1-6,11-14,17,26H,7-10H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha2A receptor expressed in MDCK cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human delta opioid receptor expressed in HEK cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human delta opioid receptor expressed in HEK cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50532691

(CHEMBL4455037)Show SMILES OC(=O)c1cc(cc(c1)-c1ccc(cc1)C1CCNCC1)C#Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H22F3NO2/c28-27(29,30)25-9-3-18(4-10-25)1-2-19-15-23(17-24(16-19)26(32)33)21-7-5-20(6-8-21)22-11-13-31-14-12-22/h3-10,15-17,22,31H,11-14H2,(H,32,33) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to sigma 2 receptor in rat PC3 cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Rattus norvegicus (Rat)) | BDBM50532691

(CHEMBL4455037)Show SMILES OC(=O)c1cc(cc(c1)-c1ccc(cc1)C1CCNCC1)C#Cc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C27H22F3NO2/c28-27(29,30)25-9-3-18(4-10-25)1-2-19-15-23(17-24(16-19)26(32)33)21-7-5-20(6-8-21)22-11-13-31-14-12-22/h3-10,15-17,22,31H,11-14H2,(H,32,33) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to sigma 2 receptor in rat PC3 cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to rat D3 dopamine receptor expressed in HEK293T cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to rat D3 dopamine receptor expressed in HEK293T cells by PDSP assay |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity human P2Y14R expressed in African green monkey COS7 cells assessed as inhibition of UDPG-induced [3H]inositol phosphate accumulat... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Antagonist activity human P2Y14R expressed in African green monkey COS7 cells assessed as inhibition of UDPG-induced [3H]inositol phosphate accumulat... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM87119

((1R,2R)-N-[(1S)-2-[4-(5-bromo-2-keto-3H-benzimidaz...)Show SMILES C[C@@H](CN1CCC(CC1)n1c2ccc(Br)cc2[nH]c1=O)NC(=O)[C@@H]1C[C@H]1c1ccccc1 Show InChI InChI=1S/C25H29BrN4O2/c1-16(27-24(31)21-14-20(21)17-5-3-2-4-6-17)15-29-11-9-19(10-12-29)30-23-8-7-18(26)13-22(23)28-25(30)32/h2-8,13,16,19-21H,9-12,14-15H2,1H3,(H,27,31)(H,28,32)/t16-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
| Assay Description
Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... |
ACS Chem Biol 10: 421-32 (2015)
Article DOI: 10.1021/cb500828m
BindingDB Entry DOI: 10.7270/Q2H70DK8 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50427800
