 Found 901 hits with Last Name = 'thomas' and Initial = 'l'
Found 901 hits with Last Name = 'thomas' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50520121
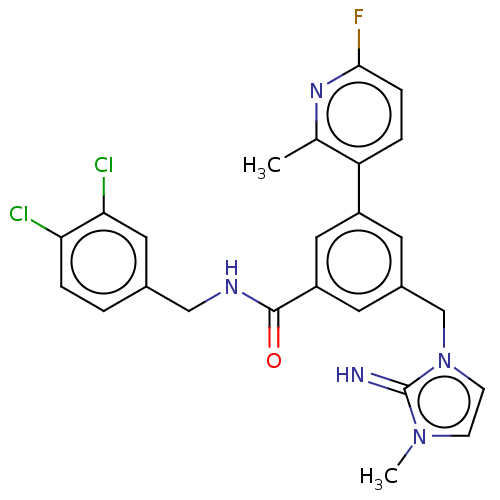
(CHEMBL4441671 | US10844044, Example 1)Show SMILES Cc1nc(F)ccc1-c1cc(Cn2ccn(C)c2=N)cc(c1)C(=O)NCc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H22Cl2FN5O/c1-15-20(4-6-23(28)31-15)18-9-17(14-33-8-7-32(2)25(33)29)10-19(12-18)24(34)30-13-16-3-5-21(26)22(27)11-16/h3-12,29H,13-14H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Competitive inhibition of human N-terminal His6-SUMO tagged WDR5 (22 to 334 residues) expressed in Escherichia coli BL21-Gold(DE3) cells using 10-mer... |
J Med Chem 63: 656-675 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01608
BindingDB Entry DOI: 10.7270/Q2HD801W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227815
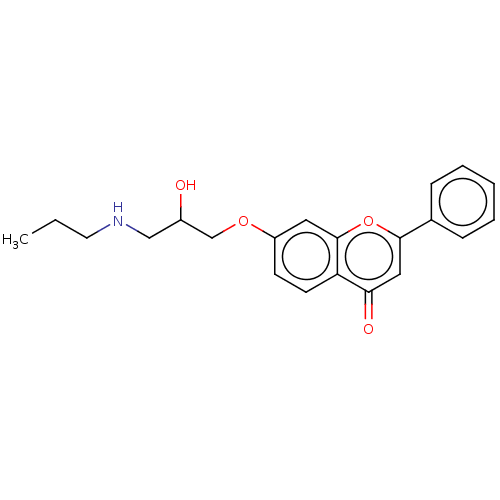
(Flavodilol)Show InChI InChI=1S/C21H23NO4/c1-2-10-22-13-16(23)14-25-17-8-9-18-19(24)12-20(26-21(18)11-17)15-6-4-3-5-7-15/h3-9,11-12,16,22-23H,2,10,13-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM142122
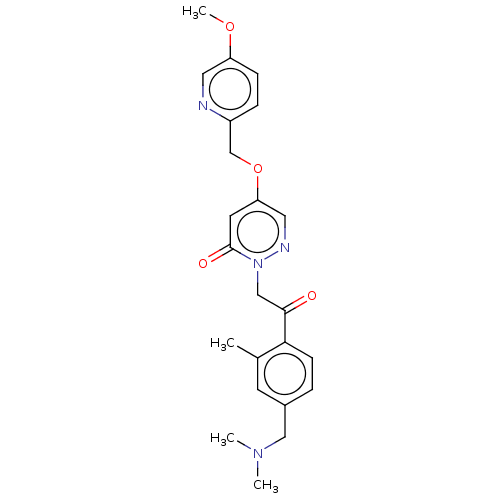
(US8933079, 149 | US8933079, 150 | US8933079, 9.1)Show SMILES COc1ccc(COc2cnn(CC(=O)c3ccc(CN(C)C)cc3C)c(=O)c2)nc1 Show InChI InChI=1S/C23H26N4O4/c1-16-9-17(13-26(2)3)5-8-21(16)22(28)14-27-23(29)10-20(12-25-27)31-15-18-6-7-19(30-4)11-24-18/h5-12H,13-15H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human MCH-R1 expressed in CHO/Galpha16 cells |
Bioorg Med Chem Lett 25: 3275-80 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.065
BindingDB Entry DOI: 10.7270/Q2T43VV4 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227820
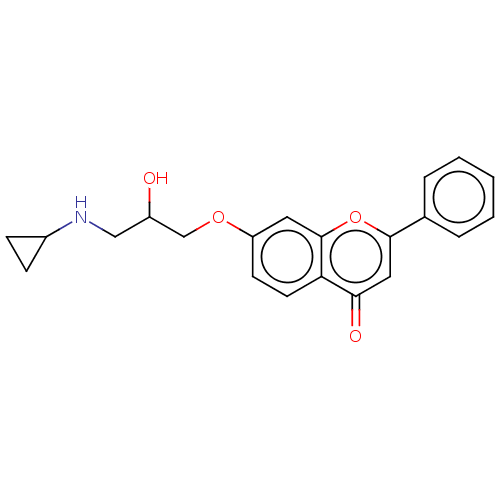
(CHEMBL57544)Show InChI InChI=1S/C21H21NO4/c23-16(12-22-15-6-7-15)13-25-17-8-9-18-19(24)11-20(26-21(18)10-17)14-4-2-1-3-5-14/h1-5,8-11,15-16,22-23H,6-7,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227814
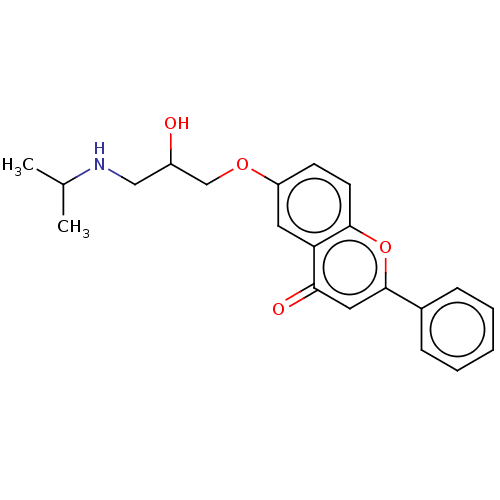
(CHEMBL291999)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-17-8-9-20-18(10-17)19(24)11-21(26-20)15-6-4-3-5-7-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227817
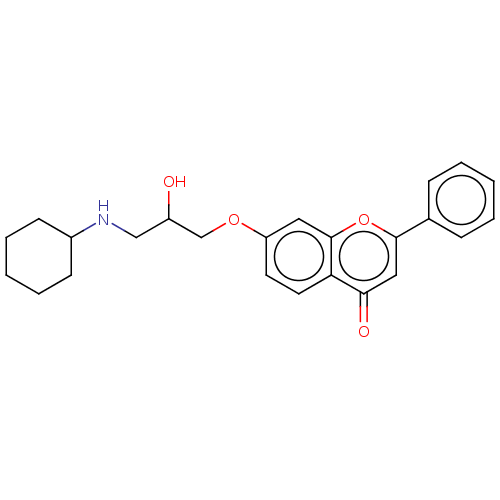
(CHEMBL57611)Show SMILES OC(CNC1CCCCC1)COc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C24H27NO4/c26-19(15-25-18-9-5-2-6-10-18)16-28-20-11-12-21-22(27)14-23(29-24(21)13-20)17-7-3-1-4-8-17/h1,3-4,7-8,11-14,18-19,25-26H,2,5-6,9-10,15-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant obtained from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227819
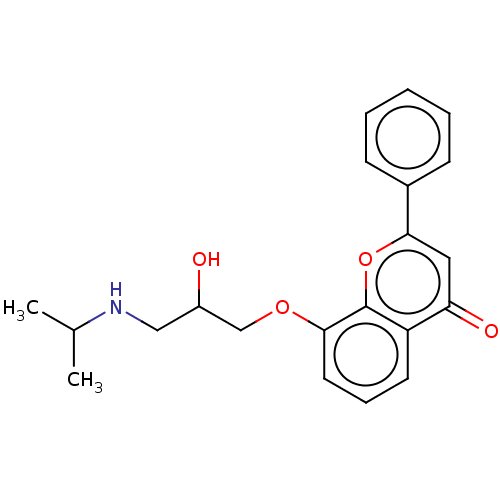
(CHEMBL273690)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-19-10-6-9-17-18(24)11-20(26-21(17)19)15-7-4-3-5-8-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227816
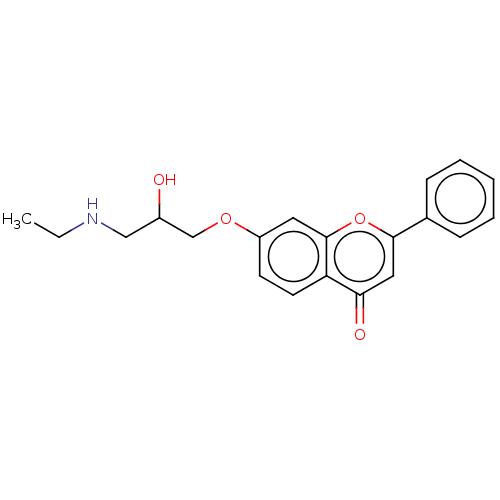
(CHEMBL57659)Show InChI InChI=1S/C20H21NO4/c1-2-21-12-15(22)13-24-16-8-9-17-18(23)11-19(25-20(17)10-16)14-6-4-3-5-7-14/h3-11,15,21-22H,2,12-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227818
