 Found 2224 hits with Last Name = 'kong' and Initial = 'l'
Found 2224 hits with Last Name = 'kong' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577958
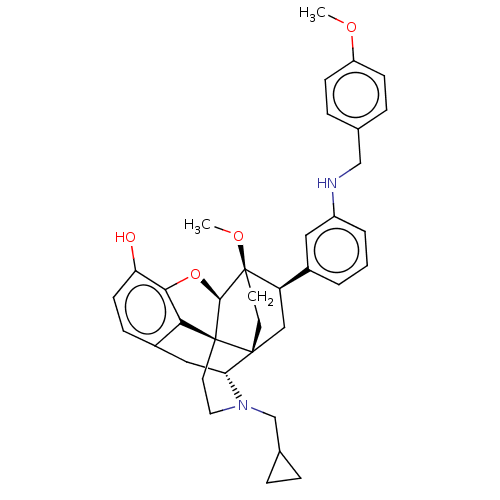
(CHEMBL4859512)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3O |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50598866
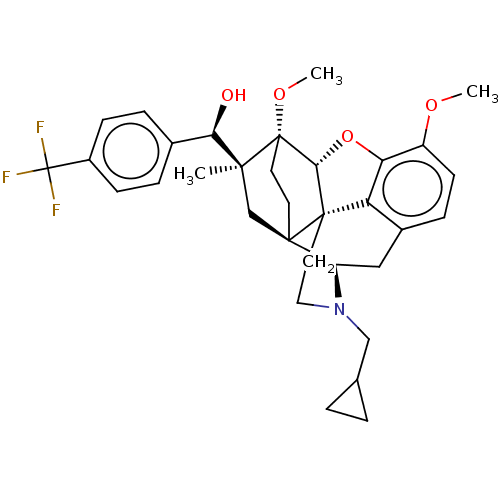
(CHEMBL5205530)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(cc1)C(F)(F)F)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598866

(CHEMBL5205530)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(cc1)C(F)(F)F)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50577958

(CHEMBL4859512)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3O |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from rat delta opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50577958

(CHEMBL4859512)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3O |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463294

(CHEMBL4249256)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@H](C(=O)c4ccccc4)[C@]2(OC)C=C1)ccc3OC |r,wU:16.16,1.0,wD:17.38,28.36,7.7,19.23,c:37,THB:10:9:17:5.6.4,(9.78,-11.07,;9.03,-9.74,;7.65,-10.81,;5.94,-9.74,;6.72,-8.4,;5.95,-7.07,;6.71,-5.74,;8.25,-5.74,;9.79,-5.74,;9.03,-4.41,;9.78,-3.07,;11.32,-3.05,;12.66,-3.81,;12.64,-2.27,;7.47,-5.14,;7.41,-7.18,;8.25,-8.41,;9.02,-7.07,;10.56,-7.06,;11.34,-8.4,;12.88,-8.4,;13.65,-7.07,;13.65,-9.73,;12.87,-11.06,;13.64,-12.4,;15.18,-12.4,;15.95,-11.05,;15.18,-9.72,;10.59,-9.7,;11.34,-11.07,;10.57,-12.41,;9.25,-8.93,;10.35,-7.84,;4.41,-7.06,;3.64,-8.38,;4.4,-9.72,;3.62,-11.05,;2.08,-11.04,)| Show InChI InChI=1S/C31H33NO4/c1-34-23-11-10-21-16-24-29-12-13-31(35-2,22(17-29)26(33)20-6-4-3-5-7-20)28-30(29,25(21)27(23)36-28)14-15-32(24)18-19-8-9-19/h3-7,10-13,19,22,24,28H,8-9,14-18H2,1-2H3/t22-,24-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506108

(CHEMBL4449252)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC(=O)c2ccccc2)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C37H40N2O4/c1-41-29-15-12-26-20-30-35-16-17-37(42-2,34-36(35,31(26)32(29)43-34)18-19-39(30)22-23-8-9-23)28(21-35)24-10-13-27(14-11-24)38-33(40)25-6-4-3-5-7-25/h3-7,10-15,23,28,30,34H,8-9,16-22H2,1-2H3,(H,38,40)/t28-,30-,34-,35-,36+,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506108

(CHEMBL4449252)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC(=O)c2ccccc2)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C37H40N2O4/c1-41-29-15-12-26-20-30-35-16-17-37(42-2,34-36(35,31(26)32(29)43-34)18-19-39(30)22-23-8-9-23)28(21-35)24-10-13-27(14-11-24)38-33(40)25-6-4-3-5-7-25/h3-7,10-15,23,28,30,34H,8-9,16-22H2,1-2H3,(H,38,40)/t28-,30-,34-,35-,36+,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463297