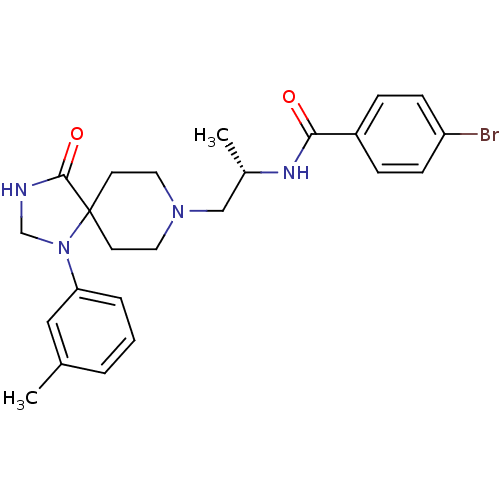
(CHEMBL2325485)Show SMILES C[C@@H](CN1CCC2(CC1)N(CNC2=O)c1cccc(C)c1)NC(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C24H29BrN4O2/c1-17-4-3-5-21(14-17)29-16-26-23(31)24(29)10-12-28(13-11-24)15-18(2)27-22(30)19-6-8-20(25)9-7-19/h3-9,14,18H,10-13,15-16H2,1-2H3,(H,26,31)(H,27,30)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 in human Calu-1 cells assessed as deuterated 1-butanol incorporation pretreated for 5 mins prior to substrate addition measured af... |
J Med Chem 56: 2695-9 (2013)
Article DOI: 10.1021/jm301782e
BindingDB Entry DOI: 10.7270/Q2RJ4KT8 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 14
(Homo sapiens (Human)) | BDBM50456168

(CHEMBL1800685)Show SMILES OC(=O)c1cc(-c2ccc(cc2)C2CCNCC2)c2ccc(cc2c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H24F3NO2/c30-29(31,32)25-8-5-19(6-9-25)22-7-10-26-23(15-22)16-24(28(34)35)17-27(26)21-3-1-18(2-4-21)20-11-13-33-14-12-20/h1-10,15-17,20,33H,11-14H2,(H,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... |
J Med Chem 59: 6149-68 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00044
BindingDB Entry DOI: 10.7270/Q2DJ5K34 |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM154517

(ML299 (5))Show SMILES C[C@@H](CN1CCC2(CC1)N(CNC2=O)c1cccc(F)c1)NC(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C23H26BrFN4O2/c1-16(27-21(30)17-5-7-18(24)8-6-17)14-28-11-9-23(10-12-28)22(31)26-15-29(23)20-4-2-3-19(25)13-20/h2-8,13,16H,9-12,14-15H2,1H3,(H,26,31)(H,27,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
| Assay Description
Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... |
ACS Chem Biol 10: 421-32 (2015)
Article DOI: 10.1021/cb500828m
BindingDB Entry DOI: 10.7270/Q2H70DK8 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50206160
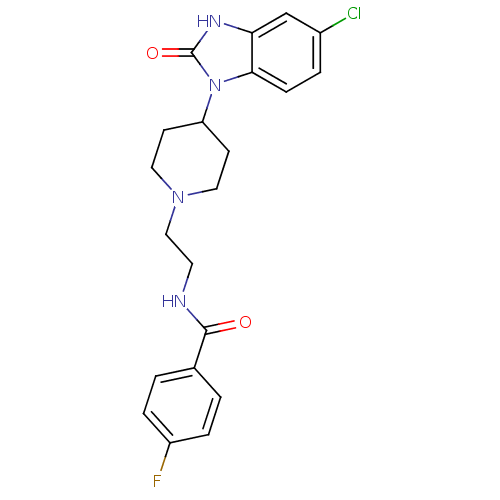
(CHEMBL245621 | Halopemide | Halopemide, 8 | N-(2-(...)Show SMILES Fc1ccc(cc1)C(=O)NCCN1CCC(CC1)n1c2ccc(Cl)cc2[nH]c1=O Show InChI InChI=1S/C21H22ClFN4O2/c22-15-3-6-19-18(13-15)25-21(29)27(19)17-7-10-26(11-8-17)12-9-24-20(28)14-1-4-16(23)5-2-14/h1-6,13,17H,7-12H2,(H,24,28)(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of dopamine receptor D2 (unknown origin) |
J Med Chem 56: 2695-9 (2013)
Article DOI: 10.1021/jm301782e
BindingDB Entry DOI: 10.7270/Q2RJ4KT8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246874
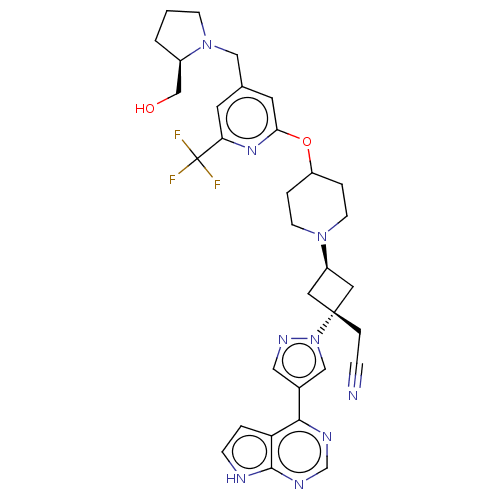