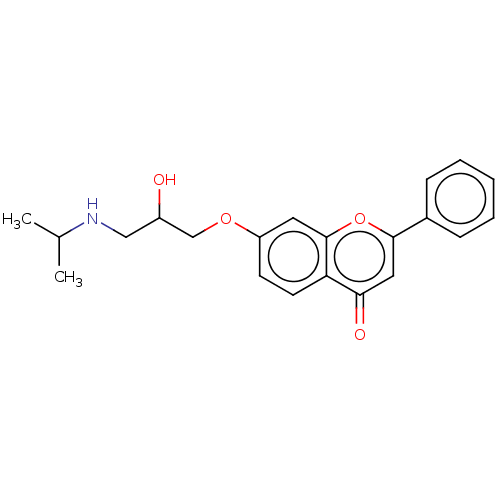
(CHEMBL56023)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-17-8-9-18-19(24)11-20(26-21(18)10-17)15-6-4-3-5-7-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227813

(CHEMBL293974)Show SMILES CC(C)(C)NCC(O)COc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C22H25NO4/c1-22(2,3)23-13-16(24)14-26-17-9-10-18-19(25)12-20(27-21(18)11-17)15-7-5-4-6-8-15/h4-12,16,23-24H,13-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227821
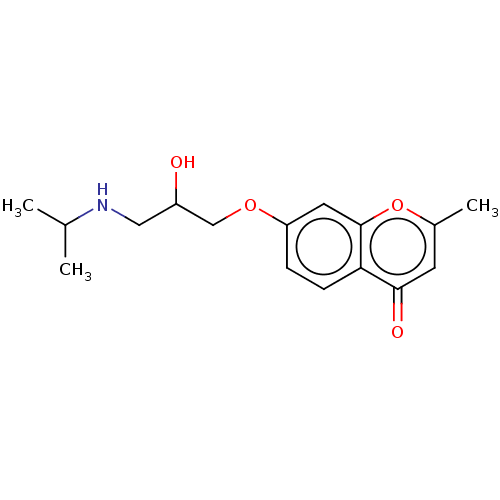
(CHEMBL56492)Show InChI InChI=1S/C16H21NO4/c1-10(2)17-8-12(18)9-20-13-4-5-14-15(19)6-11(3)21-16(14)7-13/h4-7,10,12,17-18H,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024146
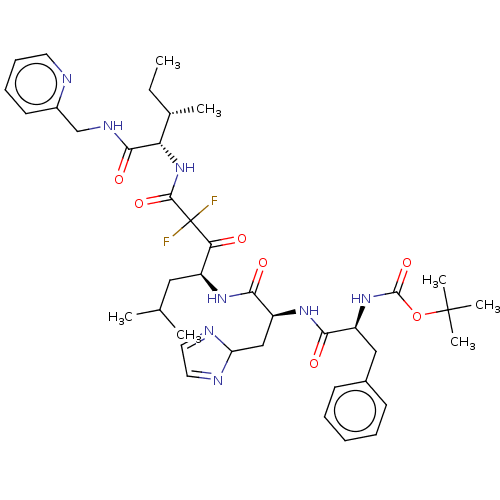
(CHEMBL3142169 | {1-[1-[1-(2,2-Difluoro-2-{2-methyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)C(F)(F)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1N=CC=N1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |c:24,26| Show InChI InChI=1S/C40H54F2N8O7/c1-8-25(4)32(36(54)46-23-27-16-12-13-17-43-27)50-37(55)40(41,42)33(51)28(20-24(2)3)47-35(53)30(22-31-44-18-19-45-31)48-34(52)29(21-26-14-10-9-11-15-26)49-38(56)57-39(5,6)7/h9-19,24-25,28-32H,8,20-23H2,1-7H3,(H,46,54)(H,47,53)(H,48,52)(H,49,56)(H,50,55) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2080-7 (1986)
BindingDB Entry DOI: 10.7270/Q25X29G7 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025927
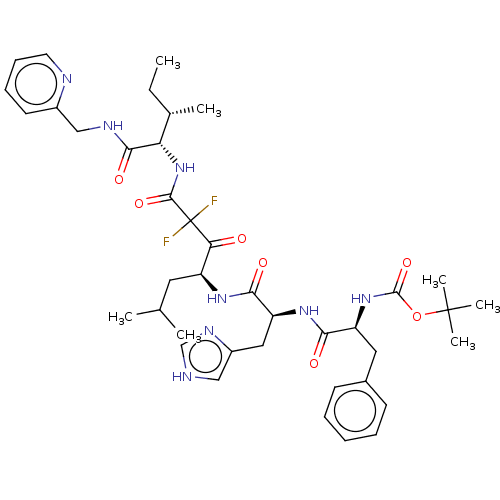
(CHEMBL3143966 | {1-[1-[1-(2,2-Difluoro-2-{2-methyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)C(F)(F)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C40H54F2N8O7/c1-8-25(4)32(36(54)45-22-27-16-12-13-17-44-27)50-37(55)40(41,42)33(51)29(18-24(2)3)47-35(53)31(20-28-21-43-23-46-28)48-34(52)30(19-26-14-10-9-11-15-26)49-38(56)57-39(5,6)7/h9-17,21,23-25,29-32H,8,18-20,22H2,1-7H3,(H,43,46)(H,45,54)(H,47,53)(H,48,52)(H,49,56)(H,50,55)/t25?,29-,30?,31?,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA |
J Med Chem 28: 1553-5 (1985)
BindingDB Entry DOI: 10.7270/Q23B60QP |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024149
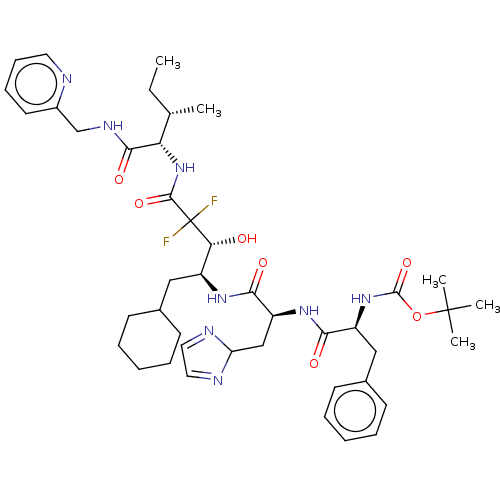
(CHEMBL3142178 | {1-[1-(1-Cyclohexylmethyl-3,3-difl...)Show SMILES CC[C@H](C)[C@H](NC(=O)C(F)(F)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC1N=CC=N1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |c:28,30| Show InChI InChI=1S/C43H60F2N8O7/c1-6-27(2)35(39(57)49-26-30-19-13-14-20-46-30)53-40(58)43(44,45)36(54)31(23-28-15-9-7-10-16-28)50-38(56)33(25-34-47-21-22-48-34)51-37(55)32(24-29-17-11-8-12-18-29)52-41(59)60-42(3,4)5/h8,11-14,17-22,27-28,31-36,54H,6-7,9-10,15-16,23-26H2,1-5H3,(H,49,57)(H,50,56)(H,51,55)(H,52,59)(H,53,58)/t27?,31-,32?,33?,35?,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2080-7 (1986)
BindingDB Entry DOI: 10.7270/Q25X29G7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50376986
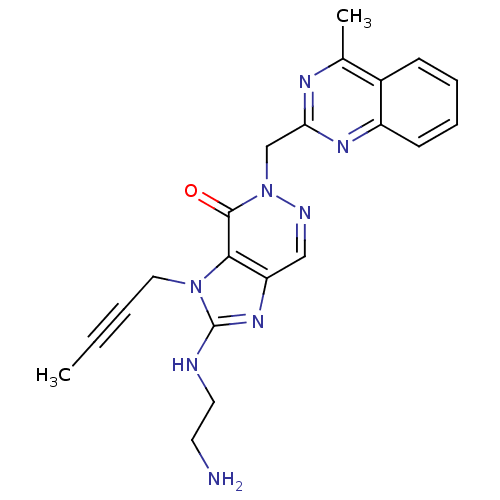