(CHEMBL4246433)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)21-7-10-23(36-2)11-8-21)19-31-13-14-33(30,38-4)29-32(31)15-16-34(18-20-5-6-20)25(31)17-22-9-12-24(37-3)27(39-29)26(22)32/h7-12,20,25,28-29,35H,5-6,13-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414626

(US10435405, Example 43 | US10934288, Example 43)Show SMILES COc1cccc(F)c1-c1cc2c(n[nH]c2cn1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C24H24FN5O/c1-29-10-12-30(13-11-29)17-8-6-16(7-9-17)24-18-14-20(26-15-21(18)27-28-24)23-19(25)4-3-5-22(23)31-2/h3-9,14-15H,10-13H2,1-2H3,(H,27,28) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase(DHFR) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50405796
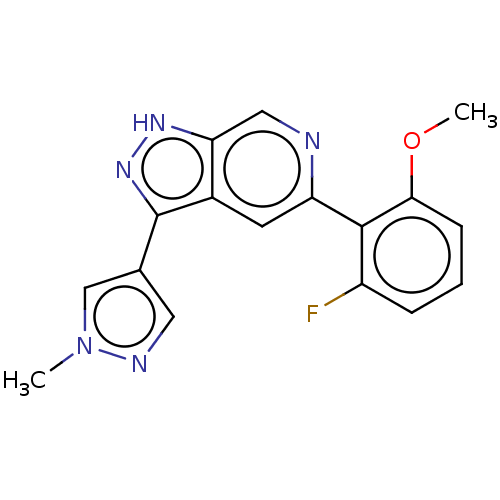
(CHEMBL5277555)Show InChI InChI=1S/C13H18N6/c1-3-9-11(12(15)19-13(16)18-9)7-4-5-10(17-2)8(14)6-7/h4-6,17H,3,14H2,1-2H3,(H4,15,16,18,19) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463293
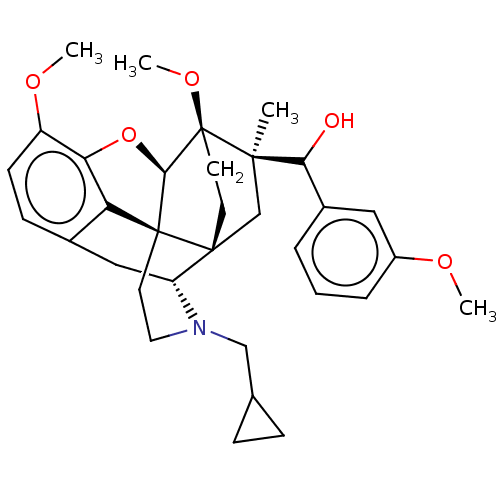
(CHEMBL4242847)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1cccc(OC)c1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)22-6-5-7-23(16-22)36-2)19-31-12-13-33(30,38-4)29-32(31)14-15-34(18-20-8-9-20)25(31)17-21-10-11-24(37-3)27(39-29)26(21)32/h5-7,10-11,16,20,25,28-29,35H,8-9,12-15,17-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM498971