(US10053465, 13 | US10065963, Compound 13 | US10125...)Show SMILES OC[C@H]1CCCN1Cc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:20.27,wD:18.19,2.1,(3.36,8.8,;1.82,8.8,;1.05,10.14,;1.52,11.59,;.29,12.48,;-.95,11.59,;-.49,10.14,;-1.58,9.05,;-1.18,7.56,;-2.27,6.47,;-1.87,4.98,;-2.96,3.9,;-2.56,2.41,;-3.65,1.32,;-3.25,-.17,;-1.77,-.57,;-.68,.52,;-1.08,2.01,;-1.37,-2.05,;-2.14,-3.39,;-.8,-4.16,;.68,-4.56,;1.77,-3.47,;2.86,-2.38,;-.03,-2.82,;-1.57,-5.49,;-1.1,-6.96,;-2.34,-7.86,;-3.59,-6.96,;-3.11,-5.49,;-2.34,-9.4,;-3.68,-10.17,;-3.68,-11.71,;-2.34,-12.48,;-1.01,-11.71,;.45,-12.19,;1.36,-10.94,;.45,-9.7,;-1.01,-10.17,;-.39,4.59,;.7,5.67,;.3,7.16,;2.19,5.28,;3.28,6.37,;2.59,3.79,;3.68,4.88,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246875
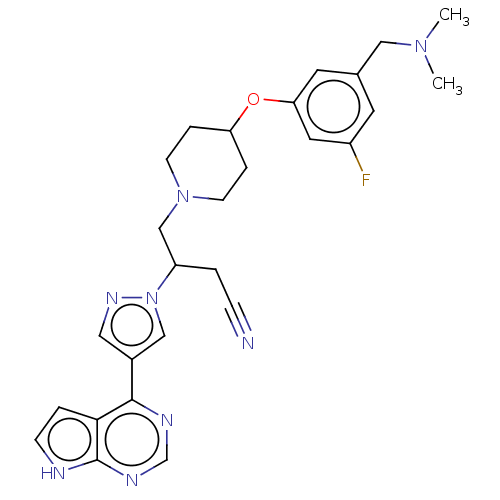
(4-(4-{3- [(dimethylamino) methyl]-5- fluorophenoxy...)Show SMILES CN(C)Cc1cc(F)cc(OC2CCN(CC(CC#N)n3cc(cn3)-c3ncnc4[nH]ccc34)CC2)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246876
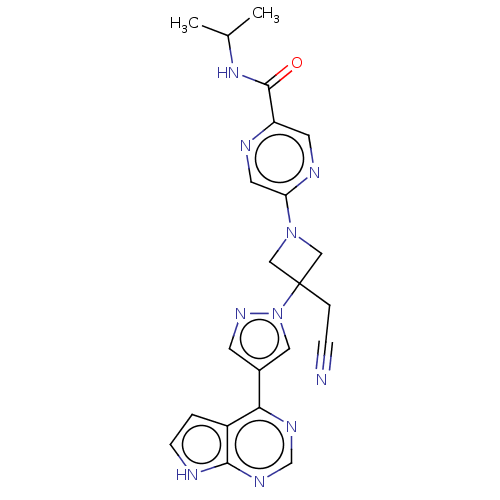
(5-{3-(cyanomethyl)- 3-[4-(7H-pyrrolo[2,3- d]pyrimi...)Show SMILES CC(C)NC(=O)c1cnc(cn1)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C22H22N10O/c1-14(2)30-21(33)17-8-26-18(9-25-17)31-11-22(12-31,4-5-23)32-10-15(7-29-32)19-16-3-6-24-20(16)28-13-27-19/h3,6-10,13-14H,4,11-12H2,1-2H3,(H,30,33)(H,24,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246877
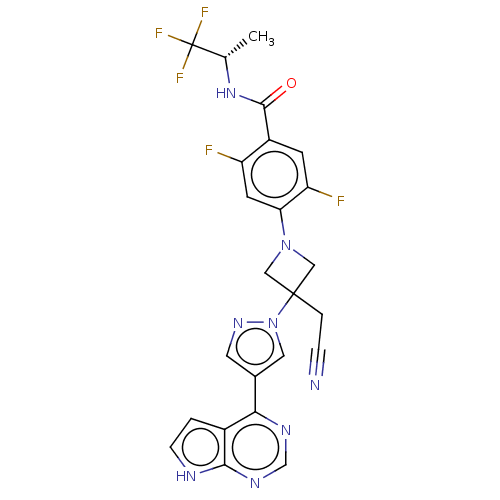
(4-{3-(cyanomethyl)- 3-[4-(7H-pyrrolo[2,3- d]pyrimi...)Show SMILES C[C@H](NC(=O)c1cc(F)c(cc1F)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12)C(F)(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM262166
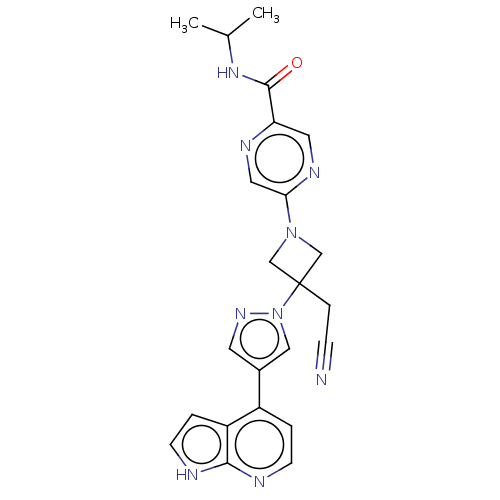
(5-{3-(cyanomethyl)- 3-[4-(1H-pyrrolo[2,3- b]pyridi...)Show SMILES CC(C)NC(=O)c1cnc(cn1)N1CC(CC#N)(C1)n1cc(cn1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C23H23N9O/c1-15(2)30-22(33)19-10-28-20(11-27-19)31-13-23(14-31,5-6-24)32-12-16(9-29-32)17-3-7-25-21-18(17)4-8-26-21/h3-4,7-12,15H,5,13-14H2,1-2H3,(H,25,26)(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246879

(US10053465, 18 | US10065963, Compound 18 | US10125...)Show SMILES OCCc1cc(O[C@H]2CC[C@H](CC2)N2CC(CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(n1)C(F)(F)F |r,wU:10.13,7.6,(9.15,6.25,;7.82,5.48,;6.48,6.25,;5.15,5.48,;3.82,6.25,;2.48,5.48,;1.15,6.25,;-.18,5.48,;-.18,3.94,;-1.52,3.17,;-2.85,3.94,;-2.85,5.48,;-1.52,6.25,;-4.19,3.17,;-4.58,1.68,;-6.07,2.08,;-7.61,2.08,;-8.38,3.41,;-9.15,4.74,;-5.67,3.56,;-6.84,.74,;-6.37,-.72,;-7.61,-1.63,;-8.86,-.72,;-8.38,.74,;-7.61,-3.17,;-8.95,-3.94,;-8.95,-5.48,;-7.61,-6.25,;-6.28,-5.48,;-4.81,-5.95,;-3.91,-4.71,;-4.81,-3.46,;-6.28,-3.94,;2.48,3.94,;3.82,3.17,;5.15,3.94,;3.82,1.63,;5.15,.86,;2.48,.86,;3.82,.09,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246880