(CHEMBL257376)Show SMILES CC#CCn1c(NCCN)nc2cnn(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C21H22N8O/c1-3-4-11-28-19-17(27-21(28)23-10-9-22)12-24-29(20(19)30)13-18-25-14(2)15-7-5-6-8-16(15)26-18/h5-8,12H,9-11,13,22H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228394
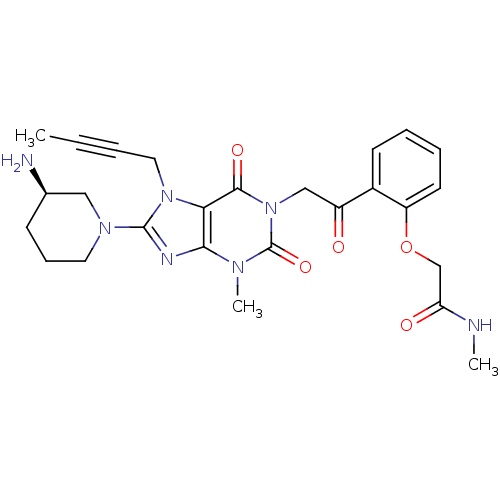
((R)-2-(2-(2-(8-(3-aminopiperidin-1-yl)-7-(but-2-yn...)Show SMILES CNC(=O)COc1ccccc1C(=O)Cn1c(=O)n(C)c2nc(N3CCC[C@@H](N)C3)n(CC#CC)c2c1=O Show InChI InChI=1S/C26H31N7O5/c1-4-5-13-32-22-23(29-25(32)31-12-8-9-17(27)14-31)30(3)26(37)33(24(22)36)15-19(34)18-10-6-7-11-20(18)38-16-21(35)28-2/h6-7,10-11,17H,8-9,12-16,27H2,1-3H3,(H,28,35)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 in Caco2 cells by fluorescene assay |
J Med Chem 50: 6450-3 (2007)
Article DOI: 10.1021/jm701280z
BindingDB Entry DOI: 10.7270/Q2639PGG |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 in Caco2 cells by fluorescene assay |
J Med Chem 50: 6450-3 (2007)
Article DOI: 10.1021/jm701280z
BindingDB Entry DOI: 10.7270/Q2639PGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50376984
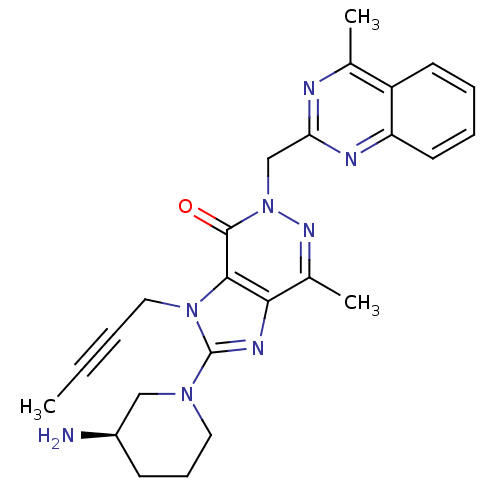
(CHEMBL256121)Show SMILES CC#CCn1c(nc2c(C)nn(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O/c1-4-5-13-32-23-22(29-25(32)31-12-8-9-18(26)14-31)17(3)30-33(24(23)34)15-21-27-16(2)19-10-6-7-11-20(19)28-21/h6-7,10-11,18H,8-9,12-15,26H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50376985
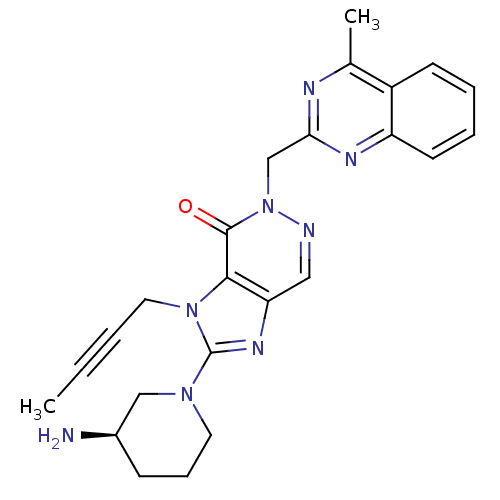
(CHEMBL403553)Show SMILES CC#CCn1c(nc2cnn(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C24H26N8O/c1-3-4-12-31-22-20(29-24(31)30-11-7-8-17(25)14-30)13-26-32(23(22)33)15-21-27-16(2)18-9-5-6-10-19(18)28-21/h5-6,9-10,13,17H,7-8,11-12,14-15,25H2,1-2H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024144
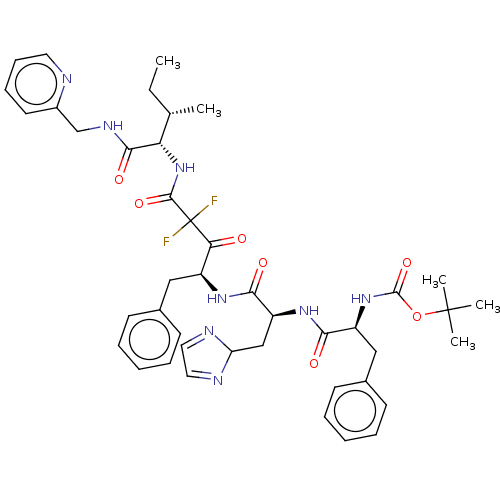
(CHEMBL3142193 | {1-[1-(1-Benzyl-3,3-difluoro-3-{2-...)Show SMILES CC[C@H](C)[C@H](NC(=O)C(F)(F)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC1N=CC=N1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r,c:28,30| Show InChI InChI=1S/C43H52F2N8O7/c1-6-27(2)35(39(57)49-26-30-19-13-14-20-46-30)53-40(58)43(44,45)36(54)31(23-28-15-9-7-10-16-28)50-38(56)33(25-34-47-21-22-48-34)51-37(55)32(24-29-17-11-8-12-18-29)52-41(59)60-42(3,4)5/h7-22,27,31-35H,6,23-26H2,1-5H3,(H,49,57)(H,50,56)(H,51,55)(H,52,59)(H,53,58) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2080-7 (1986)
BindingDB Entry DOI: 10.7270/Q25X29G7 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024145

(CHEMBL3350345 | {1-[1-(1-Cyclohexylmethyl-3,3-difl...)Show SMILES CC[C@H](C)[C@H](NC(=O)C(F)(F)C(=O)C(CC(C)C)NC(=O)[C@H](CC1N=CC=N1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |c:24,26| Show InChI InChI=1S/C43H58F2N8O7/c1-6-27(2)35(39(57)49-26-30-19-13-14-20-46-30)53-40(58)43(44,45)36(54)31(23-28-15-9-7-10-16-28)50-38(56)33(25-34-47-21-22-48-34)51-37(55)32(24-29-17-11-8-12-18-29)52-41(59)60-42(3,4)5/h8,11-14,17-22,27-28,31-35H,6-7,9-10,15-16,23-26H2,1-5H3,(H,49,57)(H,50,56)(H,51,55)(H,52,59)(H,53,58) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2080-7 (1986)
