 Found 282 hits with Last Name = 'ricci' and Initial = 'l'
Found 282 hits with Last Name = 'ricci' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589760
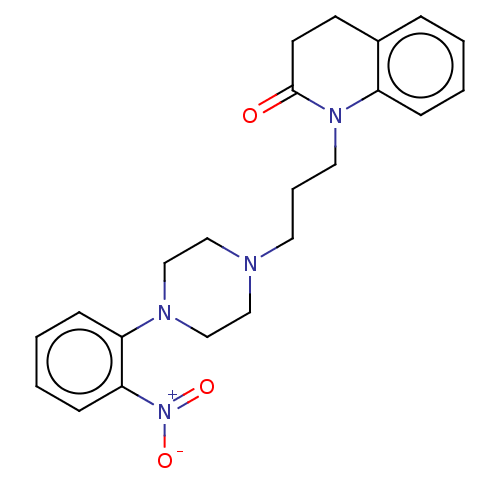
(CHEMBL5198170)Show SMILES OC(=O)C(O)=O.[O-][N+](=O)c1ccccc1N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515558
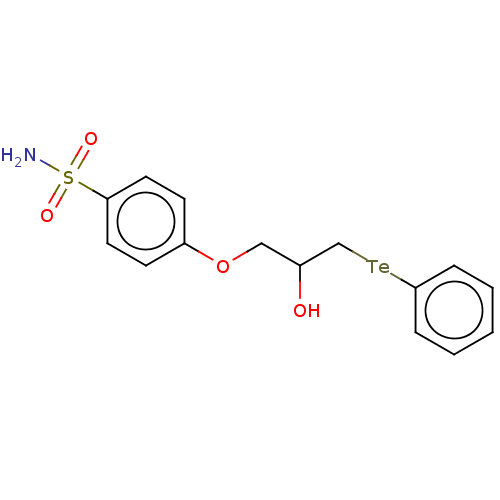
(CHEMBL4535036)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2]c2ccccc2)cc1 Show InChI InChI=1S/C15H17NO4STe/c16-21(18,19)14-8-6-13(7-9-14)20-10-12(17)11-22-15-4-2-1-3-5-15/h1-9,12,17H,10-11H2,(H2,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589757
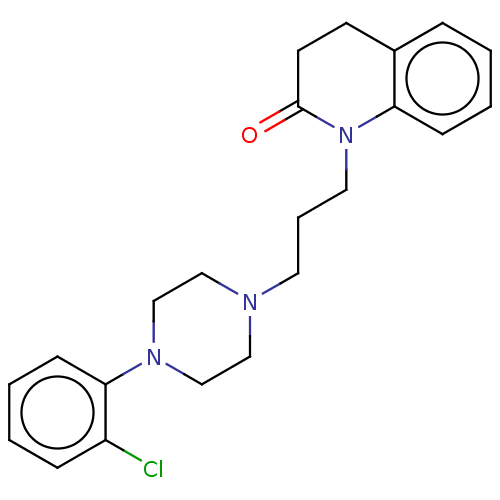
(CHEMBL5172201)Show SMILES OC(=O)C(O)=O.Clc1ccccc1N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515559
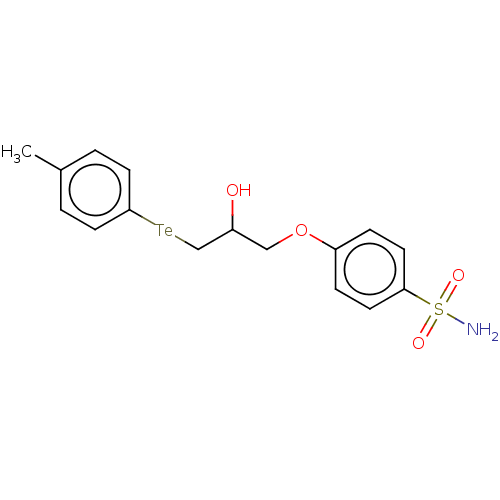
(CHEMBL4435078)Show SMILES [#6]-c1ccc([Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C16H19NO4STe/c1-12-2-8-16(9-3-12)23-11-13(18)10-21-14-4-6-15(7-5-14)22(17,19)20/h2-9,13,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589757