(US10053465, 19 | US10065963, Compound 19 | US10125...)Show SMILES CCNCc1cc(O[C@H]2CC[C@H](CC2)N2CC(CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:11.14,8.7,(9.82,5.48,;8.48,6.25,;7.15,5.48,;5.82,6.25,;4.48,5.48,;3.15,6.25,;1.82,5.48,;.48,6.25,;-.85,5.48,;-.85,3.94,;-2.18,3.17,;-3.52,3.94,;-3.52,5.48,;-2.18,6.25,;-4.85,3.17,;-5.25,1.68,;-6.74,2.08,;-8.28,2.08,;-9.05,3.41,;-9.82,4.74,;-6.34,3.56,;-7.51,.74,;-7.03,-.72,;-8.28,-1.63,;-9.52,-.72,;-9.05,.74,;-8.28,-3.17,;-9.61,-3.94,;-9.61,-5.48,;-8.28,-6.25,;-6.94,-5.48,;-5.48,-5.95,;-4.57,-4.71,;-5.48,-3.46,;-6.94,-3.94,;1.82,3.94,;3.15,3.17,;4.48,3.94,;3.15,1.63,;4.48,.86,;1.82,.86,;3.15,.09,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246881
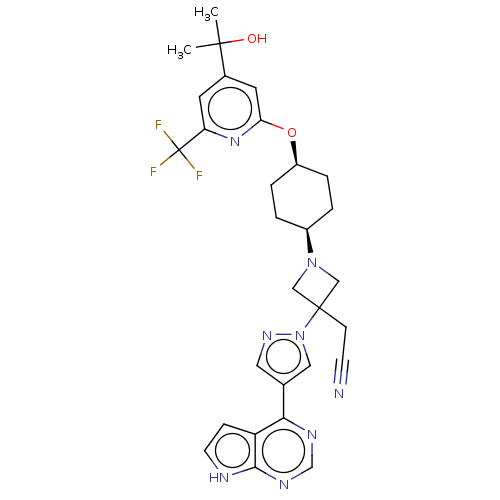
(US10053465, 20 | US10065963, Compound 20 | US10125...)Show SMILES CC(C)(O)c1cc(O[C@H]2CC[C@H](CC2)N2CC(CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:11.14,8.7,(8.48,4.71,;7.15,5.48,;7.15,7.02,;8.48,6.25,;5.82,4.71,;4.48,5.48,;3.15,4.71,;1.82,5.48,;.48,4.71,;.48,3.17,;-.85,2.4,;-2.18,3.17,;-2.18,4.71,;-.85,5.48,;-3.52,2.4,;-3.92,.91,;-5.4,1.31,;-6.94,1.31,;-7.71,2.64,;-8.48,3.97,;-5.01,2.79,;-6.17,-.03,;-5.7,-1.49,;-6.94,-2.4,;-8.19,-1.49,;-7.71,-.03,;-6.94,-3.94,;-8.28,-4.71,;-8.28,-6.25,;-6.94,-7.02,;-5.61,-6.25,;-4.15,-6.72,;-3.24,-5.48,;-4.15,-4.23,;-5.61,-4.71,;3.15,3.17,;4.48,2.4,;5.82,3.17,;4.48,.86,;5.82,.09,;3.15,.09,;4.48,-.68,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246882
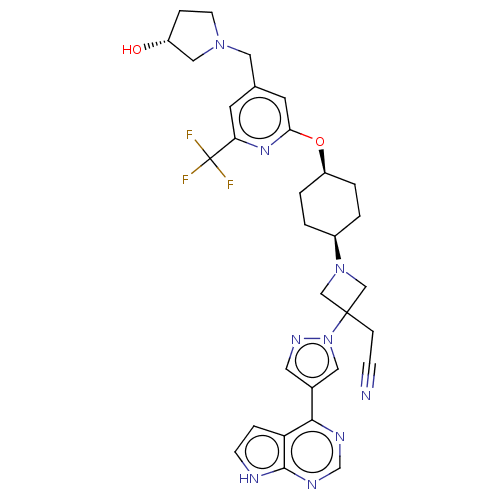
(US10053465, 21 | US10065963, Compound 21 | US10125...)Show SMILES O[C@@H]1CCN(Cc2cc(O[C@H]3CC[C@H](CC3)N3CC(CC#N)(C3)n3cc(cn3)-c3ncnc4[nH]ccc34)nc(c2)C(F)(F)F)C1 |r,wU:13.16,10.9,1.0,(.78,4.73,;-.7,5.13,;-1.18,6.59,;-2.72,6.59,;-3.2,5.13,;-4.68,4.73,;-5.08,3.24,;-3.99,2.15,;-4.39,.66,;-3.3,-.43,;-1.81,-.03,;-1.42,1.46,;.07,1.86,;1.16,.77,;.76,-.72,;-.73,-1.12,;2.65,1.17,;3.42,2.5,;4.75,1.73,;5.52,3.07,;7.06,3.07,;8.6,3.07,;3.98,.4,;5.52,.4,;5.05,-1.07,;6.29,-1.97,;7.54,-1.07,;7.06,.4,;6.29,-3.51,;4.96,-4.28,;4.96,-5.82,;6.29,-6.59,;7.63,-5.82,;9.09,-6.3,;10,-5.05,;9.09,-3.81,;7.63,-4.28,;-5.88,.27,;-6.97,1.35,;-6.57,2.84,;-8.46,.96,;-9.54,2.04,;-8.85,-.53,;-10,.96,;-1.95,4.22,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246883