BindingDB Entry DOI: 10.7270/Q25X29G7 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024147
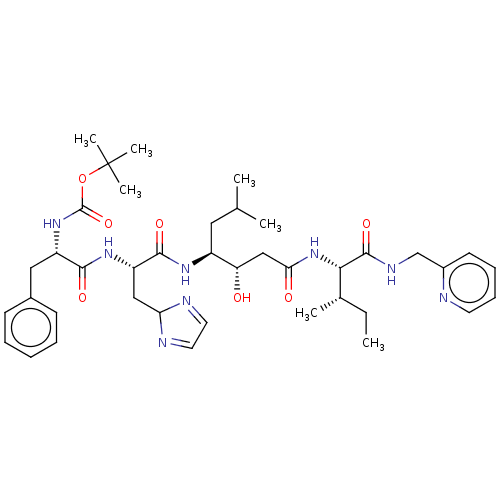
(CHEMBL3142170 | {1-[1-[1-(1-Hydroxy-2-{2-methyl-1-...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC1N=CC=N1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |c:22,24| Show InChI InChI=1S/C40H58N8O7/c1-8-26(4)35(38(53)44-24-28-16-12-13-17-41-28)48-34(50)23-32(49)29(20-25(2)3)45-37(52)31(22-33-42-18-19-43-33)46-36(51)30(21-27-14-10-9-11-15-27)47-39(54)55-40(5,6)7/h9-19,25-26,29-33,35,49H,8,20-24H2,1-7H3,(H,44,53)(H,45,52)(H,46,51)(H,47,54)(H,48,50)/t26?,29-,30?,31?,32-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2080-7 (1986)
BindingDB Entry DOI: 10.7270/Q25X29G7 |
More data for this
Ligand-Target Pair | |
Phospholipase D1
(Homo sapiens (Human)) | BDBM36423
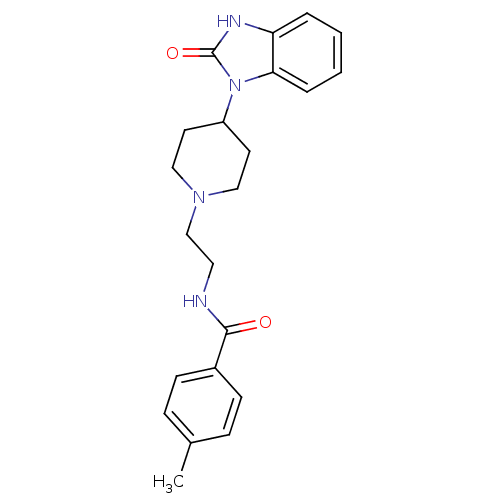
(4-Methyl-N-(2-(4-(2-oxo-2,3-dihydro-1H-benzo[d]imi...)Show SMILES Cc1ccc(cc1)C(=O)NCCN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C22H26N4O2/c1-16-6-8-17(9-7-16)21(27)23-12-15-25-13-10-18(11-14-25)26-20-5-3-2-4-19(20)24-22(26)28/h2-9,18H,10-15H2,1H3,(H,23,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University
| Assay Description
Phopholipase D1(PLD)enzymatic inhibition assay using PLD1.d311 |
Nat Chem Biol 5: 108-17 (2009)
Article DOI: 10.1038/nchembio.140
BindingDB Entry DOI: 10.7270/Q2D50KBK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025928

(CHEMBL57121 | {1-[1-[1-(1-Hydroxy-2-{2-methyl-1-[(...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C40H58N8O7/c1-8-26(4)35(38(53)43-23-28-16-12-13-17-42-28)48-34(50)21-33(49)30(18-25(2)3)45-37(52)32(20-29-22-41-24-44-29)46-36(51)31(19-27-14-10-9-11-15-27)47-39(54)55-40(5,6)7/h9-17,22,24-26,30-33,35,49H,8,18-21,23H2,1-7H3,(H,41,44)(H,43,53)(H,45,52)(H,46,51)(H,47,54)(H,48,50)/t26?,30-,31?,32?,33-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA |
J Med Chem 28: 1553-5 (1985)
BindingDB Entry DOI: 10.7270/Q23B60QP |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM120068

(US8697868, 1(6) | US8697868, 6)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3cc(C#N)c4ccccc4n3)c(=O)c12)N1CCC[C@H](C1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H34N8O4/c1-6-7-15-38-25-26(35-28(38)37-14-10-11-21(18-37)34-29(41)43-31(2,3)4)36(5)30(42)39(27(25)40)19-22-16-20(17-32)23-12-8-9-13-24(23)33-22/h8-9,12-13,16,21H,10-11,14-15,18-19H2,1-5H3,(H,34,41)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl of substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, was placed in black microtitre plate... |
US Patent US8697868 (2014)
BindingDB Entry DOI: 10.7270/Q2XD10CF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM120070
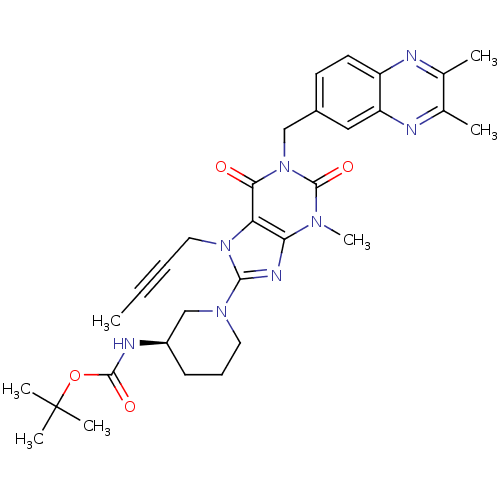