(CHEMBL5172201)Show SMILES OC(=O)C(O)=O.Clc1ccccc1N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589754
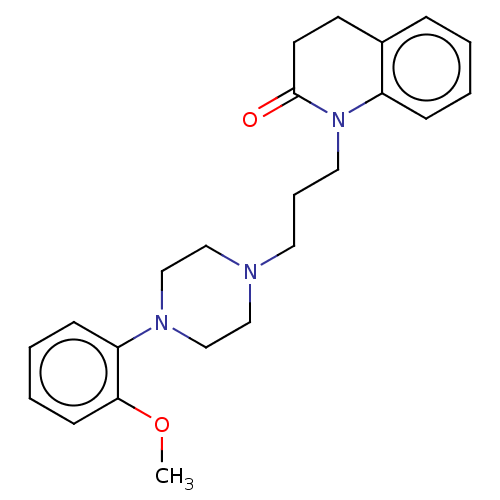
(CHEMBL5189614)Show SMILES OC(=O)C(O)=O.COc1ccccc1N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515564
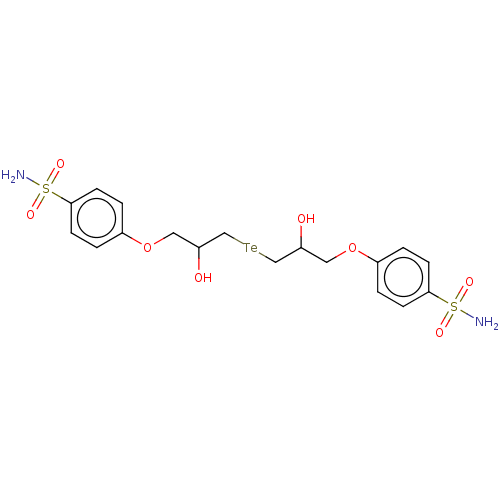
(CHEMBL4535469)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C18H24N2O8S2Te/c19-29(23,24)17-5-1-15(2-6-17)27-9-13(21)11-31-12-14(22)10-28-16-3-7-18(8-4-16)30(20,25)26/h1-8,13-14,21-22H,9-12H2,(H2,19,23,24)(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515563

(CHEMBL4436769)Show SMILES [#6]-c1ccccc1[Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c1ccc(cc1)S([#7])(=O)=O Show InChI InChI=1S/C16H19NO4STe/c1-12-4-2-3-5-16(12)23-11-13(18)10-21-14-6-8-15(9-7-14)22(17,19)20/h2-9,13,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589759
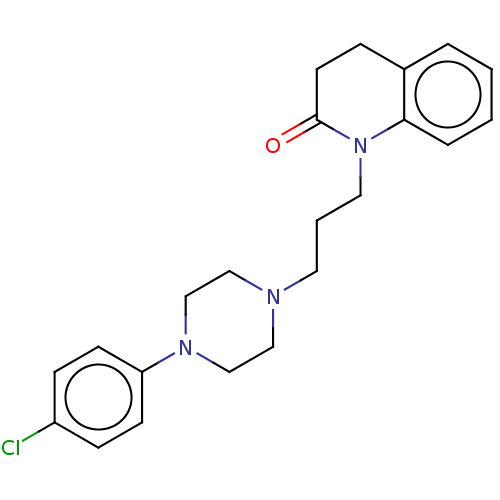
(CHEMBL5196870)Show SMILES OC(=O)C(O)=O.Clc1ccc(cc1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589767
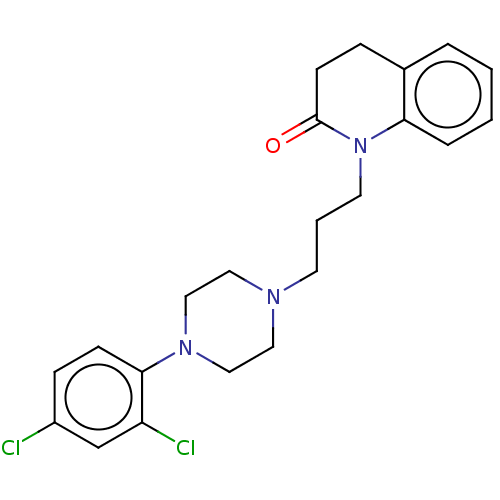
(CHEMBL5179930)Show SMILES OC(=O)C(O)=O.Clc1ccc(N2CCN(CCCN3C(=O)CCc4ccccc34)CC2)c(Cl)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589766
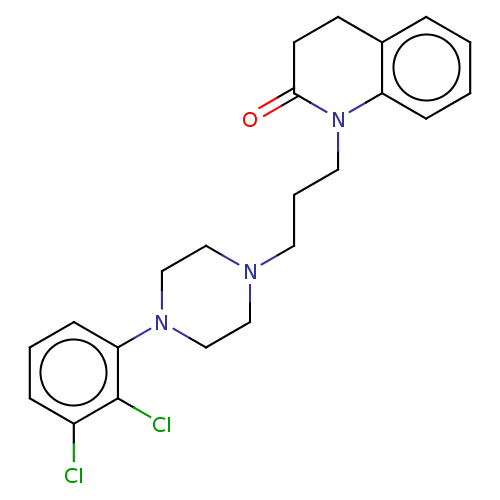
(CHEMBL5177462)Show SMILES OC(=O)C(O)=O.Clc1cccc(N2CCN(CCCN3C(=O)CCc4ccccc34)CC2)c1Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50515563