(US11014929, Example 11 | US11542265, Example 11)Show SMILES COc1cccc(F)c1-c1ncc2[nH]nc(-c3ccc(cc3)N3CCN(C)CC3)c2n1 Show InChI InChI=1S/C23H23FN6O/c1-29-10-12-30(13-11-29)16-8-6-15(7-9-16)21-22-18(27-28-21)14-25-23(26-22)20-17(24)4-3-5-19(20)31-2/h3-9,14H,10-13H2,1-2H3,(H,27,28) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463284
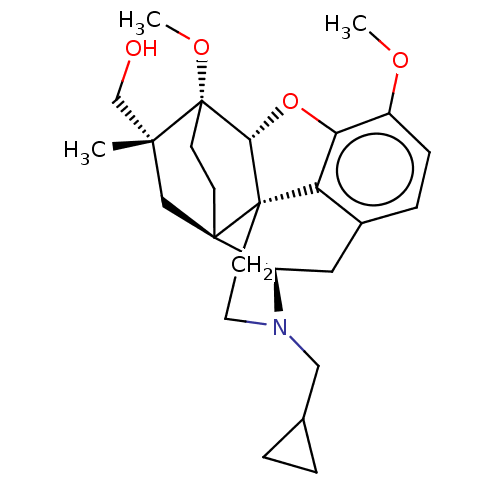
(CHEMBL4245818)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(CO)C1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C26H35NO4/c1-23(15-28)14-24-8-9-26(23,30-3)22-25(24)10-11-27(13-16-4-5-16)19(24)12-17-6-7-18(29-2)21(31-22)20(17)25/h6-7,16,19,22,28H,4-5,8-15H2,1-3H3/t19-,22-,23-,24-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414751
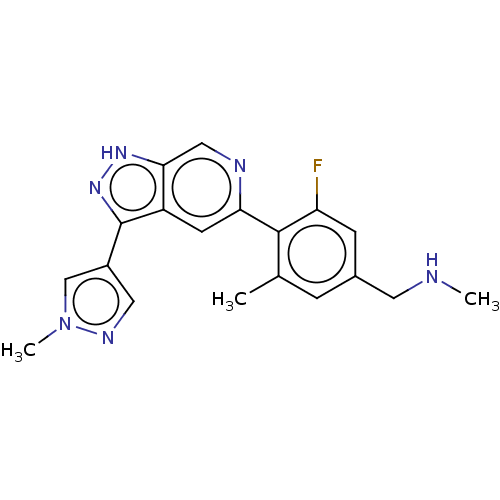
(US10435405, Example 168 | US10934288, Example 168)Show SMILES CNCc1cc(C)c(c(F)c1)-c1cc2c(n[nH]c2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C19H19FN6/c1-11-4-12(7-21-2)5-15(20)18(11)16-6-14-17(9-22-16)24-25-19(14)13-8-23-26(3)10-13/h4-6,8-10,21H,7H2,1-3H3,(H,24,25) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM536101