(US8697868, 1(12) | US8697868, 12)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3ccc4nc(C)c(C)nc4c3)c(=O)c12)N1CCC[C@H](C1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H38N8O4/c1-8-9-15-38-25-26(35-28(38)37-14-10-11-22(18-37)34-29(41)43-31(4,5)6)36(7)30(42)39(27(25)40)17-21-12-13-23-24(16-21)33-20(3)19(2)32-23/h12-13,16,22H,10-11,14-15,17-18H2,1-7H3,(H,34,41)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl of substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, was placed in black microtitre plate... |
US Patent US8697868 (2014)
BindingDB Entry DOI: 10.7270/Q2XD10CF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM120071

(US8697868, 1(21) | US8697868, 21)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CC(=O)c3ccccc3Br)c(=O)c12)N1CCC[C@H](C1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C28H33BrN6O5/c1-6-7-15-34-22-23(31-25(34)33-14-10-11-18(16-33)30-26(38)40-28(2,3)4)32(5)27(39)35(24(22)37)17-21(36)19-12-8-9-13-20(19)29/h8-9,12-13,18H,10-11,14-17H2,1-5H3,(H,30,38)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl of substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, was placed in black microtitre plate... |
US Patent US8697868 (2014)
BindingDB Entry DOI: 10.7270/Q2XD10CF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM120072

(US8697868, 1(26) | US8697868, 26)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc4ccccc4c(C)c3C#N)c(=O)c12)N1CCC[C@H](C1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C32H36N8O4/c1-7-8-16-39-26-27(36-29(39)38-15-11-12-21(18-38)34-30(42)44-32(3,4)5)37(6)31(43)40(28(26)41)19-25-23(17-33)20(2)22-13-9-10-14-24(22)35-25/h9-10,13-14,21H,11-12,15-16,18-19H2,1-6H3,(H,34,42)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl of substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, was placed in black microtitre plate... |
US Patent US8697868 (2014)
BindingDB Entry DOI: 10.7270/Q2XD10CF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228406

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)cc4ccccc34)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C26H29N7O2/c1-4-5-13-32-22-23(29-25(32)31-12-8-10-19(27)15-31)30(3)26(35)33(24(22)34)16-21-20-11-7-6-9-18(20)14-17(2)28-21/h6-7,9,11,14,19H,8,10,12-13,15-16,27H2,1-3H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228392
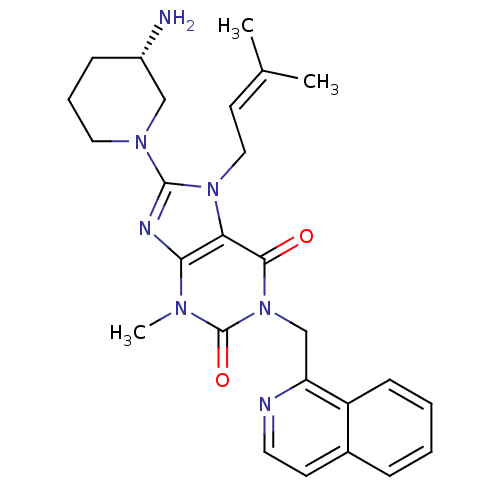
((S)-8-(3-aminopiperidin-1-yl)-1-(isoquinolin-1-ylm...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-1 Show InChI InChI=1S/C26H31N7O2/c1-17(2)11-14-32-22-23(29-25(32)31-13-6-8-19(27)15-31)30(3)26(35)33(24(22)34)16-21-20-9-5-4-7-18(20)10-12-28-21/h4-5,7,9-12,19H,6,8,13-16,27H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 in Caco2 cells by fluorescene assay |
J Med Chem 50: 6450-3 (2007)
Article DOI: 10.1021/jm701280z
BindingDB Entry DOI: 10.7270/Q2639PGG |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM120073
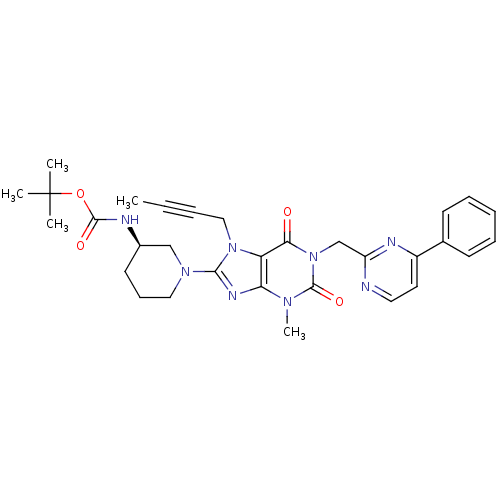