(CHEMBL4436769)Show SMILES [#6]-c1ccccc1[Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c1ccc(cc1)S([#7])(=O)=O Show InChI InChI=1S/C16H19NO4STe/c1-12-4-2-3-5-16(12)23-11-13(18)10-21-14-6-8-15(9-7-14)22(17,19)20/h2-9,13,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589758
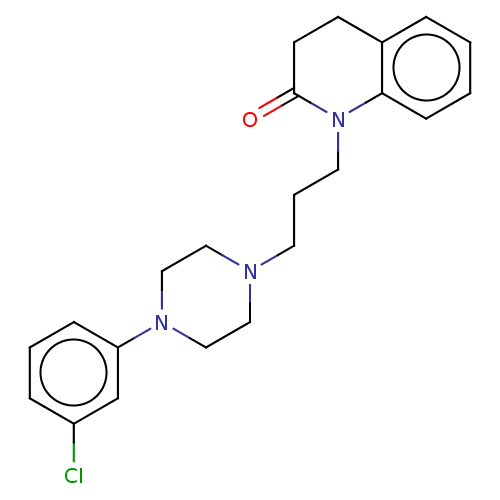
(CHEMBL5204418)Show SMILES OC(=O)C(O)=O.Clc1cccc(c1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50515558

(CHEMBL4535036)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2]c2ccccc2)cc1 Show InChI InChI=1S/C15H17NO4STe/c16-21(18,19)14-8-6-13(7-9-14)20-10-12(17)11-22-15-4-2-1-3-5-15/h1-9,12,17H,10-11H2,(H2,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50515564

(CHEMBL4535469)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C18H24N2O8S2Te/c19-29(23,24)17-5-1-15(2-6-17)27-9-13(21)11-31-12-14(22)10-28-16-3-7-18(8-4-16)30(20,25)26/h1-8,13-14,21-22H,9-12H2,(H2,19,23,24)(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589768
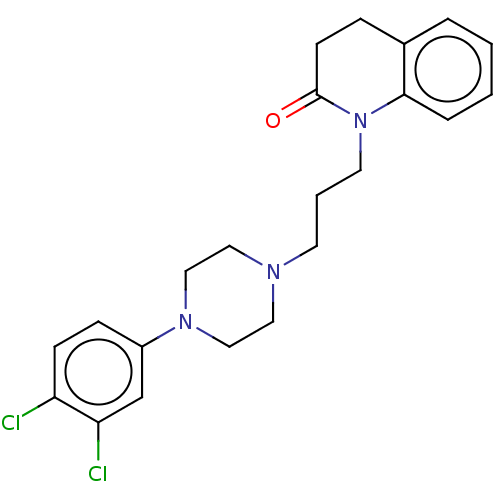
(CHEMBL5175054)Show SMILES OC(=O)C(O)=O.Clc1ccc(cc1Cl)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50030196
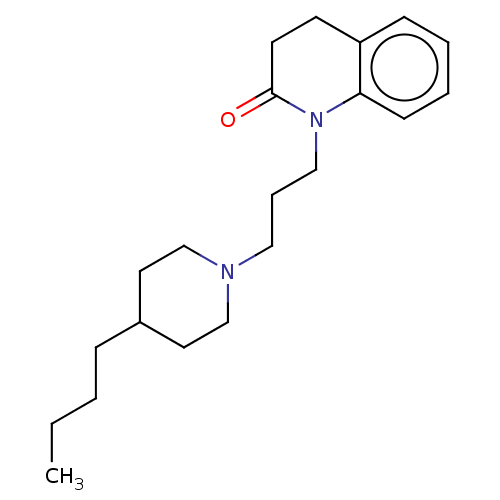
(CHEMBL3354065)Show InChI InChI=1S/C21H32N2O/c1-2-3-7-18-12-16-22(17-13-18)14-6-15-23-20-9-5-4-8-19(20)10-11-21(23)24/h4-5,8-9,18H,2-3,6-7,10-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589753
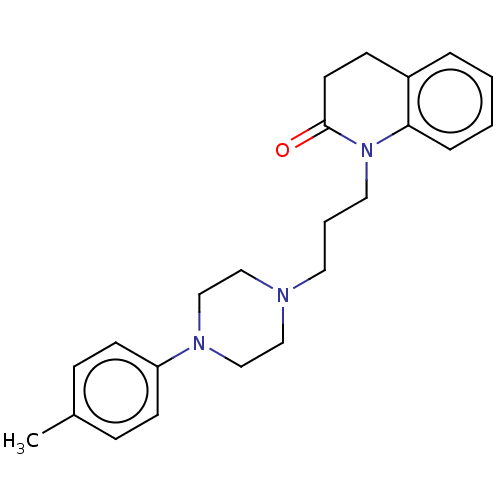
(CHEMBL5171439)Show SMILES OC(=O)C(O)=O.Cc1ccc(cc1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589758