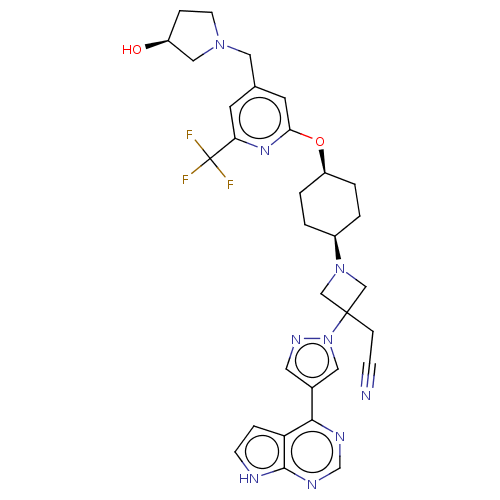
(US10005788, 1 (2nd peak, cis-) | US10053465, 22 | ...)Show SMILES O[C@H]1CCN(Cc2cc(O[C@H]3CC[C@H](CC3)N3CC(CC#N)(C3)n3cc(cn3)-c3ncnc4[nH]ccc34)nc(c2)C(F)(F)F)C1 |r,wU:13.16,10.9,wD:1.0,(.78,4.73,;-.7,5.13,;-1.18,6.59,;-2.72,6.59,;-3.2,5.13,;-4.68,4.73,;-5.08,3.24,;-3.99,2.15,;-4.39,.66,;-3.3,-.43,;-1.81,-.03,;-1.42,1.46,;.07,1.86,;1.16,.77,;.76,-.72,;-.73,-1.12,;2.65,1.17,;3.42,2.5,;4.75,1.73,;5.52,3.07,;7.06,3.07,;8.6,3.07,;3.98,.4,;5.52,.4,;5.05,-1.07,;6.29,-1.97,;7.54,-1.07,;7.06,.4,;6.29,-3.51,;4.96,-4.28,;4.96,-5.82,;6.29,-6.59,;7.63,-5.82,;9.09,-6.3,;10,-5.05,;9.09,-3.81,;7.63,-4.28,;-5.88,.27,;-6.97,1.35,;-6.57,2.84,;-8.46,.96,;-9.54,2.04,;-8.85,-.53,;-10,.96,;-1.95,4.22,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246884
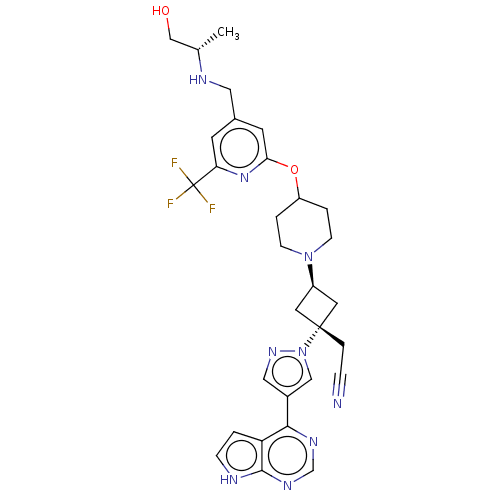
(US10053465, 23 | US10065963, Compound 23 | US10125...)Show SMILES C[C@@H](CO)NCc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:18.24,1.0,wD:16.16,(-2.38,11.91,;-.89,11.51,;.2,12.6,;1.69,12.2,;-.49,10.02,;-1.58,8.93,;-1.18,7.44,;-2.27,6.36,;-1.87,4.87,;-2.96,3.78,;-2.56,2.29,;-3.65,1.2,;-3.25,-.28,;-1.77,-.68,;-.68,.41,;-1.08,1.89,;-1.37,-2.17,;-2.14,-3.5,;-.8,-4.27,;.68,-4.67,;1.77,-3.58,;2.86,-2.49,;-.03,-2.94,;-1.57,-5.61,;-1.1,-7.07,;-2.34,-7.98,;-3.59,-7.07,;-3.11,-5.61,;-2.34,-9.52,;-3.68,-10.29,;-3.68,-11.83,;-2.34,-12.6,;-1.01,-11.83,;.45,-12.3,;1.36,-11.06,;.45,-9.81,;-1.01,-10.29,;-.39,4.47,;.7,5.56,;.3,7.05,;2.19,5.16,;3.28,6.25,;2.59,3.67,;3.68,4.76,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246885
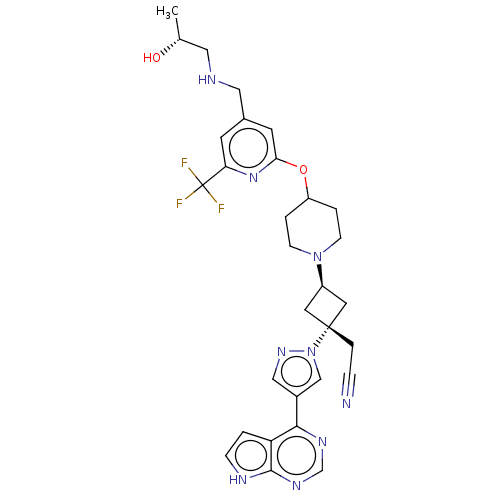
(US10053465, 24 | US10065963, Compound 25 | US10125...)Show SMILES C[C@@H](O)CNCc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:18.24,1.1,wD:16.16,(-.2,13.34,;.2,11.85,;1.69,11.45,;-.89,10.76,;-.49,9.28,;-1.58,8.19,;-1.18,6.7,;-2.27,5.61,;-1.87,4.12,;-2.96,3.04,;-2.56,1.55,;-3.65,.46,;-3.25,-1.03,;-1.77,-1.43,;-.68,-.34,;-1.08,1.15,;-1.37,-2.91,;-2.14,-4.25,;-.8,-5.02,;.68,-5.42,;1.77,-4.33,;2.86,-3.24,;-.03,-3.68,;-1.57,-6.35,;-1.1,-7.82,;-2.34,-8.72,;-3.59,-7.82,;-3.11,-6.35,;-2.34,-10.26,;-3.68,-11.03,;-3.68,-12.57,;-2.34,-13.34,;-1.01,-12.57,;.45,-13.05,;1.36,-11.8,;.45,-10.56,;-1.01,-11.03,;-.39,3.73,;.7,4.81,;.3,6.3,;2.19,4.42,;3.28,5.51,;2.59,2.93,;3.68,4.02,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246886
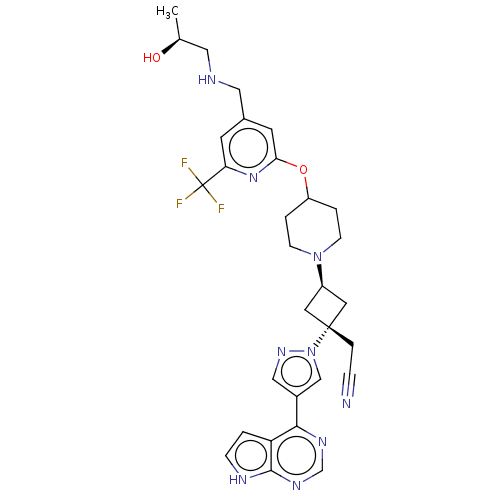
