 Found 381 hits with Last Name = 'kalinowsky' and Initial = 'l'
Found 381 hits with Last Name = 'kalinowsky' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009073
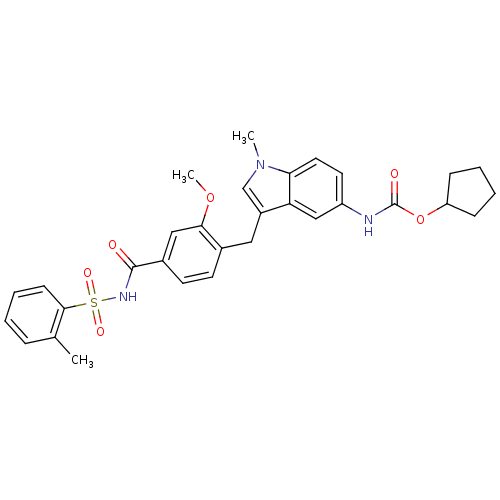
(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count... |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50227367
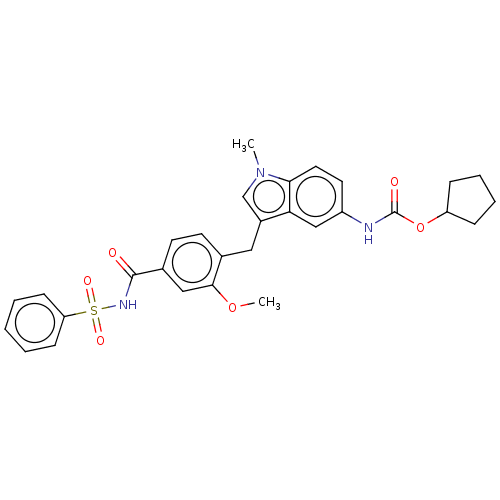
(CHEMBL48435)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C30H31N3O6S/c1-33-19-22(26-18-23(14-15-27(26)33)31-30(35)39-24-8-6-7-9-24)16-20-12-13-21(17-28(20)38-2)29(34)32-40(36,37)25-10-4-3-5-11-25/h3-5,10-15,17-19,24H,6-9,16H2,1-2H3,(H,31,35)(H,32,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count... |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538
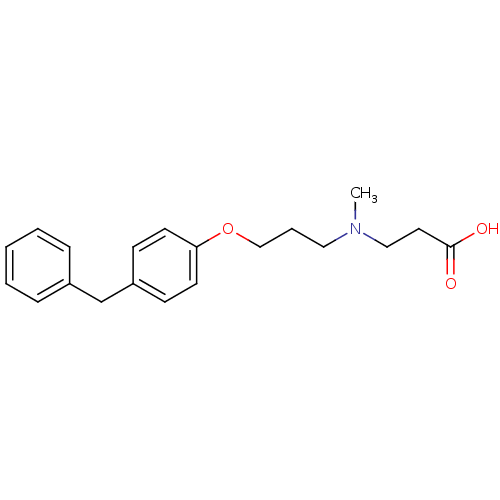
(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50015529
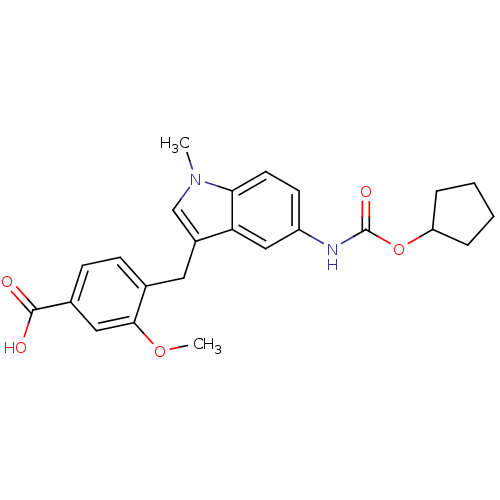
(4-(5-Cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(O)=O Show InChI InChI=1S/C24H26N2O5/c1-26-14-17(11-15-7-8-16(23(27)28)12-22(15)30-2)20-13-18(9-10-21(20)26)25-24(29)31-19-5-3-4-6-19/h7-10,12-14,19H,3-6,11H2,1-2H3,(H,25,29)(H,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count... |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197085
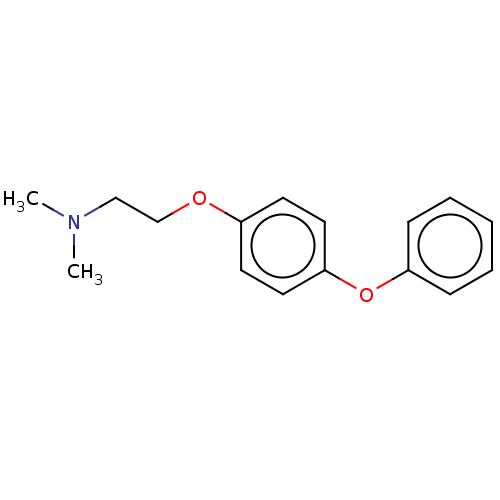