(US11242343, Example 8 | US11891388, Example 8)Show SMILES COc1cccc(F)c1-c1ccc2[nH]nc(-c3ccc(cc3)N3CCN(C)CC3)c2n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50405792
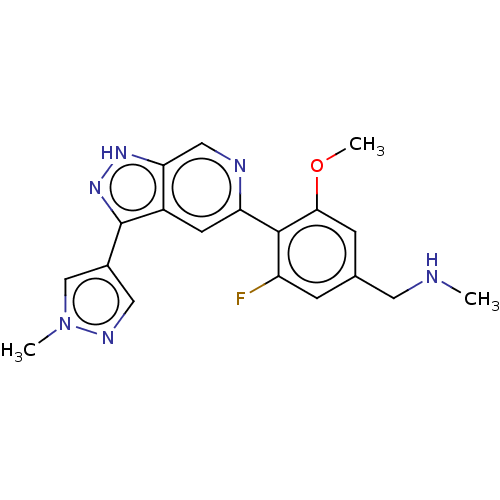
(CHEMBL5281791)Show InChI InChI=1S/C17H24N6/c1-3-14-15(16(18)21-17(19)20-14)12-4-6-13(7-5-12)23-10-8-22(2)9-11-23/h4-7H,3,8-11H2,1-2H3,(H4,18,19,20,21) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50598860
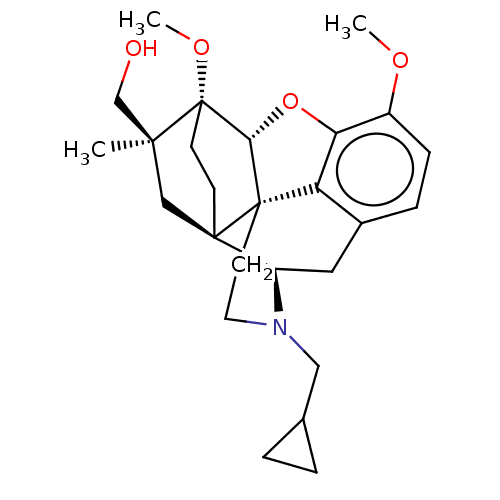
(CHEMBL5177908)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(CO)C1)ccc3OC |r,THB:10:9:17:4.6.5| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598862
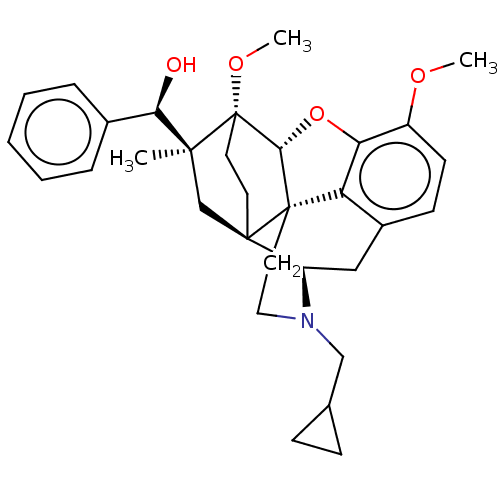
(CHEMBL5178795)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccccc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414714
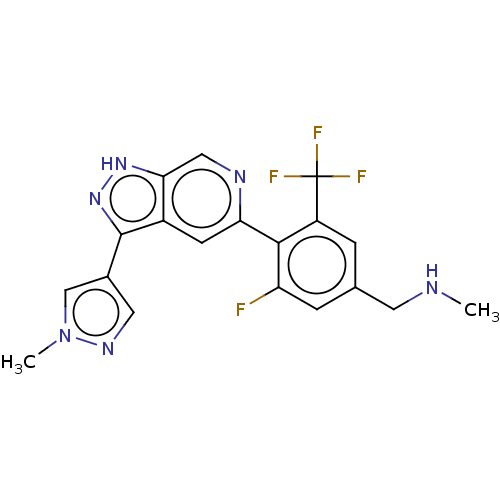
(US10435405, Example 131 | US10934288, Example 131)Show SMILES CNCc1cc(F)c(-c2cc3c(n[nH]c3cn2)-c2cnn(C)c2)c(c1)C(F)(F)F Show InChI InChI=1S/C19H16F4N6/c1-24-6-10-3-13(19(21,22)23)17(14(20)4-10)15-5-12-16(8-25-15)27-28-18(12)11-7-26-29(2)9-11/h3-5,7-9,24H,6H2,1-2H3,(H,27,28) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50598864
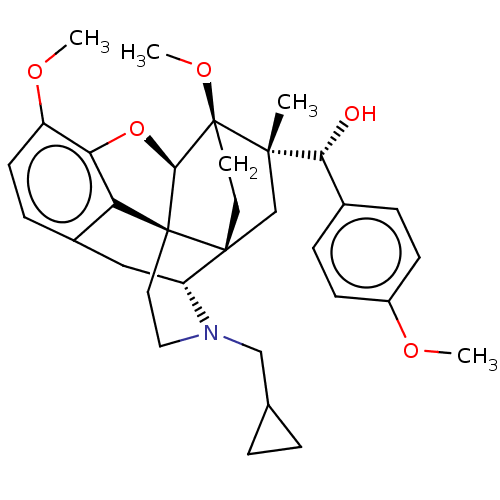
(CHEMBL5171735)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414772
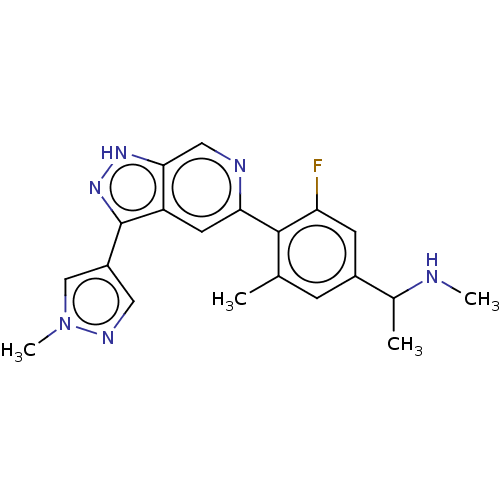
(US10435405, Example 189 | US10435405, Example 190 ...)Show SMILES CNC(C)c1cc(C)c(c(F)c1)-c1cc2c(n[nH]c2cn1)-c1cnn(C)c1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase(DHFR) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414765
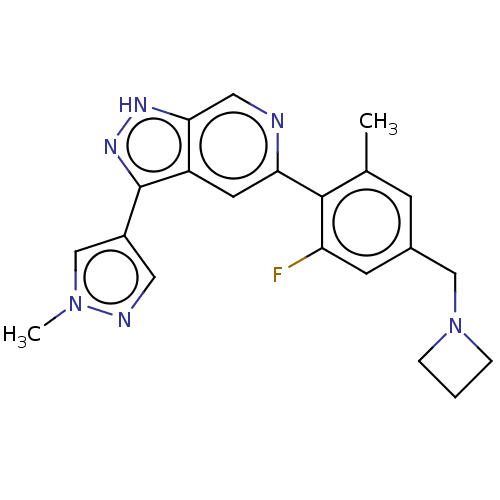
(5-(4-(Azetidin-1-ylmethyl)- 2-fluoro-6-methylpheny...)Show SMILES Cc1cc(CN2CCC2)cc(F)c1-c1cc2c(n[nH]c2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C21H21FN6/c1-13-6-14(11-28-4-3-5-28)7-17(22)20(13)18-8-16-19(10-23-18)25-26-21(16)15-9-24-27(2)12-15/h6-10,12H,3-5,11H2,1-2H3,(H,25,26) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506097
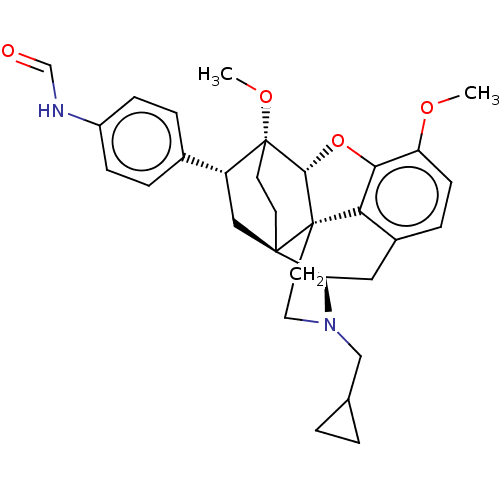
(CHEMBL4546243)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC=O)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C31H36N2O4/c1-35-24-10-7-21-15-25-29-11-12-31(36-2,23(16-29)20-5-8-22(9-6-20)32-18-34)28-30(29,26(21)27(24)37-28)13-14-33(25)17-19-3-4-19/h5-10,18-19,23,25,28H,3-4,11-17H2,1-2H3,(H,32,34)/t23-,25-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463292
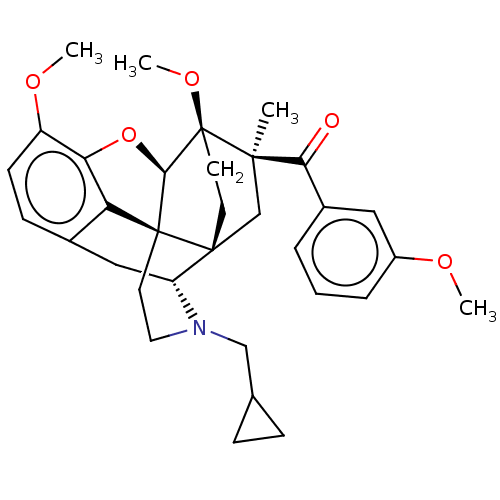
(CHEMBL4243649)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(=O)c1cccc(OC)c1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H39NO5/c1-30(28(35)22-6-5-7-23(16-22)36-2)19-31-12-13-33(30,38-4)29-32(31)14-15-34(18-20-8-9-20)25(31)17-21-10-11-24(37-3)27(39-29)26(21)32/h5-7,10-11,16,20,25,29H,8-9,12-15,17-19H2,1-4H3/t25-,29-,30-,31-,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414757