(US8697868, 1(30) | US8697868, 30)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nccc(n3)-c3ccccc3)c(=O)c12)N1CCC[C@H](C1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C31H36N8O4/c1-6-7-18-38-25-26(35-28(38)37-17-11-14-22(19-37)33-29(41)43-31(2,3)4)36(5)30(42)39(27(25)40)20-24-32-16-15-23(34-24)21-12-9-8-10-13-21/h8-10,12-13,15-16,22H,11,14,17-20H2,1-5H3,(H,33,41)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl of substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, was placed in black microtitre plate... |
US Patent US8697868 (2014)
BindingDB Entry DOI: 10.7270/Q2XD10CF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50376982
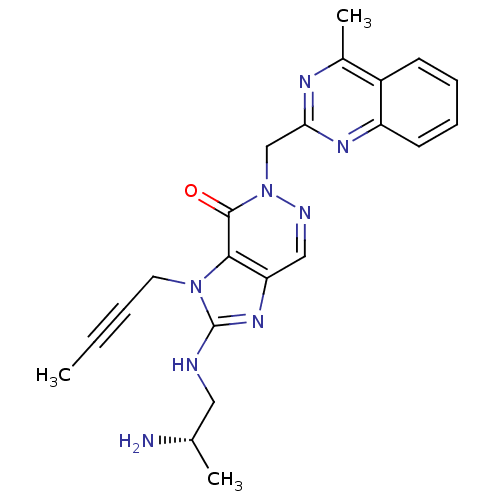
(CHEMBL403408)Show SMILES CC#CCn1c(NC[C@H](C)N)nc2cnn(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C22H24N8O/c1-4-5-10-29-20-18(28-22(29)24-11-14(2)23)12-25-30(21(20)31)13-19-26-15(3)16-8-6-7-9-17(16)27-19/h6-9,12,14H,10-11,13,23H2,1-3H3,(H,24,28)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024143
(CHEMBL3142184 | {1-[1-[1-(2,2-Difluoro-2-{2-methyl...)Show SMILES CC[C@H](C)[C@H](NC(=O)C(F)(F)C(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC1N=CC=N1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r,c:28,30| Show InChI InChI=1S/C40H54F2N8O7/c1-8-25(4)32(36(54)46-23-27-16-12-13-17-43-27)50-37(55)40(41,42)33(51)28(20-24(2)3)47-35(53)30(22-31-44-18-19-45-31)48-34(52)29(21-26-14-10-9-11-15-26)49-38(56)57-39(5,6)7/h9-19,24-25,28-32H,8,20-23H2,1-7H3,(H,46,54)(H,47,53)(H,48,52)(H,49,56)(H,50,55)/t25?,28-,29?,30?,32?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2080-7 (1986)
BindingDB Entry DOI: 10.7270/Q25X29G7 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM30645
(US8853193, 32-28)Show SMILES CC(CF)Oc1cc(F)ccc1Nc1ncnc2sc(C(N)=O)c(C)c12 Show InChI InChI=1S/C16H10ClN3O2S3/c17-13-7-8-14(23-13)25(21,22)20-11-5-3-10(4-6-11)15-19-12-2-1-9-18-16(12)24-15/h1-9,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
Inhibition of kinase activity of Mnk1 and Mnk2a was assessed with the same assay system, using pre-activated GST-Mnk1 or GST-Mnk2a, respectively. The... |
US Patent US8853193 (2014)
BindingDB Entry DOI: 10.7270/Q2GX4983 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM120074

(US8697868, 1(31) | US8697868, 31)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(CC(=O)c3ccccc3[N+]([O-])=O)c(=O)c12)N1CCC[C@H](C1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C28H33N7O7/c1-6-7-15-33-22-23(30-25(33)32-14-10-11-18(16-32)29-26(38)42-28(2,3)4)31(5)27(39)34(24(22)37)17-21(36)19-12-8-9-13-20(19)35(40)41/h8-9,12-13,18H,10-11,14-17H2,1-5H3,(H,29,38)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
50 μl of substrate solution (AFC; AFC is amido-4-trifluoromethylcoumarin), final concentration 100 μM, was placed in black microtitre plate... |
US Patent US8697868 (2014)
BindingDB Entry DOI: 10.7270/Q2XD10CF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228397
(8-((S)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 in Caco2 cells by fluorescene assay |
J Med Chem 50: 6450-3 (2007)
Article DOI: 10.1021/jm701280z
BindingDB Entry DOI: 10.7270/Q2639PGG |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50376991