(CHEMBL3921982)Show InChI InChI=1S/C16H19NO2/c1-17(2)12-13-18-14-8-10-16(11-9-14)19-15-6-4-3-5-7-15/h3-11H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197084
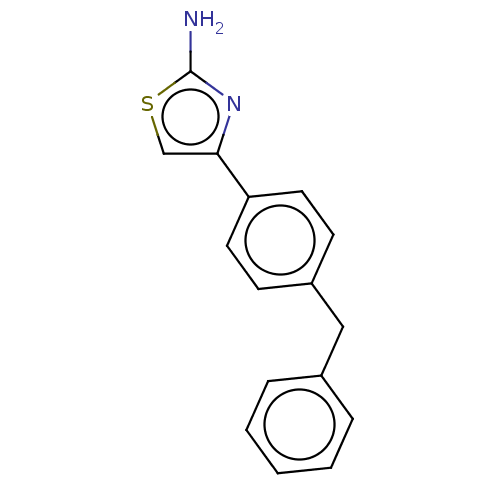
(CHEMBL3883608)Show InChI InChI=1S/C16H14N2S/c17-16-18-15(11-19-16)14-8-6-13(7-9-14)10-12-4-2-1-3-5-12/h1-9,11H,10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50023198
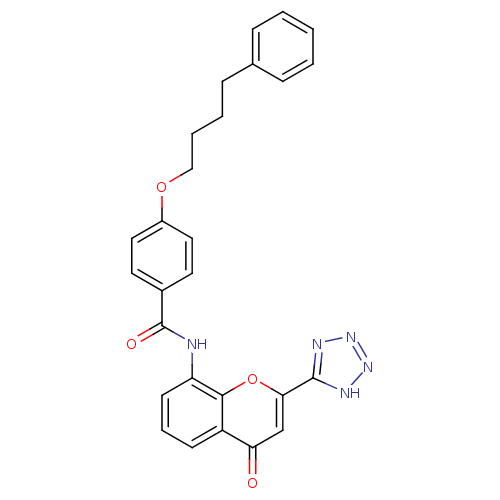
(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Displacement of [3H]ICI from cysteinyl leukotriene receptor 1 in Hartley guinea pig lung membrane after 30 mins by liquid scintillation counting meth... |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometry |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50239015
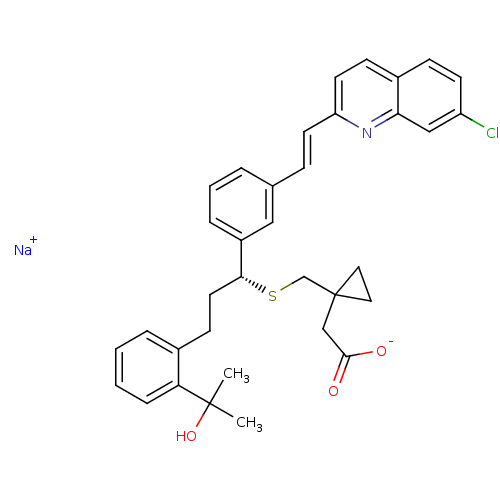
(CHEBI:6993 | MK-476 | Montelukast Sodium | Singula...)Show SMILES [Na+].CC(C)(O)c1ccccc1CC[C@@H](SCC1(CC([O-])=O)CC1)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 |r| Show InChI InChI=1S/C35H36ClNO3S.Na/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29;/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39);/q;+1/p-1/b15-10+;/t32-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometry |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50023198