(CHEMBL5204418)Show SMILES OC(=O)C(O)=O.Clc1cccc(c1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589763

(CHEMBL5191299)Show SMILES OC(=O)C(O)=O.O=C1CCc2ccccc2N1CCCN1CCN(CC1)c1ccccc1C#N | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589751
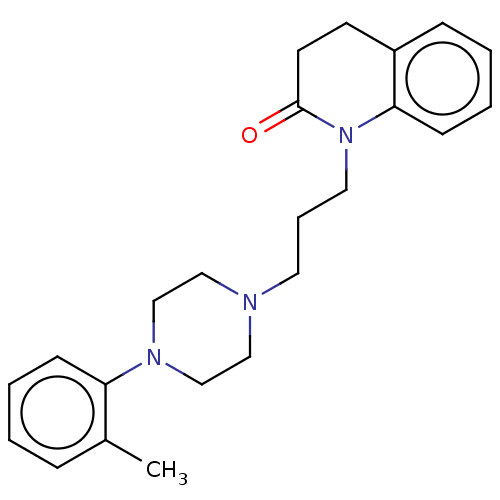
(CHEMBL5209074)Show SMILES OC(=O)C(O)=O.Cc1ccccc1N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589759

(CHEMBL5196870)Show SMILES OC(=O)C(O)=O.Clc1ccc(cc1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515559