(US10053465, 25 | US10077277, Example 25 | US101251...)Show SMILES C[C@H](O)CNCc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:18.24,wD:16.16,1.1,(-.2,13.34,;.2,11.85,;1.69,11.45,;-.89,10.76,;-.49,9.28,;-1.58,8.19,;-1.18,6.7,;-2.27,5.61,;-1.87,4.12,;-2.96,3.04,;-2.56,1.55,;-3.65,.46,;-3.25,-1.03,;-1.77,-1.43,;-.68,-.34,;-1.08,1.15,;-1.37,-2.91,;-2.14,-4.25,;-.8,-5.02,;.68,-5.42,;1.77,-4.33,;2.86,-3.24,;-.03,-3.68,;-1.57,-6.35,;-1.1,-7.82,;-2.34,-8.72,;-3.59,-7.82,;-3.11,-6.35,;-2.34,-10.26,;-3.68,-11.03,;-3.68,-12.57,;-2.34,-13.34,;-1.01,-12.57,;.45,-13.05,;1.36,-11.8,;.45,-10.56,;-1.01,-11.03,;-.39,3.73,;.7,4.81,;.3,6.3,;2.19,4.42,;3.28,5.51,;2.59,2.93,;3.68,4.02,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246888
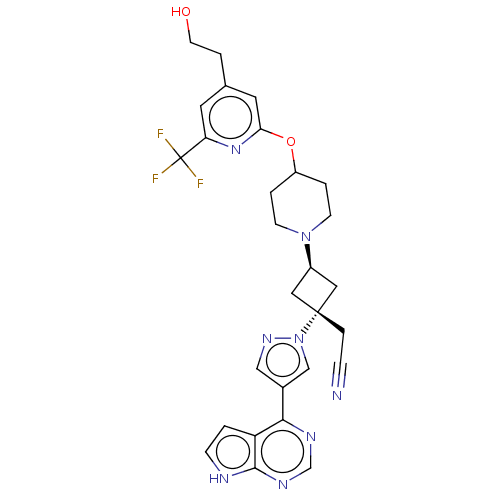
(US10053465, 26 | US10065963, Compound 26 | US10125...)Show SMILES OCCc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:15.21,wD:13.13,(-.89,12.05,;-.49,10.57,;-1.58,9.48,;-1.18,7.99,;-2.27,6.9,;-1.87,5.41,;-2.96,4.32,;-2.56,2.84,;-3.65,1.75,;-3.25,.26,;-1.77,-.14,;-.68,.95,;-1.08,2.44,;-1.37,-1.63,;-2.14,-2.96,;-.8,-3.73,;.68,-4.13,;1.77,-3.04,;2.86,-1.95,;-.03,-2.4,;-1.57,-5.06,;-1.1,-6.53,;-2.34,-7.43,;-3.59,-6.53,;-3.11,-5.06,;-2.34,-8.97,;-3.68,-9.74,;-3.68,-11.28,;-2.34,-12.05,;-1.01,-11.28,;.45,-11.76,;1.36,-10.51,;.45,-9.27,;-1.01,-9.74,;-.39,5.01,;.7,6.1,;.3,7.59,;2.19,5.7,;3.28,6.79,;2.59,4.22,;3.68,5.31,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246873
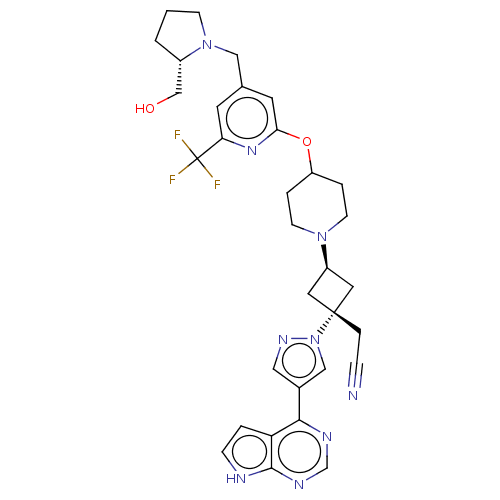
(US10053465, 12 | US10065963, Compound 12 | US10125...)Show SMILES OC[C@@H]1CCCN1Cc1cc(OC2CCN(CC2)[C@H]2C[C@@](CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)nc(c1)C(F)(F)F |r,wU:20.27,2.1,wD:18.19,(3.36,8.8,;1.82,8.8,;1.05,10.14,;1.52,11.59,;.29,12.48,;-.95,11.59,;-.49,10.14,;-1.58,9.05,;-1.18,7.56,;-2.27,6.47,;-1.87,4.98,;-2.96,3.9,;-2.56,2.41,;-3.65,1.32,;-3.25,-.17,;-1.77,-.57,;-.68,.52,;-1.08,2.01,;-1.37,-2.05,;-2.14,-3.39,;-.8,-4.16,;.68,-4.56,;1.77,-3.47,;2.86,-2.38,;-.03,-2.82,;-1.57,-5.49,;-1.1,-6.96,;-2.34,-7.86,;-3.59,-6.96,;-3.11,-5.49,;-2.34,-9.4,;-3.68,-10.17,;-3.68,-11.71,;-2.34,-12.48,;-1.01,-11.71,;.45,-12.19,;1.36,-10.94,;.45,-9.7,;-1.01,-10.17,;-.39,4.59,;.7,5.67,;.3,7.16,;2.19,5.28,;3.28,6.37,;2.59,3.79,;3.68,4.88,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246872
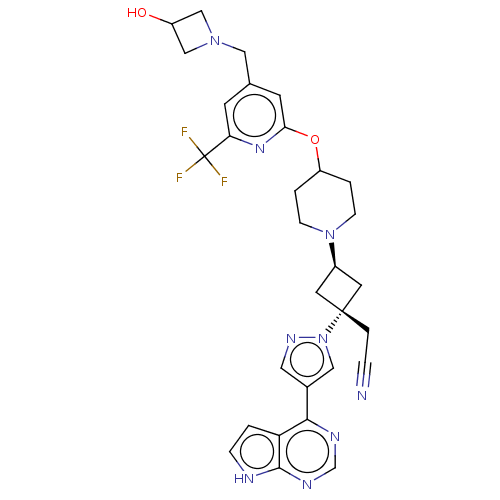
