

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50529214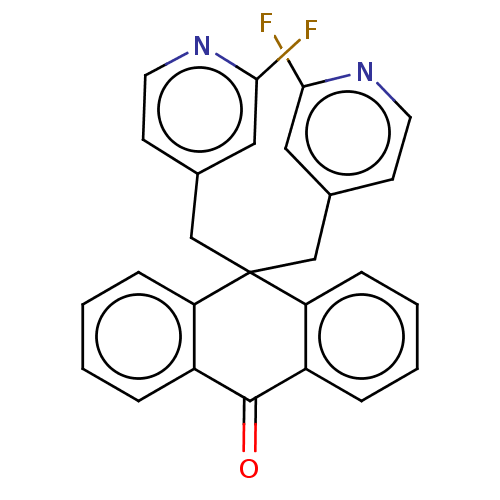 (CHEMBL343822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of alpha1A adrenergic receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50529212 (CHEMBL4471453) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of alpha1 adrenergic receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein polybromo-1 (Homo sapiens (Human)) | BDBM50529212 (CHEMBL4471453) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of PBR receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50529214 (CHEMBL343822) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of alpha1 adrenergic receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50529213 (CHEMBL4465132) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of D3 receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50529213 (CHEMBL4465132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of alpha1 adrenergic receptor (unknown origin) | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50048864 (CHEMBL3310505) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC3 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01928 BindingDB Entry DOI: 10.7270/Q2H41WB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50048864 (CHEMBL3310505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC1 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01928 BindingDB Entry DOI: 10.7270/Q2H41WB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50503927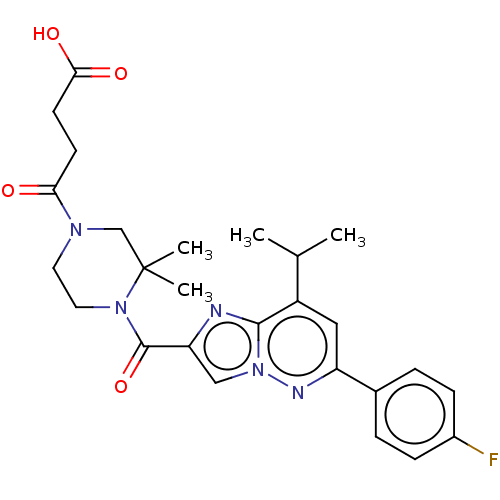 (CHEMBL4470628) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human EAhy926 cells assessed as inhibition of trypsin-induced intracellular calcium mobilization preincubated for 15 m... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50048864 (CHEMBL3310505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC2 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01928 BindingDB Entry DOI: 10.7270/Q2H41WB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human MDA-MB-231 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated fo... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50048864 (CHEMBL3310505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC8 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01928 BindingDB Entry DOI: 10.7270/Q2H41WB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503928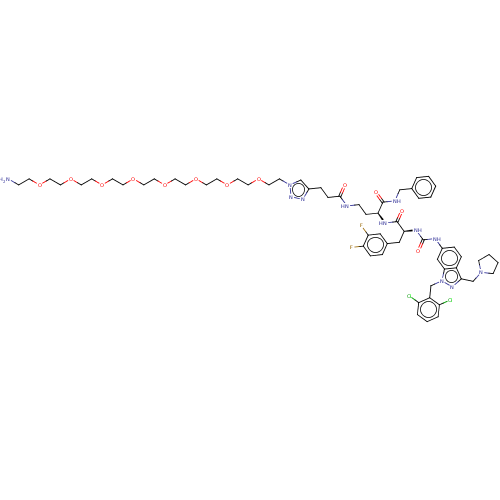 (CHEMBL4459024) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human MDA-MB-231 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated fo... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kaposi's sarcoma-associated herpesvirus cyclin homolog (Human herpesvirus 8 (HHV-8) (Kaposi's sarcoma-...) | BDBM36461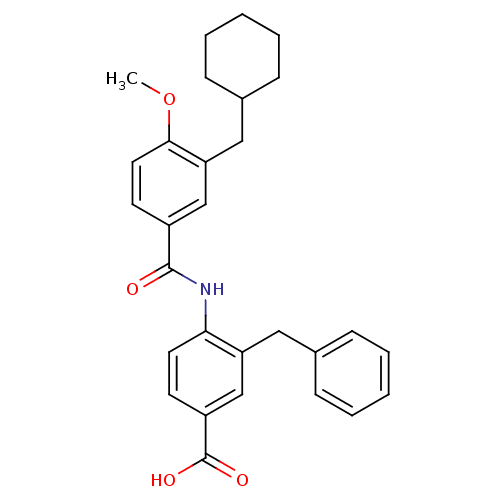 (3-Benzyl-4-(3-(cyclohexylmethyl)-4-methoxybenzamid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.4 | n/a | n/a | n/a | n/a | 8.0 | 30 |
