

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50025148 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase with CB3717 as control | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025149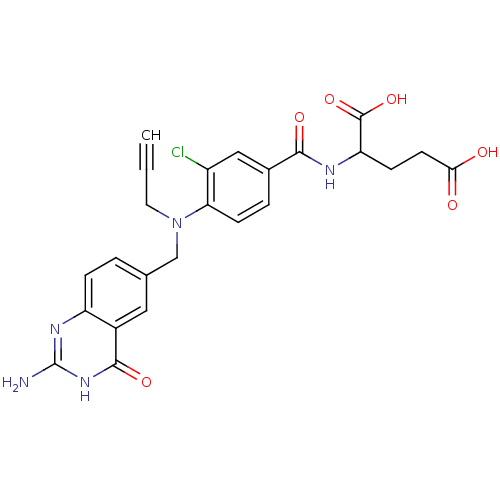 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025150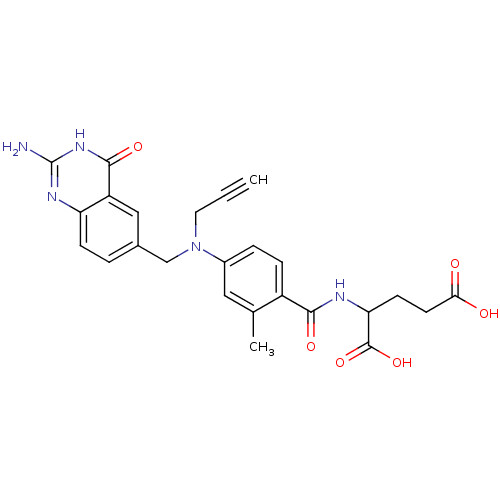 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase with CB3717 as control | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025147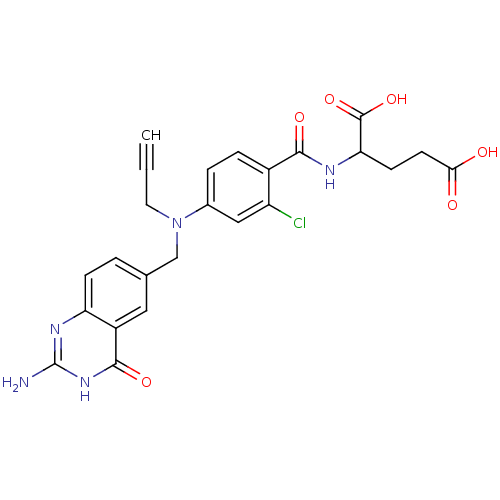 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025147 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025150 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase with CB3717 as control | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50025149 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of purified L1210 dihydrofolate reductase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50025148 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of purified L1210 dihydrofolate reductase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367702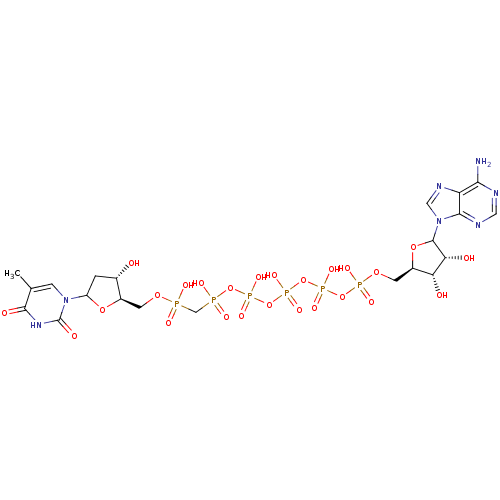 (CHEMBL606084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025149 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50025148 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated in vitro for the inhibition of L1210 thymidylate synthase with CB3717 as control | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367696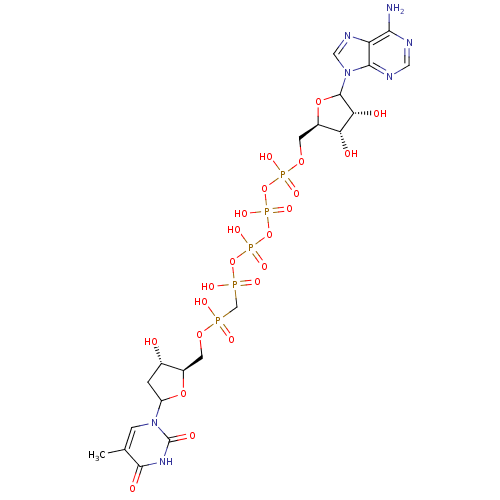 (CHEMBL605435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367698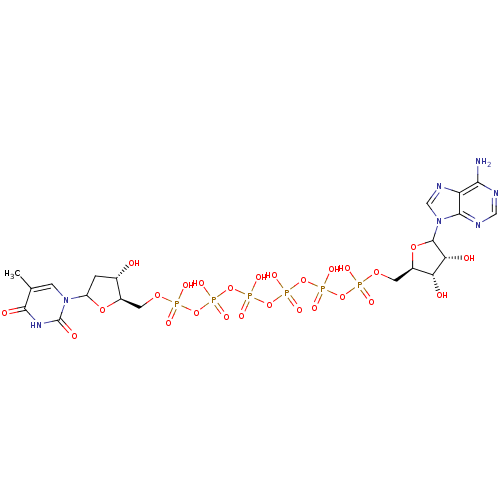 (CHEMBL604405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008294 (2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of purified L1210 dihydrofolate reductase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50366828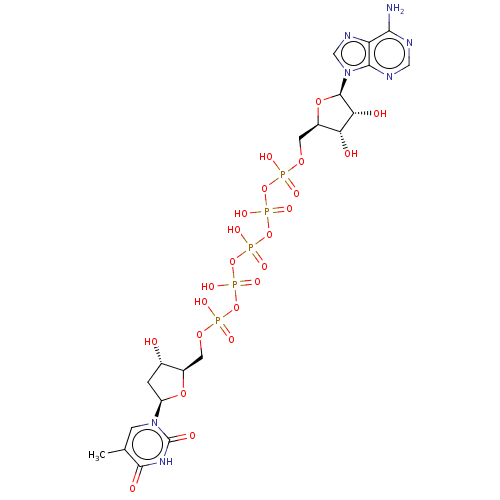 (CHEMBL1236157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50025147 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of purified L1210 dihydrofolate reductase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50025150 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated in vitro for inhibition of purified L1210 dihydrofolate reductase | J Med Chem 29: 468-72 (1986) BindingDB Entry DOI: 10.7270/Q2HH6J3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367703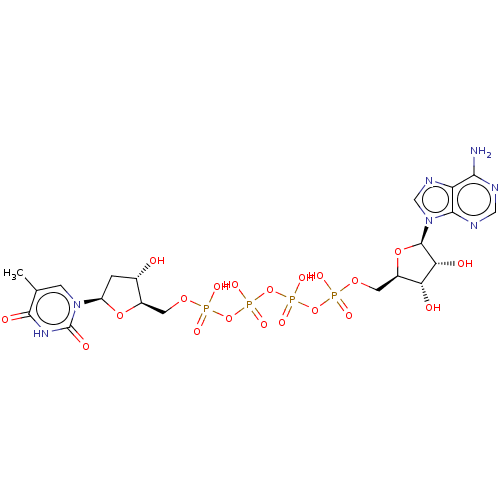 (CHEMBL1794614 | CHEMBL603793) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367697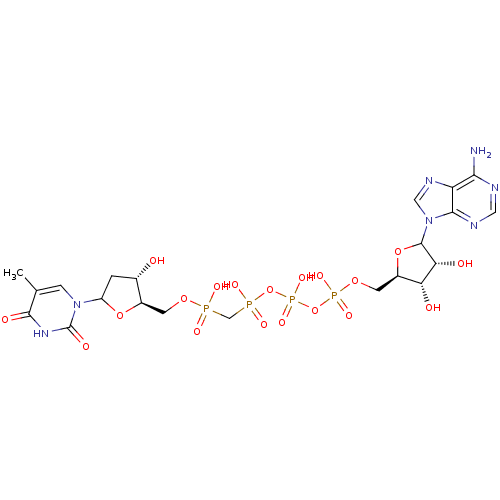 (CHEMBL606301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367704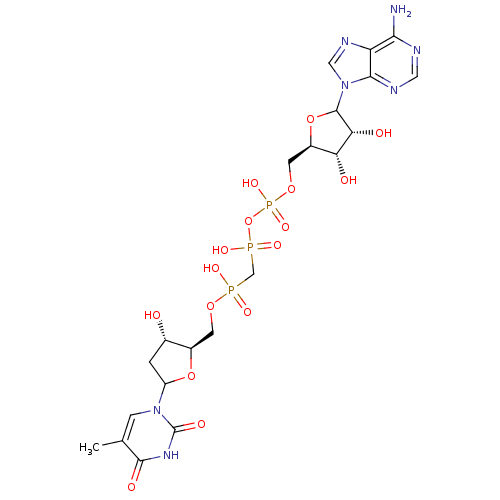 (CHEMBL604419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367699 (CHEMBL604420) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate kinase (Homo sapiens (Human)) | BDBM50367701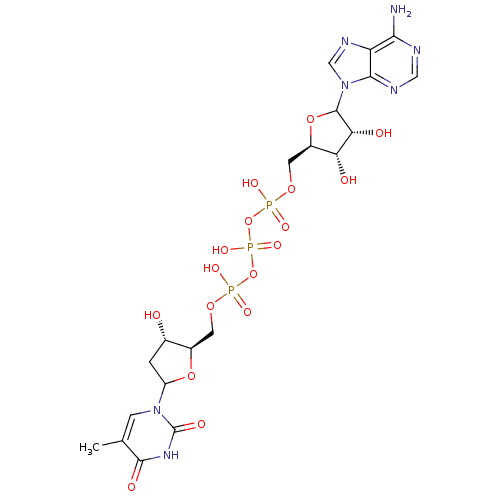 (CHEMBL605228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.23E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration required to reduce Thymidylate kinase rate by 50% in human blast cells | J Med Chem 31: 1305-8 (1988) BindingDB Entry DOI: 10.7270/Q2FT8MNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||