(CHEMBL4435078)Show SMILES [#6]-c1ccc([Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C16H19NO4STe/c1-12-2-8-16(9-3-12)23-11-13(18)10-21-14-4-6-15(7-5-14)22(17,19)20/h2-9,13,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589752
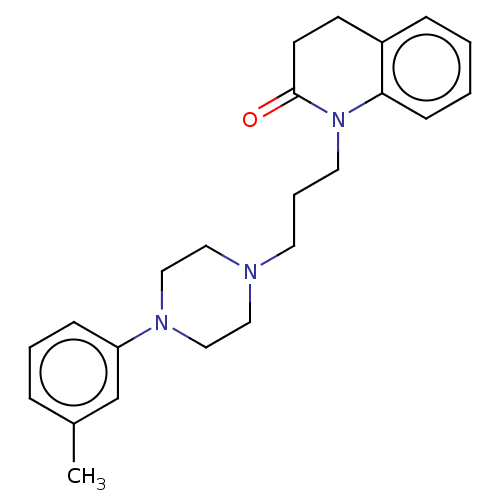
(CHEMBL5170580)Show SMILES OC(=O)C(O)=O.Cc1cccc(c1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM82354
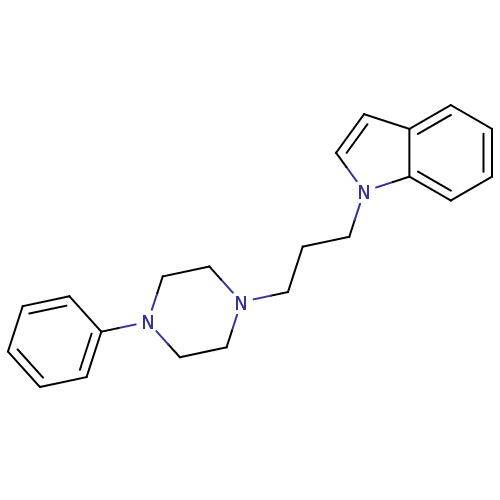
(1-{3-[4-(substitutedphenyl)piperazin1-yl]propyl}-1...)Show InChI InChI=1S/C21H25N3/c1-2-8-20(9-3-1)23-17-15-22(16-18-23)12-6-13-24-14-11-19-7-4-5-10-21(19)24/h1-5,7-11,14H,6,12-13,15-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM85093
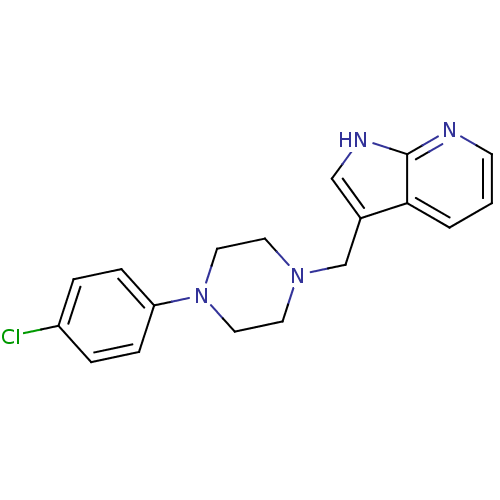
(CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...)Show InChI InChI=1S/C18H19ClN4/c19-15-3-5-16(6-4-15)23-10-8-22(9-11-23)13-14-12-21-18-17(14)2-1-7-20-18/h1-7,12H,8-11,13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589764
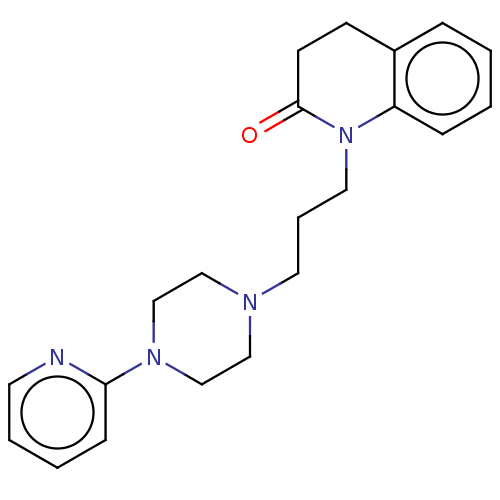
(CHEMBL5196873)Show SMILES OC(=O)C(O)=O.O=C1CCc2ccccc2N1CCCN1CCN(CC1)c1ccccn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589747

(CHEMBL5169993)Show SMILES OC(=O)C(O)=O.C(CN1CCN(CC1)c1ccccc1)CN1CCCc2ccccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50515559

(CHEMBL4435078)Show SMILES [#6]-c1ccc([Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C16H19NO4STe/c1-12-2-8-16(9-3-12)23-11-13(18)10-21-14-4-6-15(7-5-14)22(17,19)20/h2-9,13,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50263488
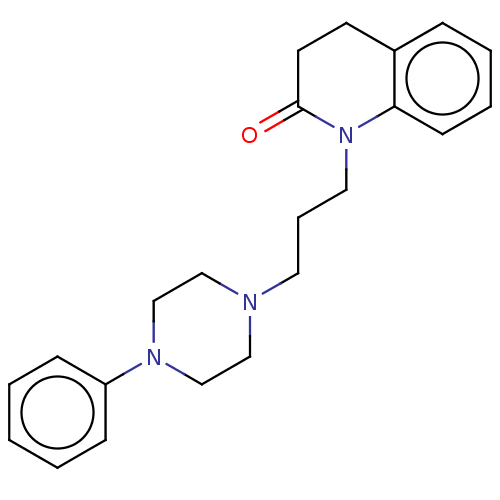
(CHEMBL4085780)Show InChI InChI=1S/C22H27N3O/c26-22-12-11-19-7-4-5-10-21(19)25(22)14-6-13-23-15-17-24(18-16-23)20-8-2-1-3-9-20/h1-5,7-10H,6,11-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589761
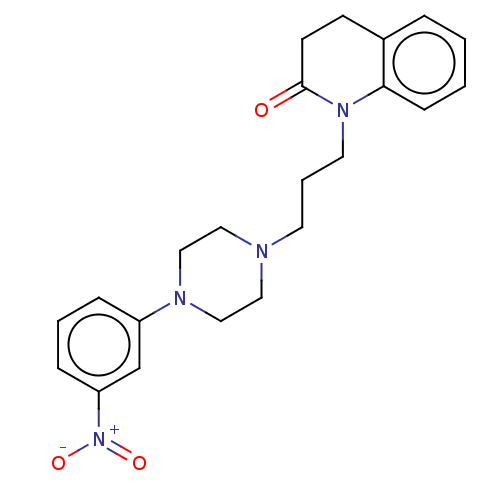
(CHEMBL5176100)Show SMILES OC(=O)C(O)=O.[O-][N+](=O)c1cccc(c1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515561