(CHEMBL401502)Show SMILES CC#CCn1c(nc2cnn(CC3=Nc4ccccc4Oc4ccccc34)c(=O)c12)N1CCC[C@@H](N)C1 |t:12| Show InChI InChI=1S/C28H27N7O2/c1-2-3-15-34-26-22(32-28(34)33-14-8-9-19(29)17-33)16-30-35(27(26)36)18-23-20-10-4-6-12-24(20)37-25-13-7-5-11-21(25)31-23/h4-7,10-13,16,19H,8-9,14-15,17-18,29H2,1H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50106579
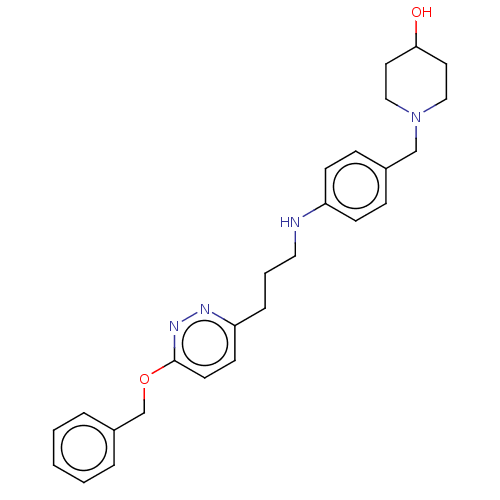
(CHEMBL3601036)Show SMILES OC1CCN(Cc2ccc(NCCCc3ccc(OCc4ccccc4)nn3)cc2)CC1 Show InChI InChI=1S/C26H32N4O2/c31-25-14-17-30(18-15-25)19-21-8-10-23(11-9-21)27-16-4-7-24-12-13-26(29-28-24)32-20-22-5-2-1-3-6-22/h1-3,5-6,8-13,25,27,31H,4,7,14-20H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method |
Bioorg Med Chem Lett 25: 3270-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.074
BindingDB Entry DOI: 10.7270/Q2MK6FN7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228406

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)cc4ccccc34)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C26H29N7O2/c1-4-5-13-32-22-23(29-25(32)31-12-8-10-19(27)15-31)30(3)26(35)33(24(22)34)16-21-20-11-7-6-9-18(20)14-17(2)28-21/h6-7,9,11,14,19H,8,10,12-13,15-16,27H2,1-3H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 in Caco2 cells by fluorescene assay |
J Med Chem 50: 6450-3 (2007)
Article DOI: 10.1021/jm701280z
BindingDB Entry DOI: 10.7270/Q2639PGG |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50376987
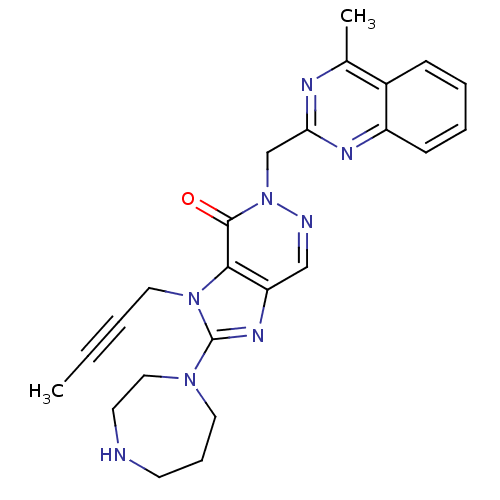
(CHEMBL257999)Show SMILES CC#CCn1c(nc2cnn(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCCNCC1 Show InChI InChI=1S/C24H26N8O/c1-3-4-13-31-22-20(29-24(31)30-12-7-10-25-11-14-30)15-26-32(23(22)33)16-21-27-17(2)18-8-5-6-9-19(18)28-21/h5-6,8-9,15,25H,7,10-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50106487
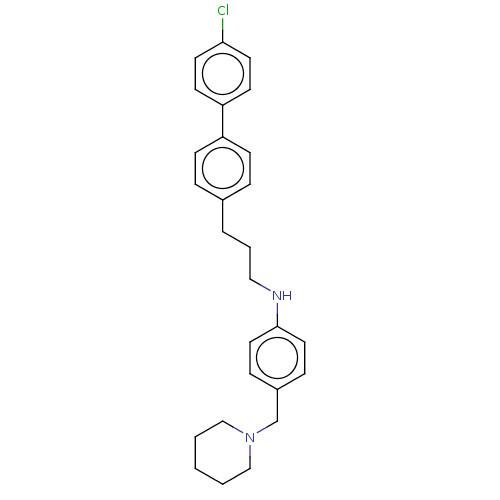
(CHEMBL3600970)Show SMILES Clc1ccc(cc1)-c1ccc(CCCNc2ccc(CN3CCCCC3)cc2)cc1 Show InChI InChI=1S/C27H31ClN2/c28-26-14-12-25(13-15-26)24-10-6-22(7-11-24)5-4-18-29-27-16-8-23(9-17-27)21-30-19-2-1-3-20-30/h6-17,29H,1-5,18-21H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method |
Bioorg Med Chem Lett 25: 3270-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.074
BindingDB Entry DOI: 10.7270/Q2MK6FN7 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM98036
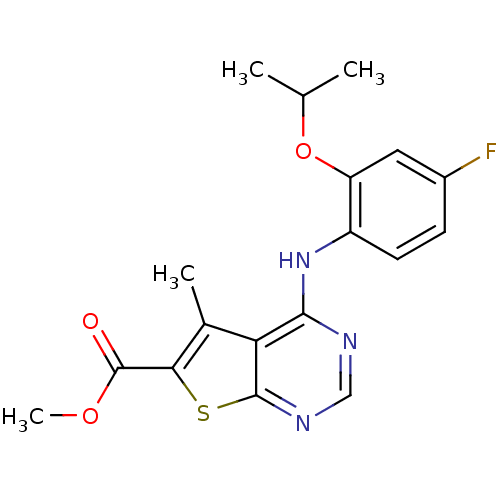
(US8486953, 19)Show InChI InChI=1S/C18H18FN3O3S/c1-9(2)25-13-7-11(19)5-6-12(13)22-16-14-10(3)15(18(23)24-4)26-17(14)21-8-20-16/h5-9H,1-4H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
Inhibition of kinase activity of Mnk1 and Mnk2a was assessed with the same assay system, using pre-activated GST-Mnk1 or GST-Mnk2a, respectively. The... |
US Patent US8853193 (2014)
BindingDB Entry DOI: 10.7270/Q2GX4983 |
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
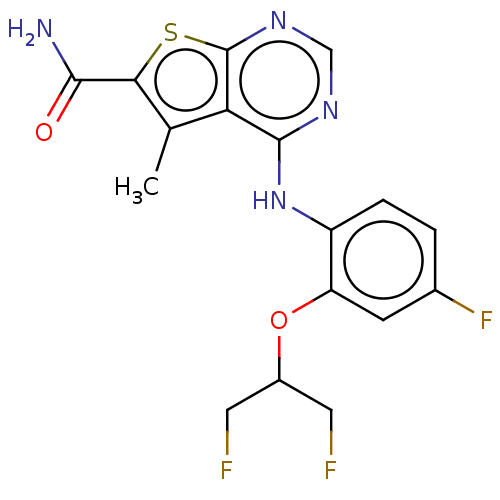
(Homo sapiens (Human)) | BDBM136194
(US8853193, 23-3)Show SMILES Cc1c(sc2ncnc(Nc3ccc(F)cc3OC(CF)CF)c12)C(N)=O Show InChI InChI=1S/C17H15F3N4O2S/c1-8-13-16(22-7-23-17(13)27-14(8)15(21)25)24-11-3-2-9(20)4-12(11)26-10(5-18)6-19/h2-4,7,10H,5-6H2,1H3,(H2,21,25)(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
