

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493576 (CHEMBL2435828) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493578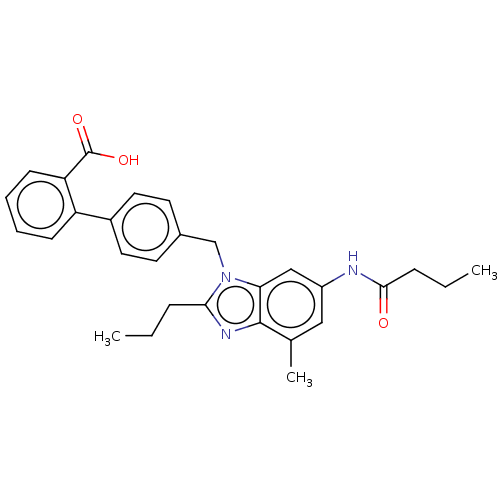 (CHEMBL2435824) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Inhibition of angiotensin AT1 receptor | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493577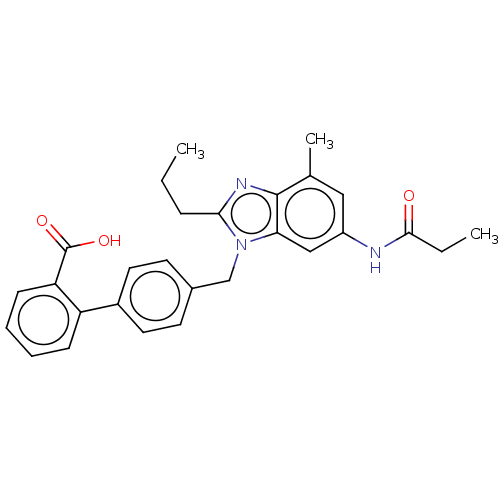 (CHEMBL2435823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493583 (CHEMBL2435829) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50388737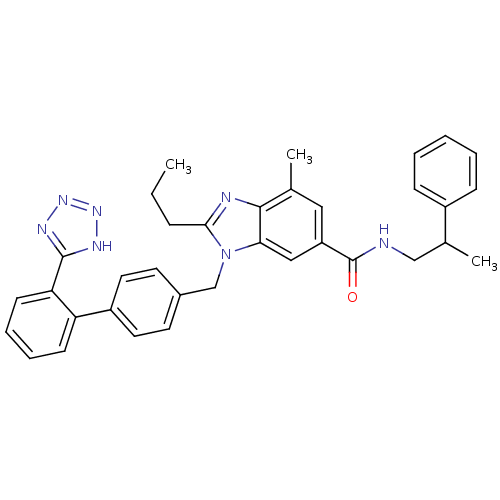 (CHEMBL2058860) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498919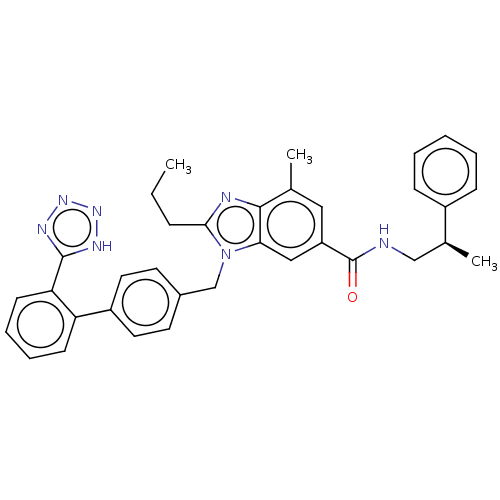 (CHEMBL3735744) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493589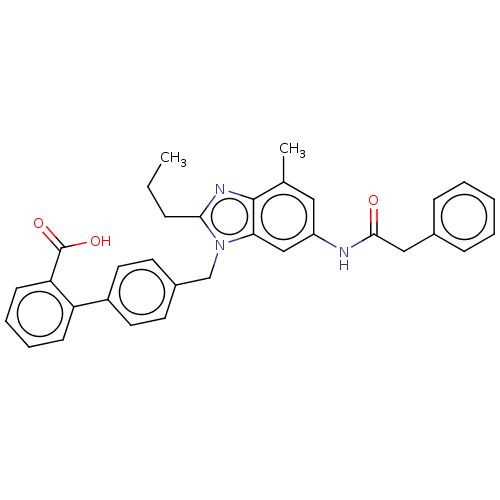 (CHEMBL2435827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50363833 (CHEMBL1945010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50388736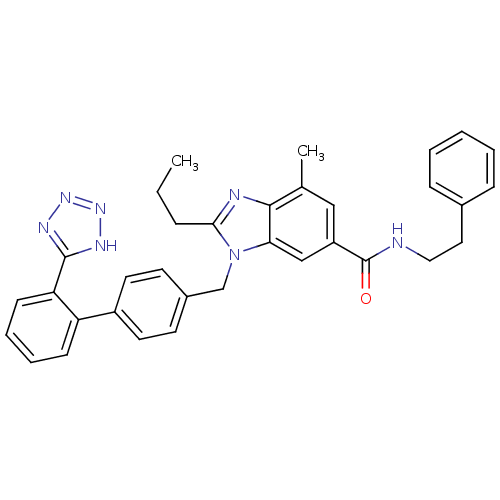 (CHEMBL2058859) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498908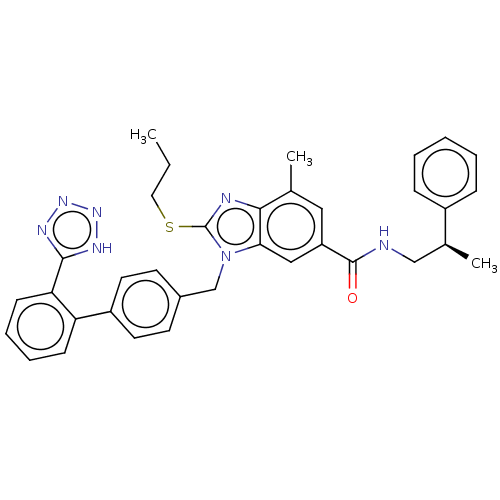 (CHEMBL3736514) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50363826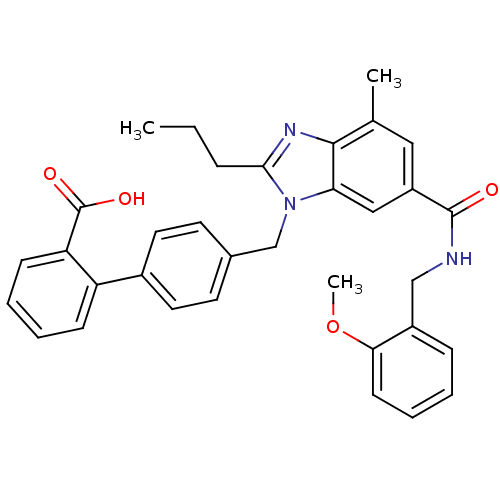 (CHEMBL1947129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50388738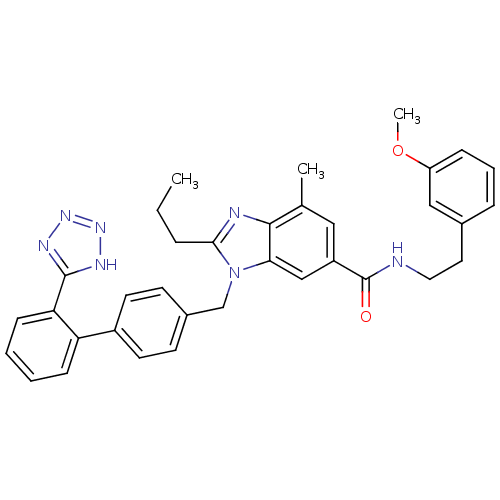 (CHEMBL2058861) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498907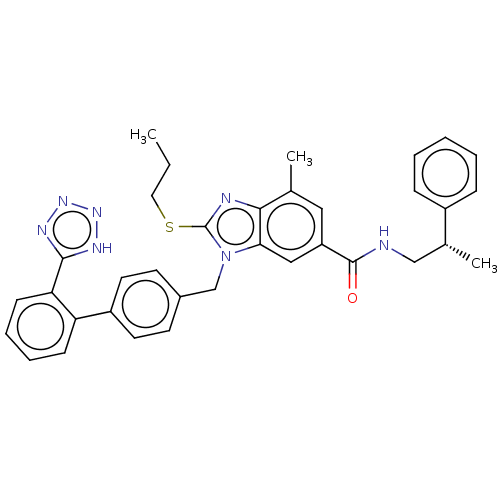 (CHEMBL3736244) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493582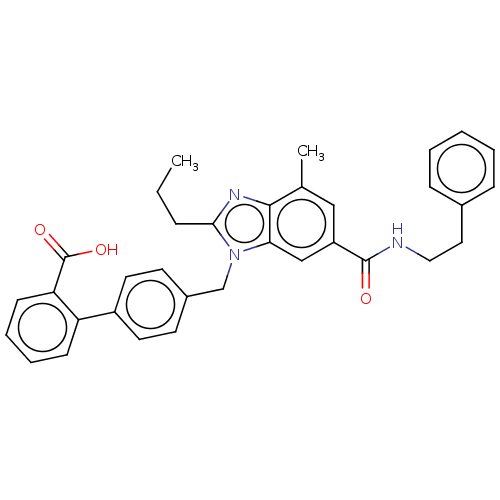 (CHEMBL2435818) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498936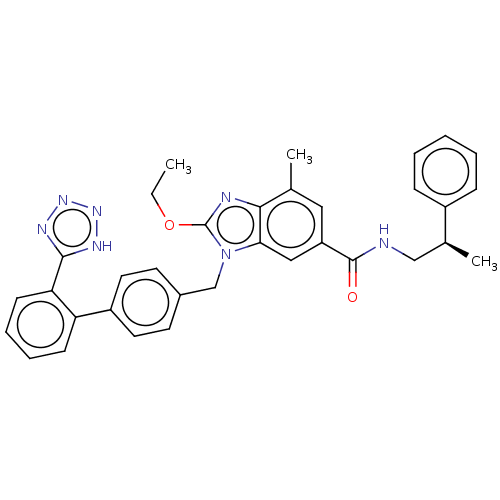 (CHEMBL3735290) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50388741 (CHEMBL2058864) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493585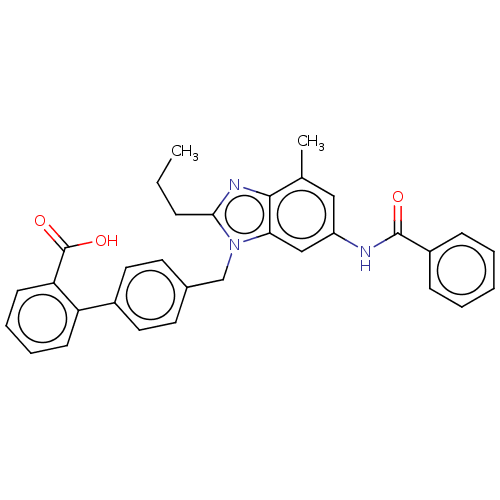 (CHEMBL2435825) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50363828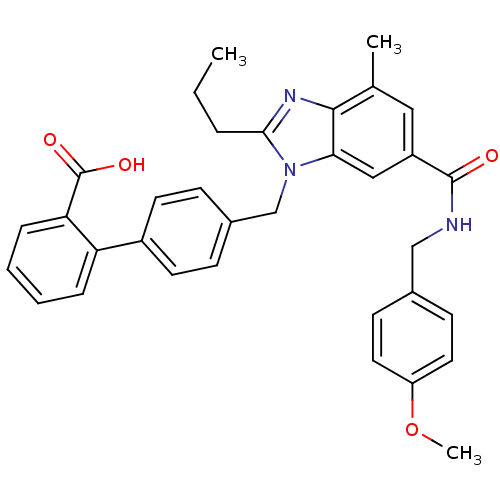 (CHEMBL1947131) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493581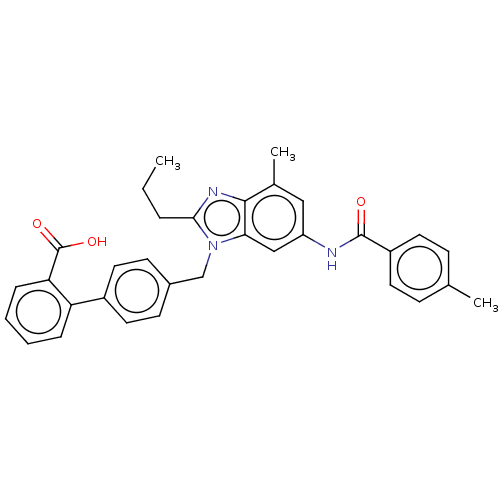 (CHEMBL2435826) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498943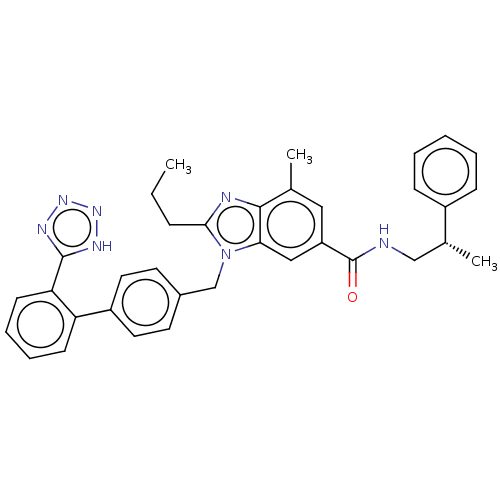 (CHEMBL3735409) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493585 (CHEMBL2435825) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498931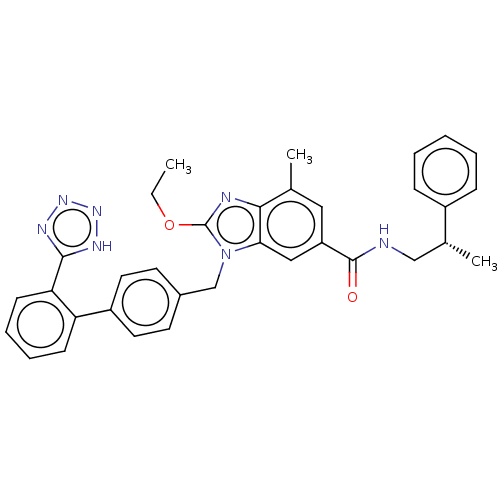 (CHEMBL3734891) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498942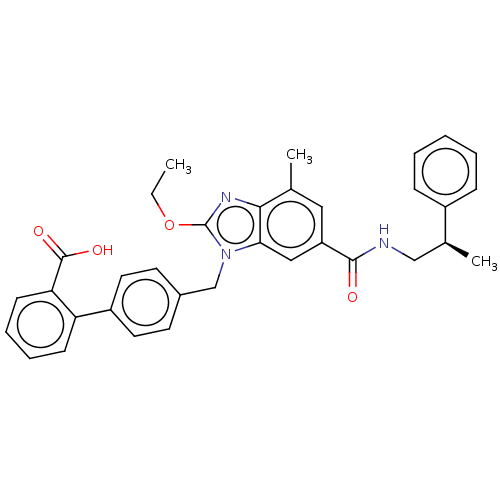 (CHEMBL3736440) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493588 (CHEMBL2435821) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493580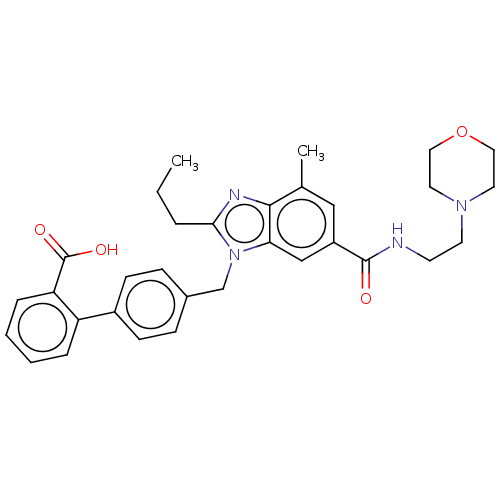 (CHEMBL2435819) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493575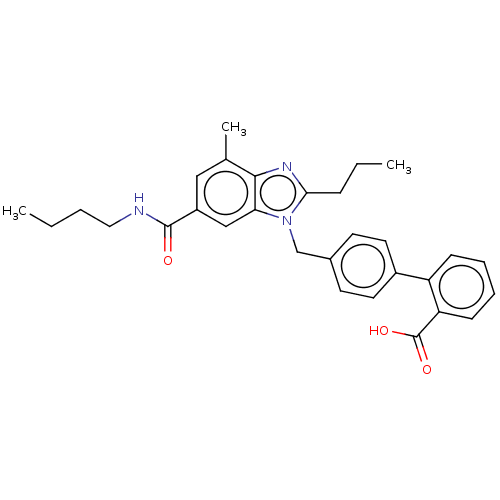 (CHEMBL2435831) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50388737 (CHEMBL2058860) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50388732 (CHEMBL2058855) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493579 (CHEMBL2435833) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498930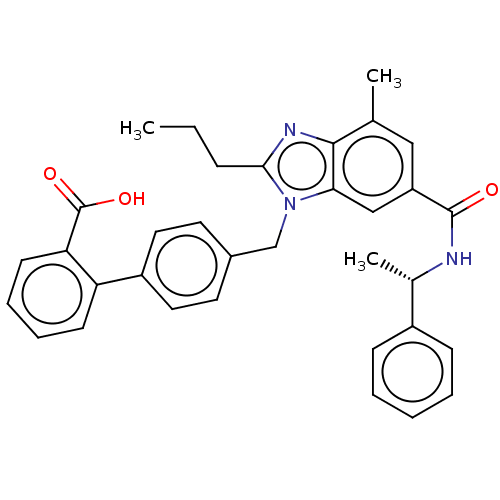 (CHEMBL3736295) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50388739 (CHEMBL2058862) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498920 (CHEMBL3735588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493582 (CHEMBL2435818) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50363829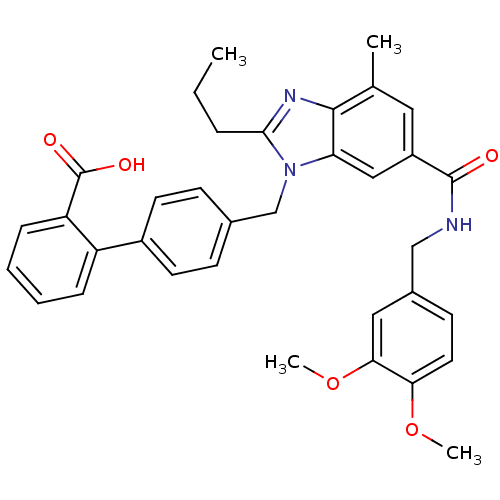 (CHEMBL1945006) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50363830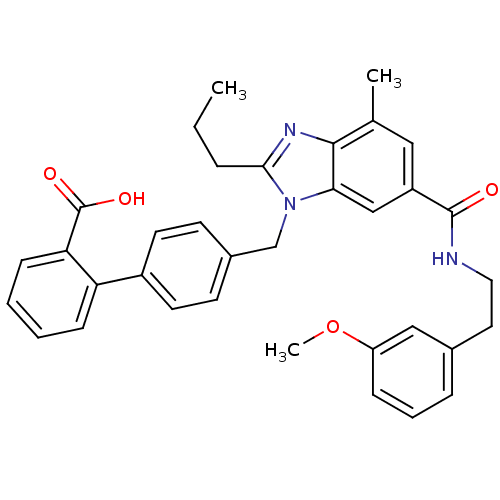 (CHEMBL1945007) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50388730 (CHEMBL2058865) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37.6 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemical Engineering& the Environment Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from angiotensin AT1 receptor after 180 mins by gamma counting | Bioorg Med Chem 20: 4208-16 (2012) Article DOI: 