(CHEMBL4442220)Show SMILES [#6]-[#6]-[#6]-[#6][Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c1ccc(cc1)S([#7])(=O)=O Show InChI InChI=1S/C13H21NO4STe/c1-2-3-8-20-10-11(15)9-18-12-4-6-13(7-5-12)19(14,16)17/h4-7,11,15H,2-3,8-10H2,1H3,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589755
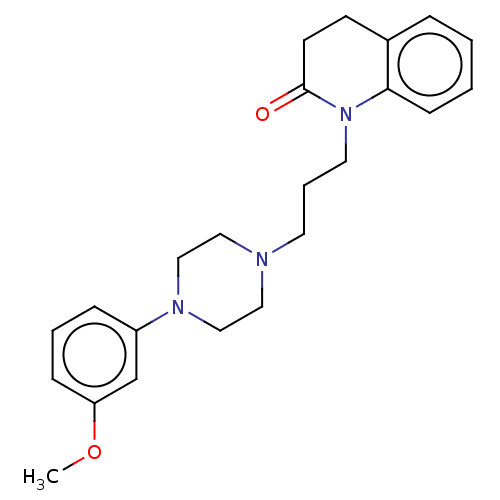
(CHEMBL5191408)Show SMILES OC(=O)C(O)=O.COc1cccc(c1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589749
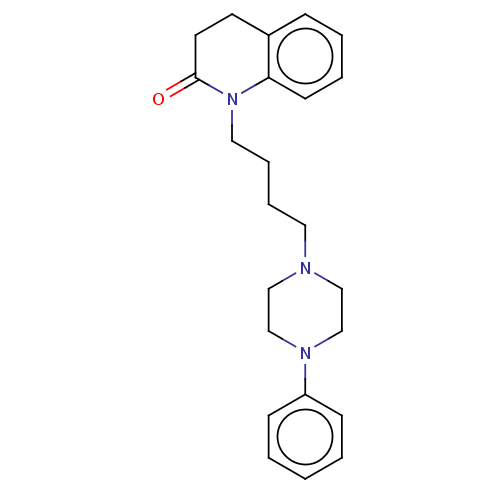
(CHEMBL5199358)Show SMILES OC(=O)C(O)=O.O=C1CCc2ccccc2N1CCCCN1CCN(CC1)c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515557

(CHEMBL4470177)Show SMILES [#6]-[#8]-c1ccc([Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C16H19NO5STe/c1-21-13-4-8-16(9-5-13)24-11-12(18)10-22-14-2-6-15(7-3-14)23(17,19)20/h2-9,12,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589765

(CHEMBL5172871)Show SMILES OC(=O)C(O)=O.O=C1CCc2ccccc2N1CCCN1CCN(CC1)c1ncccn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50515562

(CHEMBL4522383)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2]c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C19H19NO4STe/c20-25(22,23)18-8-6-17(7-9-18)24-12-16(21)13-26-19-10-5-14-3-1-2-4-15(14)11-19/h1-11,16,21H,12-13H2,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589762
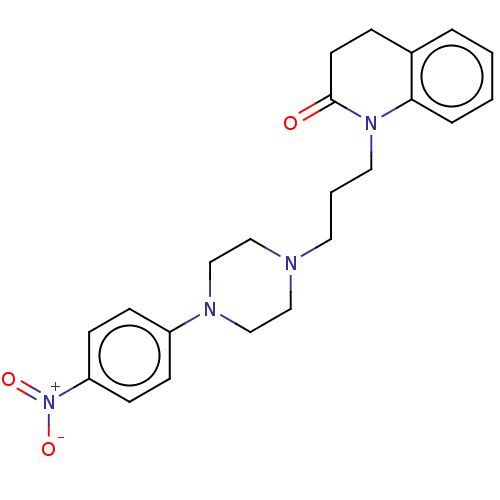
(CHEMBL5175009)Show SMILES OC(=O)C(O)=O.[O-][N+](=O)c1ccc(cc1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515558