(US10435405, Example 174 | US10435405, Example 175 ...)Show SMILES Cc1cc(cc(F)c1-c1cc2c(n[nH]c2cn1)-c1cnn(C)c1)C1CCCCN1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase(DHFR) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463289

(CHEMBL4248801)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C=O)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C26H33NO4/c1-23(15-28)14-24-8-9-26(23,30-3)22-25(24)10-11-27(13-16-4-5-16)19(24)12-17-6-7-18(29-2)21(31-22)20(17)25/h6-7,15-16,19,22H,4-5,8-14H2,1-3H3/t19-,22-,23-,24-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598860

(CHEMBL5177908)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(CO)C1)ccc3OC |r,THB:10:9:17:4.6.5| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414759
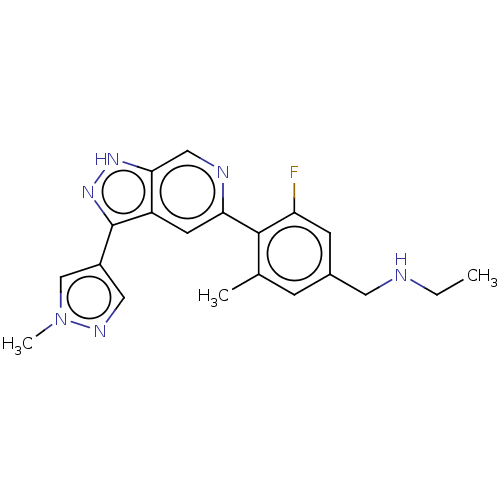
(US10435405, Example 176 | US10934288, Example 176)Show SMILES CCNCc1cc(C)c(c(F)c1)-c1cc2c(n[nH]c2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C20H21FN6/c1-4-22-8-13-5-12(2)19(16(21)6-13)17-7-15-18(10-23-17)25-26-20(15)14-9-24-27(3)11-14/h5-7,9-11,22H,4,8H2,1-3H3,(H,25,26) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase(DHFR) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM414705
(US10435405, Example 122 | US10934288, Example 122)Show SMILES CNCc1cc(F)c(c(F)c1)-c1cc2c(n[nH]c2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C18H16F2N6/c1-21-6-10-3-13(19)17(14(20)4-10)15-5-12-16(8-22-15)24-25-18(12)11-7-23-26(2)9-11/h3-5,7-9,21H,6H2,1-2H3,(H,24,25) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory concentration against rat liver dihydrofolate reductase(DHFR) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577936
(CHEMBL4864911)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCC2CC2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598864