Inhibition of kinase activity of Mnk1 and Mnk2a was assessed with the same assay system, using pre-activated GST-Mnk1 or GST-Mnk2a, respectively. The... |
US Patent US8853193 (2014)
BindingDB Entry DOI: 10.7270/Q2GX4983 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
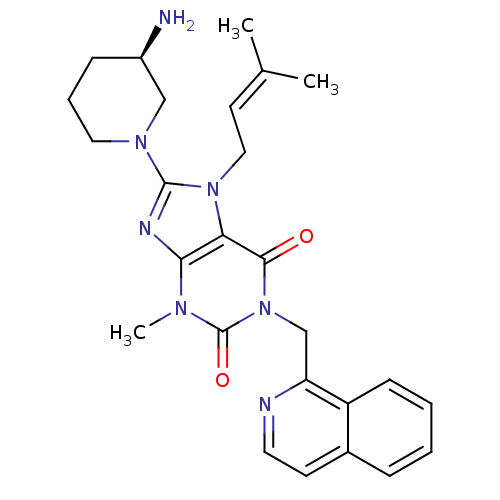
(Homo sapiens (Human)) | BDBM50228391
((R)-8-(3-aminopiperidin-1-yl)-1-(isoquinolin-1-ylm...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C26H31N7O2/c1-17(2)11-14-32-22-23(29-25(32)31-13-6-8-19(27)15-31)30(3)26(35)33(24(22)34)16-21-20-9-5-4-7-18(20)10-12-28-21/h4-5,7,9-12,19H,6,8,13-16,27H2,1-3H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 in Caco2 cells by fluorescene assay |
J Med Chem 50: 6450-3 (2007)
Article DOI: 10.1021/jm701280z
BindingDB Entry DOI: 10.7270/Q2639PGG |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50106583

(CHEMBL3601040)Show SMILES OCC1CCN(Cc2ccc(OCCCc3ccc(nn3)-c3ccc(Cl)cc3)cc2)CC1 Show InChI InChI=1S/C26H30ClN3O2/c27-23-7-5-22(6-8-23)26-12-9-24(28-29-26)2-1-17-32-25-10-3-20(4-11-25)18-30-15-13-21(19-31)14-16-30/h3-12,21,31H,1-2,13-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method |
Bioorg Med Chem Lett 25: 3270-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.074
BindingDB Entry DOI: 10.7270/Q2MK6FN7 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50106522

(CHEMBL3600978)Show SMILES Clc1ccc(cc1)-c1ccc(CCCNc2ccc(CN3CCCCC3)cc2)nn1 Show InChI InChI=1S/C25H29ClN4/c26-22-10-8-21(9-11-22)25-15-14-24(28-29-25)5-4-16-27-23-12-6-20(7-13-23)19-30-17-2-1-3-18-30/h6-15,27H,1-5,16-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Displacement of [125I-MCH] from human MCH receptor 1 expressed in CHO cell membranes by scintillation counting method |
Bioorg Med Chem Lett 25: 3270-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.074
BindingDB Entry DOI: 10.7270/Q2MK6FN7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
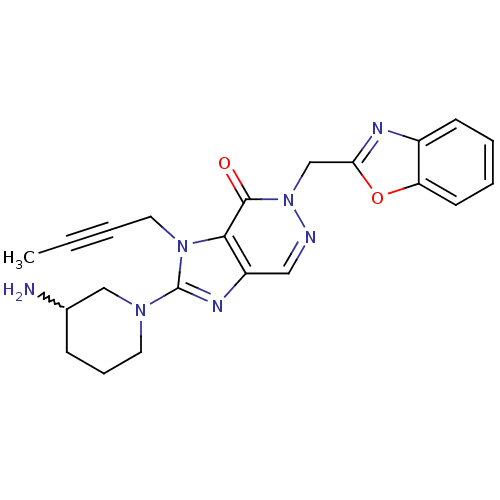
(Homo sapiens (Human)) | BDBM50376988
(CHEMBL256969)Show SMILES CC#CCn1c(nc2cnn(Cc3nc4ccccc4o3)c(=O)c12)N1CCCC(N)C1 |w:28.32| Show InChI InChI=1S/C22H23N7O2/c1-2-3-11-28-20-17(26-22(28)27-10-6-7-15(23)13-27)12-24-29(21(20)30)14-19-25-16-8-4-5-9-18(16)31-19/h4-5,8-9,12,15H,6-7,10-11,13-14,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
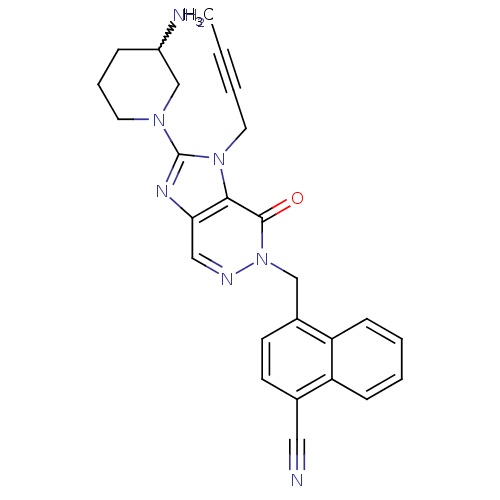
(Homo sapiens (Human)) | BDBM50376989
(CHEMBL404220)Show SMILES CC#CCn1c(nc2cnn(Cc3ccc(C#N)c4ccccc34)c(=O)c12)N1CCCC(N)C1 |w:31.35| Show InChI InChI=1S/C26H25N7O/c1-2-3-13-32-24-23(30-26(32)31-12-6-7-20(28)17-31)15-29-33(25(24)34)16-19-11-10-18(14-27)21-8-4-5-9-22(19)21/h4-5,8-11,15,20H,6-7,12-13,16-17,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco-2 cells after 1 hr |
Bioorg Med Chem Lett 18: 3158-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.075
BindingDB Entry DOI: 10.7270/Q2ZP470M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data