(US10053465, 11 | US10065963, Compound 11 | US10125...)Show SMILES OC1CN(Cc2cc(OC3CCN(CC3)[C@H]3C[C@@](CC#N)(C3)n3cc(cn3)-c3ncnc4[nH]ccc34)nc(c2)C(F)(F)F)C1 |r,wU:17.23,wD:15.15,(2.14,12.62,;1.05,11.53,;1.05,9.99,;-.49,9.99,;-1.58,8.91,;-1.18,7.42,;-2.27,6.33,;-1.87,4.84,;-2.96,3.75,;-2.56,2.27,;-3.65,1.18,;-3.25,-.31,;-1.77,-.71,;-.68,.38,;-1.08,1.87,;-1.37,-2.2,;-2.14,-3.53,;-.8,-4.3,;.68,-4.7,;1.77,-3.61,;2.86,-2.52,;-.03,-2.97,;-1.57,-5.63,;-1.1,-7.1,;-2.34,-8,;-3.59,-7.1,;-3.11,-5.63,;-2.34,-9.54,;-3.68,-10.31,;-3.68,-11.85,;-2.34,-12.62,;-1.01,-11.85,;.45,-12.33,;1.36,-11.08,;.45,-9.84,;-1.01,-10.31,;-.39,4.44,;.7,5.53,;.3,7.02,;2.19,5.13,;3.28,6.22,;2.59,3.65,;3.68,4.74,;-.49,11.53,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246871
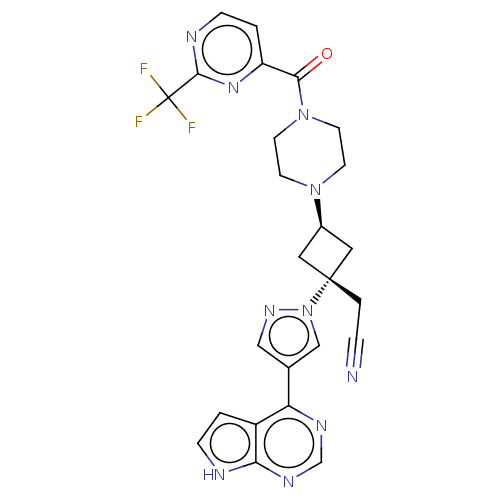
(US10053465, 10 | US10065963, Compound 10 | US10125...)Show SMILES FC(F)(F)c1nccc(n1)C(=O)N1CCN(CC1)[C@H]1C[C@@](CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r,wU:20.27,wD:18.19,(-2.27,10.39,;-.79,10,;.3,11.08,;-.79,11.54,;-.39,8.51,;1.1,8.11,;1.5,6.62,;.41,5.53,;-1.08,5.93,;-1.48,7.42,;-2.17,4.84,;-3.66,5.24,;-1.77,3.35,;-2.86,2.27,;-2.46,.78,;-.97,.38,;.12,1.47,;-.28,2.96,;-.57,-1.11,;-1.34,-2.44,;-.01,-3.21,;1.48,-3.61,;2.57,-2.52,;3.66,-1.43,;.76,-1.88,;-.78,-4.55,;-.3,-6.01,;-1.55,-6.92,;-2.8,-6.01,;-2.32,-4.55,;-1.55,-8.46,;-2.88,-9.23,;-2.88,-10.77,;-1.55,-11.54,;-.22,-10.77,;1.25,-11.24,;2.15,-10,;1.25,-8.75,;-.22,-9.23,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246867
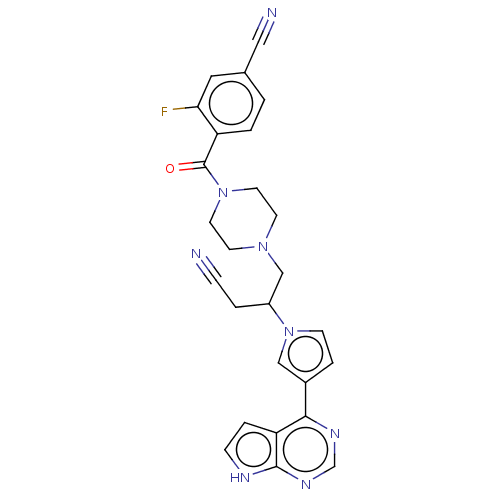
(4-[(4-{3-cyano-2-[3- (7H-pyrrolo[2,3- d]pyrimidin-...)Show SMILES Fc1cc(ccc1C(=O)N1CCN(CC(CC#N)n2ccc(c2)-c2ncnc3[nH]ccc23)CC1)C#N | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM285696
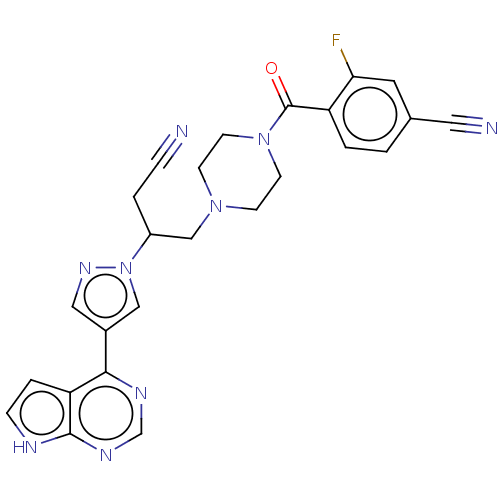
(4-[(4-{3-cyano-2-[3- (7H-pyrrolo[2,3- d]pyrimidin-...)Show SMILES Fc1cc(ccc1C(=O)N1CCN(CC(CC#N)n2cc(cn2)-c2ncnc3[nH]ccc23)CC1)C#N | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246861
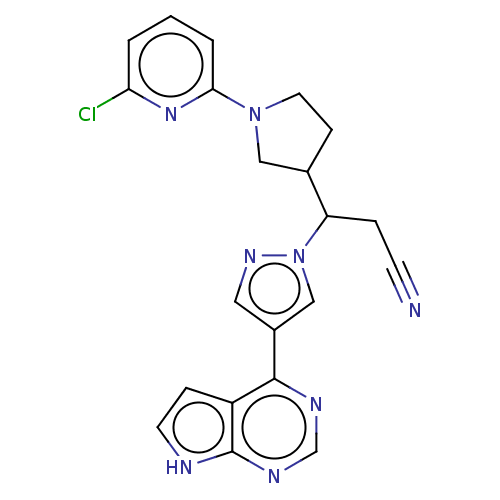
(3-[1-(6- chloropyridin-2- yl)pyrrolidin-3-yl]-3- [...)Show SMILES Clc1cccc(n1)N1CCC(C1)C(CC#N)n1cc(cn1)-c1ncnc2[nH]ccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246864
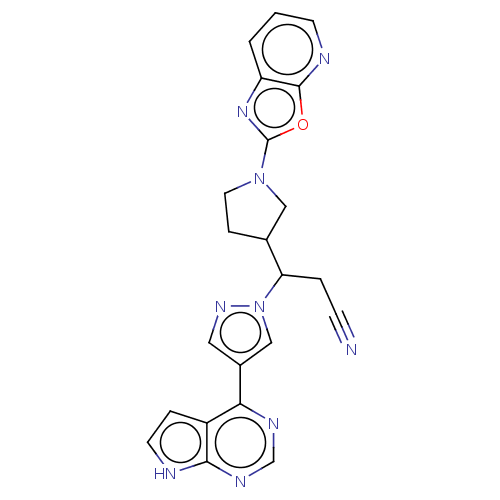
(3-(1- [1,3]oxazolo[5,4- b]pyridin-2- ylpyrrolidin-...)Show SMILES N#CCC(C1CCN(C1)c1nc2cccnc2o1)n1cc(cn1)-c1ncnc2[nH]ccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246870
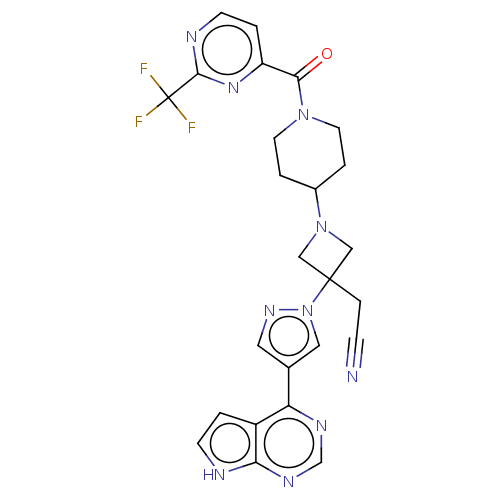