(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometry |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538

(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50239014

(CHEMBL4102154)Show SMILES CCCCCc1ccc(\C=C\C(=O)Nc2cccc3c2oc(cc3=O)-n2cnnn2)cc1 Show InChI InChI=1S/C24H23N5O3/c1-2-3-4-6-17-9-11-18(12-10-17)13-14-22(31)26-20-8-5-7-19-21(30)15-23(32-24(19)20)29-16-25-27-28-29/h5,7-16H,2-4,6H2,1H3,(H,26,31)/b14-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Displacement of [3H]ICI from cysteinyl leukotriene receptor 1 in Hartley guinea pig lung membrane after 30 mins by liquid scintillation counting meth... |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197085

(CHEMBL3921982)Show InChI InChI=1S/C16H19NO2/c1-17(2)12-13-18-14-8-10-16(11-9-14)19-15-6-4-3-5-7-15/h3-11H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50227141
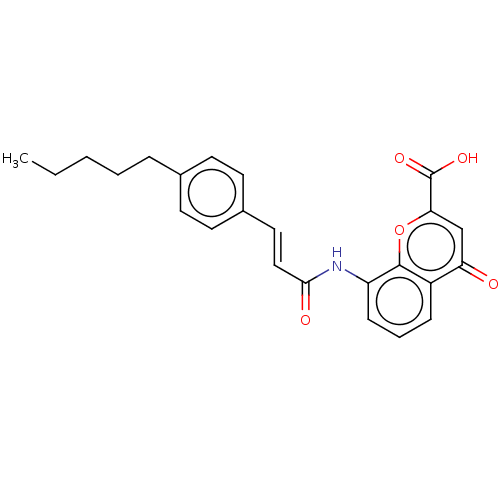
(CHEMBL174483)Show SMILES CCCCCc1ccc(\C=C\C(=O)Nc2cccc3c2oc(cc3=O)C(O)=O)cc1 Show InChI InChI=1S/C24H23NO5/c1-2-3-4-6-16-9-11-17(12-10-16)13-14-22(27)25-19-8-5-7-18-20(26)15-21(24(28)29)30-23(18)19/h5,7-15H,2-4,6H2,1H3,(H,25,27)(H,28,29)/b14-13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Displacement of [3H]ICI from cysteinyl leukotriene receptor 1 in Hartley guinea pig lung membrane after 30 mins by liquid scintillation counting meth... |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197084

(CHEMBL3883608)Show InChI InChI=1S/C16H14N2S/c17-16-18-15(11-19-16)14-8-6-13(7-9-14)10-12-4-2-1-3-5-12/h1-9,11H,10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197084

(CHEMBL3883608)Show InChI InChI=1S/C16H14N2S/c17-16-18-15(11-19-16)14-8-6-13(7-9-14)10-12-4-2-1-3-5-12/h1-9,11H,10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 35 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197084

(CHEMBL3883608)Show InChI InChI=1S/C16H14N2S/c17-16-18-15(11-19-16)14-8-6-13(7-9-14)10-12-4-2-1-3-5-12/h1-9,11H,10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 15 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197085

(CHEMBL3921982)Show InChI InChI=1S/C16H19NO2/c1-17(2)12-13-18-14-8-10-16(11-9-14)19-15-6-4-3-5-7-15/h3-11H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 15 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 25 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538