(CHEMBL4535036)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2]c2ccccc2)cc1 Show InChI InChI=1S/C15H17NO4STe/c16-21(18,19)14-8-6-13(7-9-14)20-10-12(17)11-22-15-4-2-1-3-5-15/h1-9,12,17H,10-11H2,(H2,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589756

(CHEMBL5178868)Show SMILES OC(=O)C(O)=O.COc1ccc(cc1)N1CCN(CCCN2C(=O)CCc3ccccc23)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50515557

(CHEMBL4470177)Show SMILES [#6]-[#8]-c1ccc([Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C16H19NO5STe/c1-21-13-4-8-16(9-5-13)24-11-12(18)10-22-14-2-6-15(7-3-14)23(17,19)20/h2-9,12,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50515562

(CHEMBL4522383)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2]c2ccc3ccccc3c2)cc1 Show InChI InChI=1S/C19H19NO4STe/c20-25(22,23)18-8-6-17(7-9-18)24-12-16(21)13-26-19-10-5-14-3-1-2-4-15(14)11-19/h1-11,16,21H,12-13H2,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50515563

(CHEMBL4436769)Show SMILES [#6]-c1ccccc1[Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c1ccc(cc1)S([#7])(=O)=O Show InChI InChI=1S/C16H19NO4STe/c1-12-4-2-3-5-16(12)23-11-13(18)10-21-14-6-8-15(9-7-14)22(17,19)20/h2-9,13,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589746

(CHEMBL5200816)Show SMILES OC(=O)C(O)=O.C(CN1CCN(CC1)c1ccccc1)Cc1nc2ccccc2s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50515557

(CHEMBL4470177)Show SMILES [#6]-[#8]-c1ccc([Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C16H19NO5STe/c1-21-13-4-8-16(9-5-13)24-11-12(18)10-22-14-2-6-15(7-3-14)23(17,19)20/h2-9,12,18H,10-11H2,1H3,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50589743
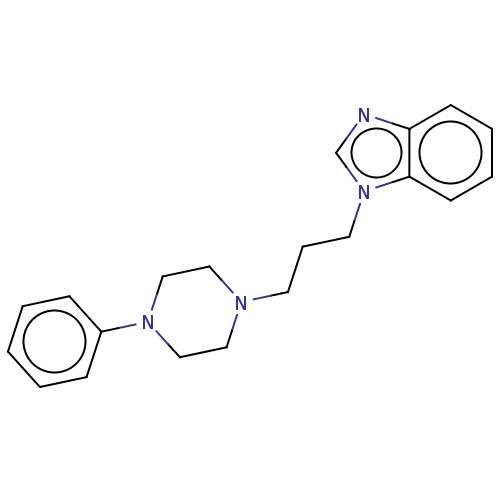
(CHEMBL5207313)Show SMILES OC(=O)C(O)=O.C(CN1CCN(CC1)c1ccccc1)Cn1cnc2ccccc12 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00840
BindingDB Entry DOI: 10.7270/Q2ST7TTC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50515558

(CHEMBL4535036)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2]c2ccccc2)cc1 Show InChI InChI=1S/C15H17NO4STe/c16-21(18,19)14-8-6-13(7-9-14)20-10-12(17)11-22-15-4-2-1-3-5-15/h1-9,12,17H,10-11H2,(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50515564

(CHEMBL4535469)Show SMILES [#7]S(=O)(=O)c1ccc(-[#8]-[#6]-[#6](-[#8])-[#6][Te;v2][#6]-[#6](-[#8])-[#6]-[#8]-c2ccc(cc2)S([#7])(=O)=O)cc1 Show InChI InChI=1S/C18H24N2O8S2Te/c19-29(23,24)17-5-1-15(2-6-17)27-9-13(21)11-31-12-14(22)10-28-16-3-7-18(8-4-16)30(20,25)26/h1-8,13-14,21-22H,9-12H2,(H2,19,23,24)(H2,20,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 4 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111586
BindingDB Entry DOI: 10.7270/Q2Z03CJ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data