(CHEMBL5171735)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000780
(2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN([C@H]1CCCC[C@@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463293

(CHEMBL4242847)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1cccc(OC)c1)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H41NO5/c1-30(28(35)22-6-5-7-23(16-22)36-2)19-31-12-13-33(30,38-4)29-32(31)14-15-34(18-20-8-9-20)25(31)17-21-10-11-24(37-3)27(39-29)26(21)32/h5-7,10-11,16,20,25,28-29,35H,8-9,12-15,17-19H2,1-4H3/t25-,28?,29-,30-,31-,32+,33+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506099
(CHEMBL4451186)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(N)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C30H36N2O3/c1-33-23-10-7-20-15-24-28-11-12-30(34-2,22(16-28)19-5-8-21(31)9-6-19)27-29(28,25(20)26(23)35-27)13-14-32(24)17-18-3-4-18/h5-10,18,22,24,27H,3-4,11-17,31H2,1-2H3/t22-,24-,27-,28-,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577953
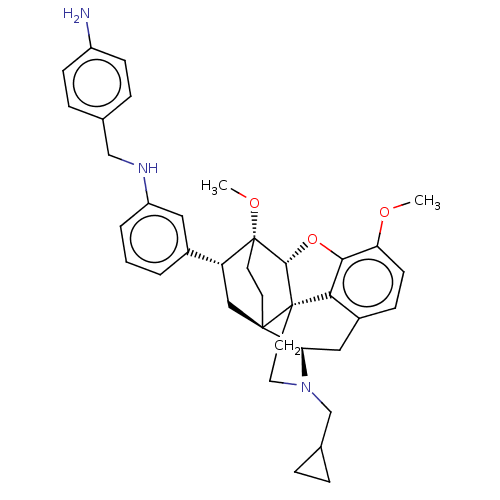
(CHEMBL4876542)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(N)cc2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577940
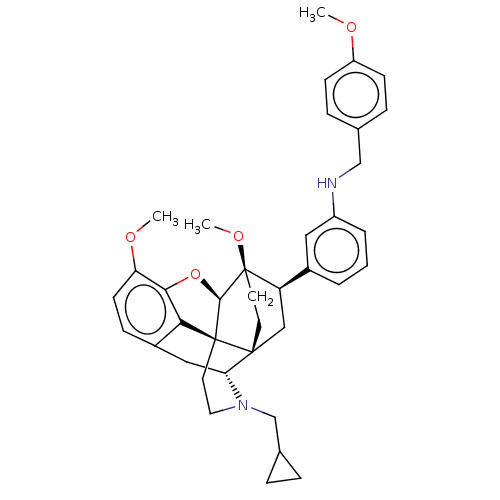
(CHEMBL4848808)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463287