(US10053465, 9 | US10065963, Compound 9 | US1012515...)Show SMILES FC(F)(F)c1nccc(n1)C(=O)N1CCC(CC1)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C25H23F3N10O/c26-25(27,28)23-31-8-2-19(35-23)22(39)36-9-3-17(4-10-36)37-13-24(14-37,5-6-29)38-12-16(11-34-38)20-18-1-7-30-21(18)33-15-32-20/h1-2,7-8,11-12,15,17H,3-5,9-10,13-14H2,(H,30,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246869
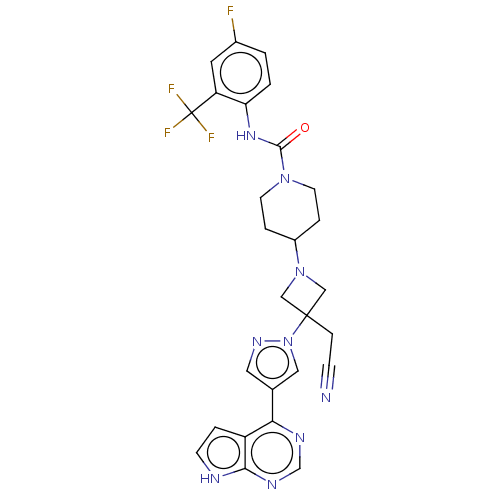
(4-{3-(Cyanomethyl)- 3-[4-(7H-pyrrolo[2,3- d]pyrimi...)Show SMILES Fc1ccc(NC(=O)N2CCC(CC2)N2CC(CC#N)(C2)n2cc(cn2)-c2ncnc3[nH]ccc23)c(c1)C(F)(F)F Show InChI InChI=1S/C27H25F4N9O/c28-18-1-2-22(21(11-18)27(29,30)31)37-25(41)38-9-4-19(5-10-38)39-14-26(15-39,6-7-32)40-13-17(12-36-40)23-20-3-8-33-24(20)35-16-34-23/h1-3,8,11-13,16,19H,4-6,9-10,14-15H2,(H,37,41)(H,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM246868

(US10053465, 7 | US10065963, Compound 7 | US1012515...)Show SMILES Fc1c(ccnc1C(F)(F)F)C(=O)N1CCC(CC1)N1CC(CC#N)(C1)n1cc(cn1)-c1ncnc2[nH]ccc12 Show InChI InChI=1S/C26H23F4N9O/c27-20-18(1-7-32-22(20)26(28,29)30)24(40)37-9-3-17(4-10-37)38-13-25(14-38,5-6-31)39-12-16(11-36-39)21-19-2-8-33-23(19)35-15-34-21/h1-2,7-8,11-12,15,17H,3-5,9-10,13-14H2,(H,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phospholipase D2
(Homo sapiens (Human)) | BDBM50427800

(CHEMBL2325485)Show SMILES C[C@@H](CN1CCC2(CC1)N(CNC2=O)c1cccc(C)c1)NC(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C24H29BrN4O2/c1-17-4-3-5-21(14-17)29-16-26-23(31)24(29)10-12-28(13-11-24)15-18(2)27-22(30)19-6-8-20(25)9-7-19/h3-9,14,18H,10-13,15-16H2,1-2H3,(H,26,31)(H,27,30)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of GFP-tagged PLD2 in human HEK293 cells assessed as deuterated 1-butanol incorporation pretreated for 5 mins prior to substrate addition ... |
J Med Chem 56: 2695-9 (2013)
Article DOI: 10.1021/jm301782e
BindingDB Entry DOI: 10.7270/Q2RJ4KT8 |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM50427802
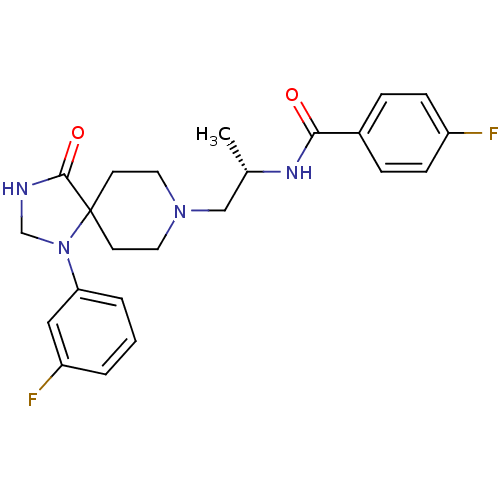
(CHEMBL2325482)Show SMILES C[C@@H](CN1CCC2(CC1)N(CNC2=O)c1cccc(F)c1)NC(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H26F2N4O2/c1-16(27-21(30)17-5-7-18(24)8-6-17)14-28-11-9-23(10-12-28)22(31)26-15-29(23)20-4-2-3-19(25)13-20/h2-8,13,16H,9-12,14-15H2,1H3,(H,26,31)(H,27,30)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of PLD1 in human Calu-1 cells assessed as deuterated 1-butanol incorporation pretreated for 5 mins prior to substrate addition measured af... |
J Med Chem 56: 2695-9 (2013)
Article DOI: 10.1021/jm301782e
BindingDB Entry DOI: 10.7270/Q2RJ4KT8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data