10.1016/j.bmc.2012.05.056 BindingDB Entry DOI: 10.7270/Q2GT5P7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493587 (CHEMBL2435834) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498922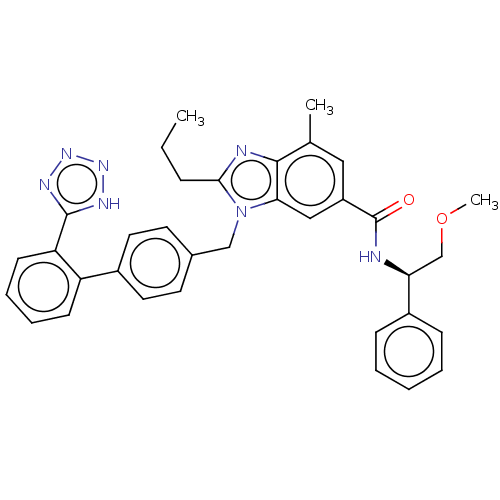 (CHEMBL3735769) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50498921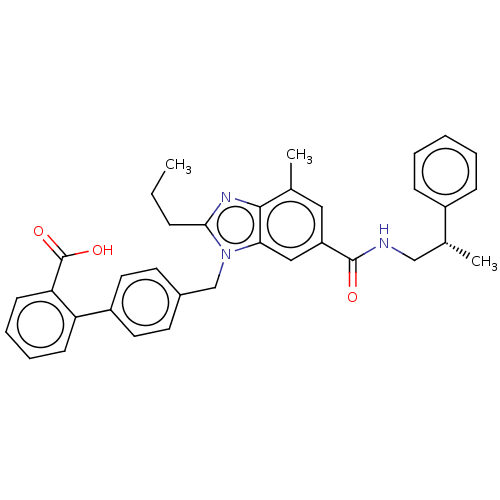 (CHEMBL3735652) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1Ile8-Ang2 from human AT1 receptor expressed in CHO-K1 cell membranes after 180 mins | Eur J Med Chem 103: 473-87 (2015) Article DOI: 10.1016/j.ejmech.2015.09.010 BindingDB Entry DOI: 10.7270/Q21J9DS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493579 (CHEMBL2435833) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50363825 (CHEMBL1947128) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting | Eur J Med Chem 49: 183-90 (2012) Article DOI: 10.1016/j.ejmech.2012.01.009 BindingDB Entry DOI: 10.7270/Q29K4BPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 126 total ) | Next | Last >> |