(CHEMBL4238309)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)OC(=O)c1ccc(cc1)C(F)(F)F)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H36F3NO5/c1-29(42-27(38)20-6-9-22(10-7-20)33(34,35)36)18-30-12-13-32(29,40-3)28-31(30)14-15-37(17-19-4-5-19)24(30)16-21-8-11-23(39-2)26(41-28)25(21)31/h6-11,19,24,28H,4-5,12-18H2,1-3H3/t24-,28-,29+,30-,31+,32+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50463290
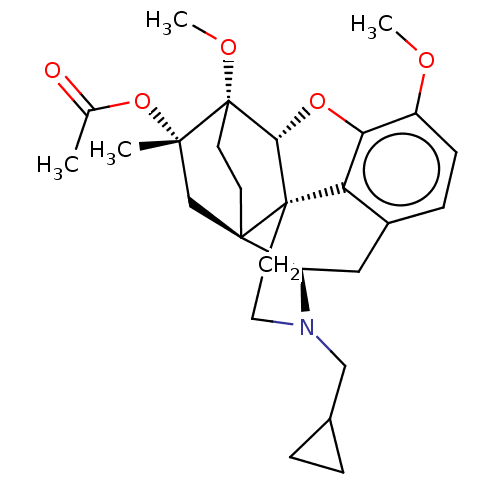
(CHEMBL4241031)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)OC(C)=O)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C27H35NO5/c1-16(29)33-24(2)15-25-9-10-27(24,31-4)23-26(25)11-12-28(14-17-5-6-17)20(25)13-18-7-8-19(30-3)22(32-23)21(18)26/h7-8,17,20,23H,5-6,9-15H2,1-4H3/t20-,23-,24+,25-,26+,27+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577937
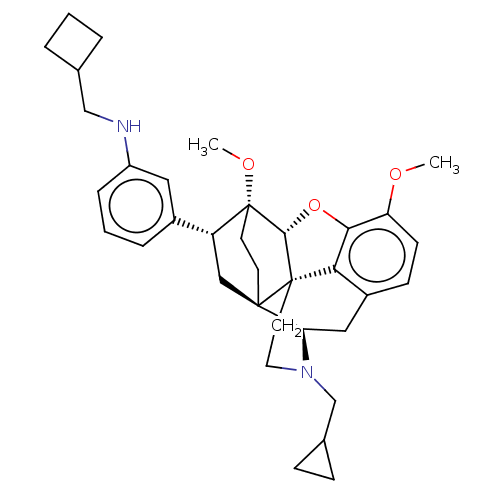
(CHEMBL4860416)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCC2CCC2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506112
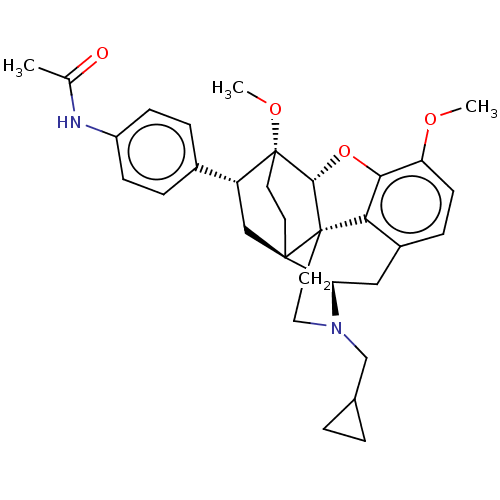
(CHEMBL4542404)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC(C)=O)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C32H38N2O4/c1-19(35)33-23-9-6-21(7-10-23)24-17-30-12-13-32(24,37-3)29-31(30)14-15-34(18-20-4-5-20)26(30)16-22-8-11-25(36-2)28(38-29)27(22)31/h6-11,20,24,26,29H,4-5,12-18H2,1-3H3,(H,33,35)/t24-,26-,29-,30-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human KOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577941
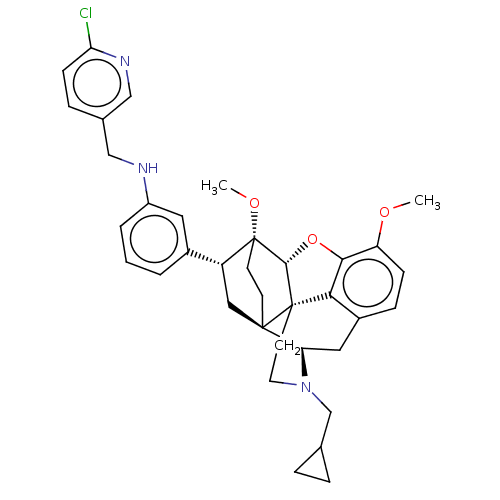
(CHEMBL4873458)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(Cl)nc2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50506112

(CHEMBL4542404)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC(C)=O)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C32H38N2O4/c1-19(35)33-23-9-6-21(7-10-23)24-17-30-12-13-32(24,37-3)29-31(30)14-15-34(18-20-4-5-20)26(30)16-22-8-11-25(36-2)28(38-29)27(22)31/h6-11,20,24,26,29H,4-5,12-18H2,1-3H3,(H,33,35)/t24-,26-,29-,30-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat MOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463290