(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 35 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197085

(CHEMBL3921982)Show InChI InChI=1S/C16H19NO2/c1-17(2)12-13-18-14-8-10-16(11-9-14)19-15-6-4-3-5-7-15/h3-11H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 35 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538

(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 15 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 15 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197084

(CHEMBL3883608)Show InChI InChI=1S/C16H14N2S/c17-16-18-15(11-19-16)14-8-6-13(7-9-14)10-12-4-2-1-3-5-12/h1-9,11H,10H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 25 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 5.15E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 35 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 8.47E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 35 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50197085

(CHEMBL3921982)Show InChI InChI=1S/C16H19NO2/c1-17(2)12-13-18-14-8-10-16(11-9-14)19-15-6-4-3-5-7-15/h3-11H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 25 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 15 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 25 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116538

(3-{[3-(4-Benzyl-phenoxy)-propyl]-methyl-amino}-pro...)Show InChI InChI=1S/C20H25NO3/c1-21(14-12-20(22)23)13-5-15-24-19-10-8-18(9-11-19)16-17-6-3-2-4-7-17/h2-4,6-11H,5,12-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 25 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM246524

(Psoralidin (5))Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc2c3oc4cc(-[#8])ccc4c3c(=O)oc2cc1-[#8] Show InChI InChI=1S/C20H16O5/c1-10(2)3-4-11-7-14-17(9-15(11)22)25-20(23)18-13-6-5-12(21)8-16(13)24-19(14)18/h3,5-9,21-22H,4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human 6His-tagged FLAP expressed in Escherichia coli BL21 (DE3) by isothermal titration calorimetric method |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21674
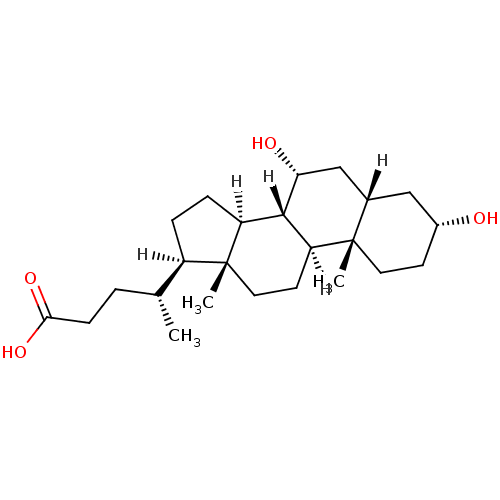
((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) assessed as recruitment of SRC1 peptide to FXR by FRET assay |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21675

((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) assessed as recruitment of SRC1 peptide to FXR by FRET assay |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Activation of human PXR expressed in DPX2 cells assessed as CYP3A4 induction after 24 hrs by luciferase reporter gene assay |
J Med Chem 60: 5235-5266 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01287
BindingDB Entry DOI: 10.7270/Q2T43WDQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50508793

(CHEMBL4554527)Show InChI InChI=1S/C21H18O3/c1-15-7-12-20(19(13-15)17-5-3-2-4-6-17)24-14-16-8-10-18(11-9-16)21(22)23/h2-13H,14H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRbeta LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50508794
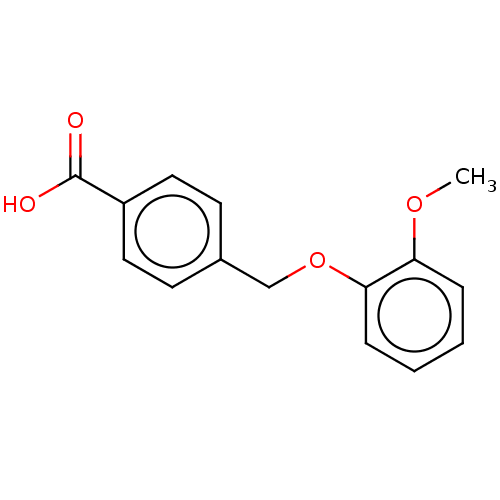
(CHEMBL4435989)Show InChI InChI=1S/C15H14O4/c1-18-13-4-2-3-5-14(13)19-10-11-6-8-12(9-7-11)15(16)17/h2-9H,10H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRbeta LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50508795