University of California, San Francisco | Assay Description Human herpesvirus assay using human Kaposi's sarcoma-associated herpesvirus protease (KSHV Pr). | Nat Chem Biol 5: 640-6 (2009) Article DOI: 10.1038/nchembio.192 BindingDB Entry DOI: 10.7270/Q2RF5SC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503926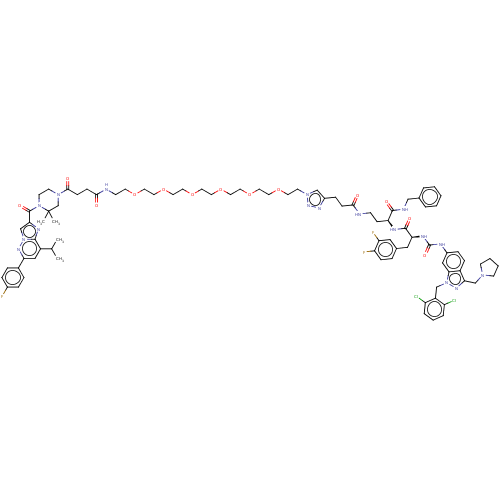 (CHEMBL4472608) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503924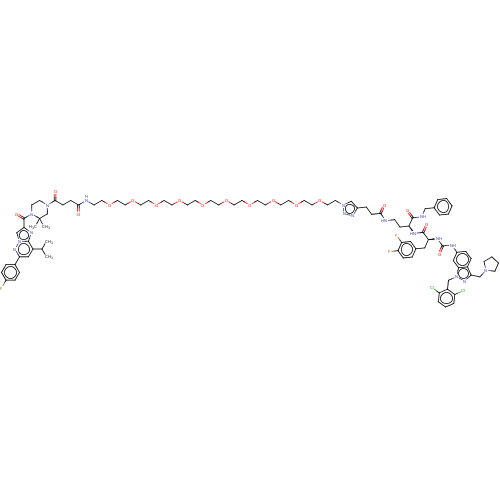 (CHEMBL4437508) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50529214 (CHEMBL343822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of Kv7.2 in human HEK293 cells incubated for 1 hr by thallium flux assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50529215 (CHEBI:35046 | CHEMBL342375) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of Kv7.2 in human HEK293 cells incubated for 1 hr by thallium flux assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503928 (CHEMBL4459024) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50503927 (CHEMBL4470628) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human EAhy926 cells assessed as inhibition of SLIGKV-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503929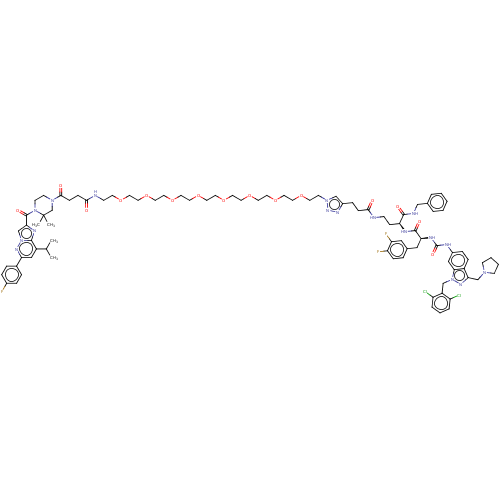 (CHEMBL4578092) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503925 (CHEMBL4540591) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human MDA-MB-231 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated fo... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50529213 (CHEMBL4465132) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of Kv7.2 in human HEK293 cells incubated for 1 hr by thallium flux assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503925 (CHEMBL4540591) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of TFLLRN-NH2-induced intracellular calcium mobilization preincubated for 1... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50048864 (CHEMBL3310505) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HDAC6 in human DU-145 cells assessed as increase in histone H3 acetylation after 24 hrs by immunofluorescence microscopy | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01928 BindingDB Entry DOI: 10.7270/Q2H41WB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50529212 (CHEMBL4471453) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of Kv7.2 in human HEK293 cells incubated for 1 hr by thallium flux assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512562 (CHEMBL4438936) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512562 (CHEMBL4438936) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512565 (CHEMBL4451525) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512565 (CHEMBL4451525) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50503925 (CHEMBL4540591) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR1 in human EAhy926 cells assessed as inhibition of thrombin-induced intracellular calcium mobilization preincubated for 15 ... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512563 (CHEMBL4514779) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512563 (CHEMBL4514779) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512561 (CHEMBL4448258) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512561 (CHEMBL4448258) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512564 (CHEMBL4594058) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50294961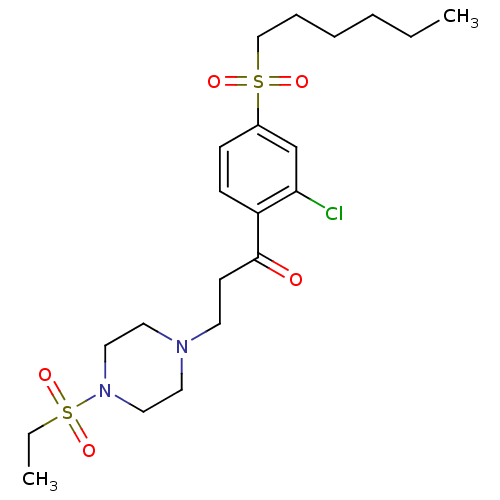 (1-(2-Chloro-4-(hexylsulfonyl)phenyl)-3-(4-(ethylsu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Hospital Curated by ChEMBL | Assay Description Inhibition of SRC2 binding to human TRbeta receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 52: 3892-901 (2009) Article DOI: 10.1021/jm9002704 BindingDB Entry DOI: 10.7270/Q2S75GCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50512564 (CHEMBL4594058) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Negative allosteric modulation of PAR1 in human EAhy926 cells assessed as reduction in TFLLRN-NH2-induced intracellular calcium mobilization incubate... | Bioorg Med Chem 27: 3788-3796 (2019) Article DOI: 10.1016/j.bmc.2019.06.043 BindingDB Entry DOI: 10.7270/Q2MP56MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 2 (Homo sapiens (Human)) | BDBM50503927 (CHEMBL4470628) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Antagonist activity at PAR2 in human MDA-MB-231 cells assessed as inhibition of SLIGKV-NH2-induced intracellular calcium mobilization preincubated fo... | ACS Med Chem Lett 10: 121-126 (2019) Article DOI: 10.1021/acsmedchemlett.8b00538 BindingDB Entry DOI: 10.7270/Q24Q7Z7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50294962 (3-(4-Acetylpiperazin-1-yl)-1-(2-chloro-4-(hexylsul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Hospital Curated by ChEMBL | Assay Description Inhibition of SRC2 binding to human TRbeta receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 52: 3892-901 (2009) Article DOI: 10.1021/jm9002704 BindingDB Entry DOI: 10.7270/Q2S75GCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 2 (Homo sapiens (Human)) | BDBM50529217 (CHEMBL4447214) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of Kv7.2 in human HEK293 cells incubated for 1 hr by thallium flux assay | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126681 BindingDB Entry DOI: 10.7270/Q20K2D1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50446373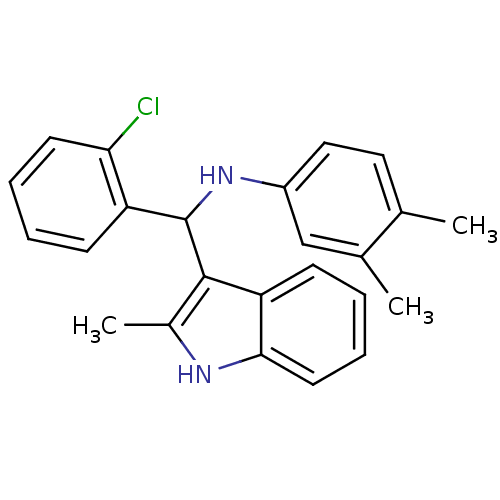 (CHEMBL3109624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of 1,25(OH)2D3-induced VDR (unknown origin)-mediated CYP24A1 transcription expressed in HEK293T cells after 16 hrs by luciferase reporter ... | ACS Med Chem Lett 5: 199-204 (2014) Article DOI: 10.1021/ml400462j BindingDB Entry DOI: 10.7270/Q2KK9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50446350 (CHEMBL3109604) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee Curated by ChEMBL | Assay Description Inhibition of 1,25(OH)2D3-induced VDR (unknown origin)-mediated CYP24A1 transcription expressed in HEK293T cells after 16 hrs by luciferase reporter ... | ACS Med Chem Lett 5: 199-204 (2014) Article DOI: 10.1021/ml400462j BindingDB Entry DOI: 10.7270/Q2KK9D8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50294969 (4-(3-(2,5-Dichloro-4-(hexylsulfonyl)phenyl)-3-oxop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Hospital Curated by ChEMBL | Assay Description Inhibition of SRC2 binding to human TRbeta receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 52: 3892-901 (2009) Article DOI: 10.1021/jm9002704 BindingDB Entry DOI: 10.7270/Q2S75GCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50294964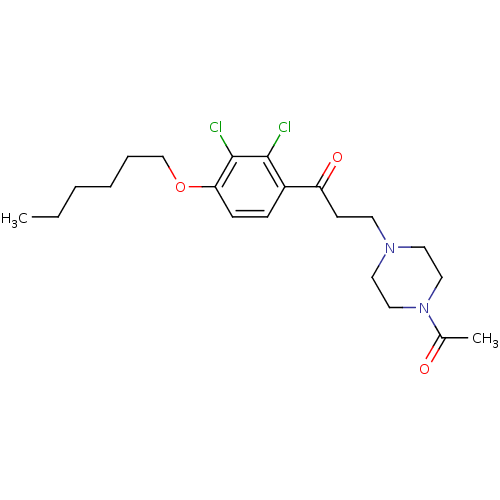 (3-(4-Acetylpiperazin-1-yl)-1-(2,3-dichloro-4-(hexy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Hospital Curated by ChEMBL | Assay Description Inhibition of SRC2 binding to human TRbeta receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 52: 3892-901 (2009) Article DOI: 10.1021/jm9002704 BindingDB Entry DOI: 10.7270/Q2S75GCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50294967 (4-(3-(2,5-Dichloro-4-(hexylthio)phenyl)-3-oxopropy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Hospital Curated by ChEMBL | Assay Description Inhibition of SRC2 binding to human TRbeta receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 52: 3892-901 (2009) Article DOI: 10.1021/jm9002704 BindingDB Entry DOI: 10.7270/Q2S75GCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50294966 (3-(4-Acetylpiperazin-1-yl)-1-(2-chloro-4-(hexylthi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Hospital Curated by ChEMBL | Assay Description Inhibition of SRC2 binding to human TRbeta receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 52: 3892-901 (2009) Article DOI: 10.1021/jm9002704 BindingDB Entry DOI: 10.7270/Q2S75GCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50294965 (3-(4-acetylpiperazin-1-yl)-1-(2,6-dichloro-4-(hexy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Hospital Curated by ChEMBL | Assay Description Inhibition of SRC2 binding to human TRbeta receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 52: 3892-901 (2009) Article DOI: 10.1021/jm9002704 BindingDB Entry DOI: 10.7270/Q2S75GCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50294968 (1-(2-Chloro-4-(hexylsulfonyl)phenyl)-3-morpholinop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Hospital Curated by ChEMBL | Assay Description Inhibition of SRC2 binding to human TRbeta receptor ligand binding domain expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 52: 3892-901 (2009) Article DOI: 10.1021/jm9002704 BindingDB Entry DOI: 10.7270/Q2S75GCS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 632 total ) | Next | Last >> |