(CHEMBL4241031)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)OC(C)=O)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C27H35NO5/c1-16(29)33-24(2)15-25-9-10-27(24,31-4)23-26(25)11-12-28(14-17-5-6-17)20(25)13-18-7-8-19(30-3)22(32-23)21(18)26/h7-8,17,20,23H,5-6,9-15H2,1-4H3/t20-,23-,24+,25-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50463286
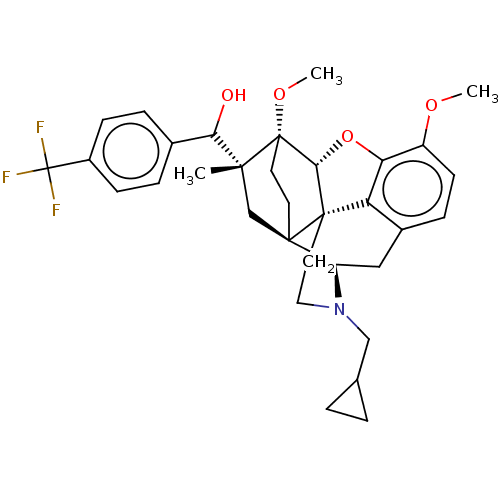
(CHEMBL4239240)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)C(O)c1ccc(cc1)C(F)(F)F)ccc3OC |r,THB:10:9:17:5.6.4| Show InChI InChI=1S/C33H38F3NO4/c1-29(27(38)20-6-9-22(10-7-20)33(34,35)36)18-30-12-13-32(29,40-3)28-31(30)14-15-37(17-19-4-5-19)24(30)16-21-8-11-23(39-2)26(41-28)25(21)31/h6-11,19,24,27-28,38H,4-5,12-18H2,1-3H3/t24-,27?,28-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 26: 4254-4263 (2018)
Article DOI: 10.1016/j.bmc.2018.07.020
BindingDB Entry DOI: 10.7270/Q28W3GZJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50506095
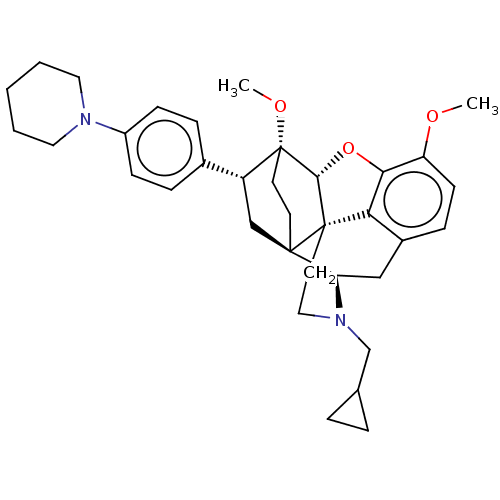
(CHEMBL4446668)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(cc1)N1CCCCC1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C35H44N2O3/c1-38-28-13-10-25-20-29-33-14-15-35(39-2,27(21-33)24-8-11-26(12-9-24)36-17-4-3-5-18-36)32-34(33,30(25)31(28)40-32)16-19-37(29)22-23-6-7-23/h8-13,23,27,29,32H,3-7,14-22H2,1-2H3/t27-,29-,32-,33-,34+,35-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat MOR expressed in CHO cell membranes incubated for 30 mins by liquid scintillation counting |
J Med Chem 62: 11054-11070 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00857
BindingDB Entry DOI: 10.7270/Q2VD72RV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577947
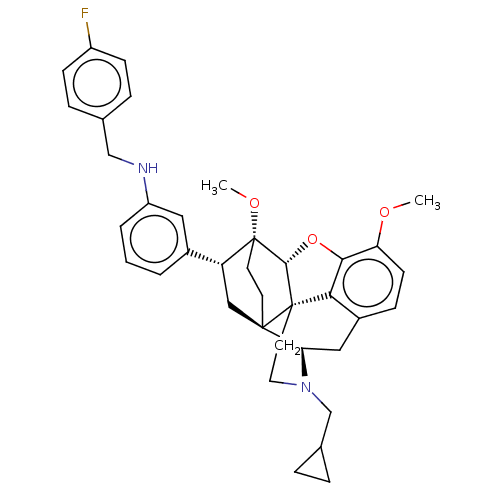
(CHEMBL4875933)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(F)cc2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50598863
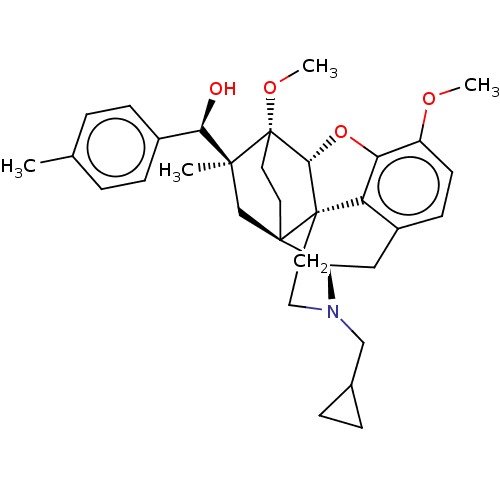
(CHEMBL5188701)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(C)cc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data