(CHEMBL4563945)Show InChI InChI=1S/C14H11IO3/c15-12-5-7-13(8-6-12)18-9-10-1-3-11(4-2-10)14(16)17/h1-8H,9H2,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRalpha LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50508796

(CHEMBL4447727)Show InChI InChI=1S/C14H11ClO3/c15-12-5-7-13(8-6-12)18-9-10-1-3-11(4-2-10)14(16)17/h1-8H,9H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRbeta LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50508797

(CHEMBL4514278)Show InChI InChI=1S/C18H19ClO3/c1-18(2,3)14-8-9-16(15(19)10-14)22-11-12-4-6-13(7-5-12)17(20)21/h4-10H,11H2,1-3H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRalpha LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50508798

(CHEMBL4570663)Show InChI InChI=1S/C18H20O3/c1-18(2,3)15-6-4-5-7-16(15)21-12-13-8-10-14(11-9-13)17(19)20/h4-11H,12H2,1-3H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRalpha LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50508799
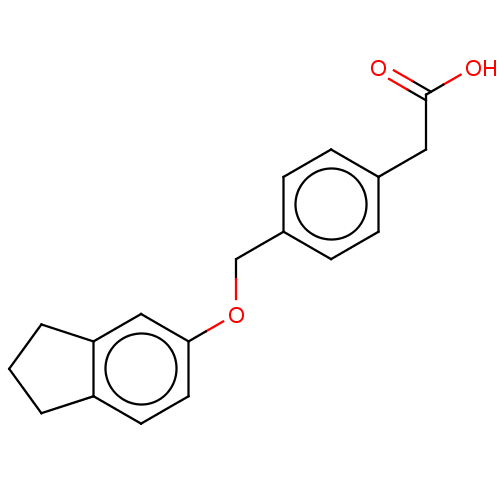
(CHEMBL4519831)Show InChI InChI=1S/C18H18O3/c19-18(20)10-13-4-6-14(7-5-13)12-21-17-9-8-15-2-1-3-16(15)11-17/h4-9,11H,1-3,10,12H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRgamma LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50508800

(CHEMBL4551159)Show InChI InChI=1S/C15H11F3O3/c16-15(17,18)12-3-1-2-4-13(12)21-9-10-5-7-11(8-6-10)14(19)20/h1-8H,9H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRgamma LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50508801

(CHEMBL4464986)Show InChI InChI=1S/C18H19NO2/c1-19(12-13-5-7-15(8-6-13)18(20)21)17-10-9-14-3-2-4-16(14)11-17/h5-11H,2-4,12H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRgamma LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50508802

(CHEMBL4455253)Show InChI InChI=1S/C20H16O3/c21-20(22)17-12-10-15(11-13-17)14-23-19-9-5-4-8-18(19)16-6-2-1-3-7-16/h1-13H,14H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRgamma LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50508803

(CHEMBL4513660)Show InChI InChI=1S/C15H11F3O4/c16-15(17,18)22-13-4-2-1-3-12(13)21-9-10-5-7-11(8-6-10)14(19)20/h1-8H,9H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRgamma LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50508794

(CHEMBL4435989)Show InChI InChI=1S/C15H14O4/c1-18-13-4-2-3-5-14(13)19-10-11-6-8-12(9-7-11)15(16)17/h2-9H,10H2,1H3,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Agonist activity at human Gal4-DBD fused RXRgamma LBD expressed in HEK293T cells after 12 to 14 hrs by luciferase reporter gene assay |
ACS Med Chem Lett 10: 203-208 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00551
BindingDB Entry DOI: 10.7270/Q27S7S37 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data