 Found 410 hits with Last Name = 'hieke' and Initial = 'm'
Found 410 hits with Last Name = 'hieke' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50497566
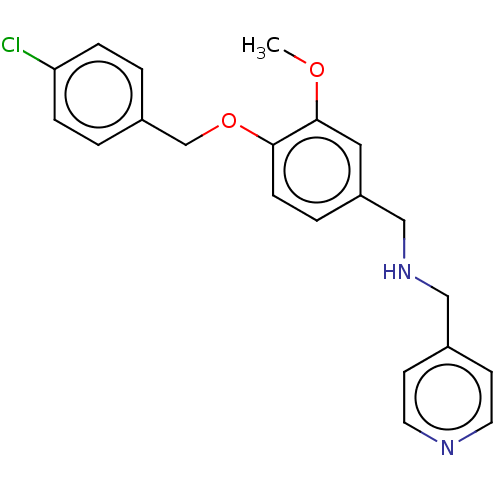
(CHEMBL3344193)Show InChI InChI=1S/C21H21ClN2O2/c1-25-21-12-18(14-24-13-16-8-10-23-11-9-16)4-7-20(21)26-15-17-2-5-19(22)6-3-17/h2-12,24H,13-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann-Wolfgang-Goethe University of Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of Plk1 immunoprecipitated from human HeLa cells using casein substrate after 12 to 26 hrs by autoradiography |
Bioorg Med Chem Lett 24: 5063-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.015
BindingDB Entry DOI: 10.7270/Q26Q2171 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as residual activity by measuring formation of 12-HHT from arachidonic acid by HPLC analysis |
Bioorg Med Chem 19: 5372-82 (2011)
Article DOI: 10.1016/j.bmc.2011.08.003
BindingDB Entry DOI: 10.7270/Q2JQ11D0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as residual activity by measuring formation of 12-HHT from arachidonic acid by HPLC analysis |
Bioorg Med Chem 19: 5372-82 (2011)
Article DOI: 10.1016/j.bmc.2011.08.003
BindingDB Entry DOI: 10.7270/Q2JQ11D0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365616
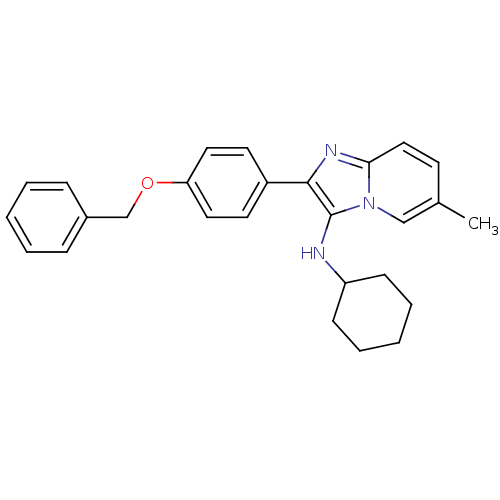
(CHEMBL1958141)Show SMILES Cc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C27H29N3O/c1-20-12-17-25-29-26(27(30(25)18-20)28-23-10-6-3-7-11-23)22-13-15-24(16-14-22)31-19-21-8-4-2-5-9-21/h2,4-5,8-9,12-18,23,28H,3,6-7,10-11,19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365618
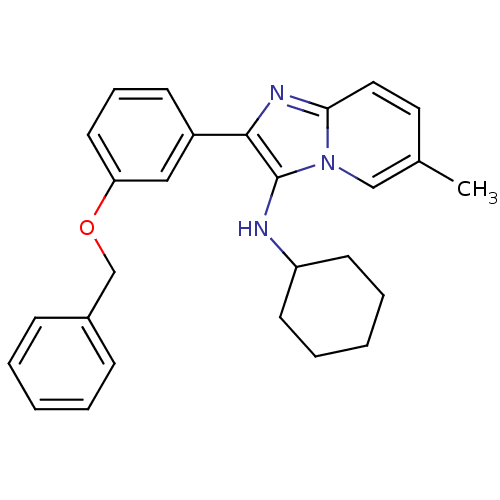
(CHEMBL1958143)Show SMILES Cc1ccc2nc(c(NC3CCCCC3)n2c1)-c1cccc(OCc2ccccc2)c1 Show InChI InChI=1S/C27H29N3O/c1-20-15-16-25-29-26(27(30(25)18-20)28-23-12-6-3-7-13-23)22-11-8-14-24(17-22)31-19-21-9-4-2-5-10-21/h2,4-5,8-11,14-18,23,28H,3,6-7,12-13,19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365646
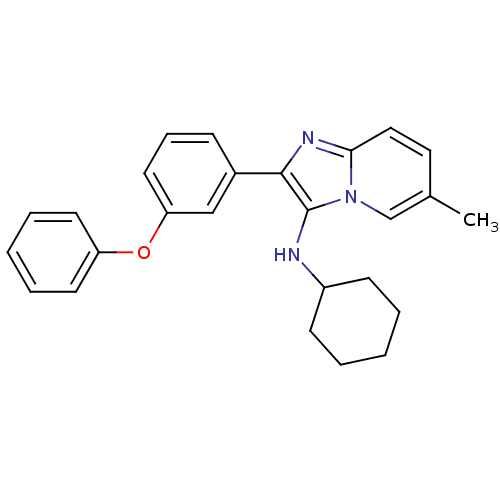
(CHEMBL1958140)Show SMILES Cc1ccc2nc(c(NC3CCCCC3)n2c1)-c1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C26H27N3O/c1-19-15-16-24-28-25(26(29(24)18-19)27-21-10-4-2-5-11-21)20-9-8-14-23(17-20)30-22-12-6-3-7-13-22/h3,6-9,12-18,21,27H,2,4-5,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50110164
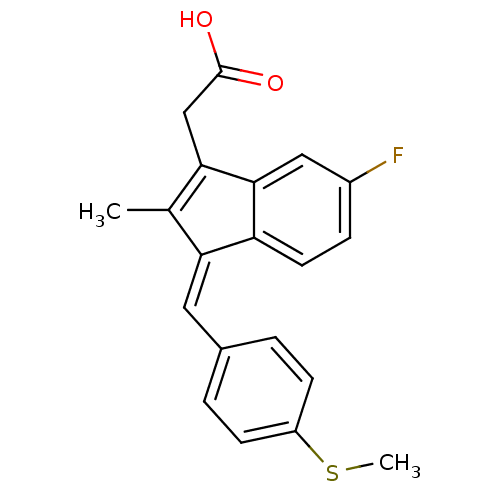
((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
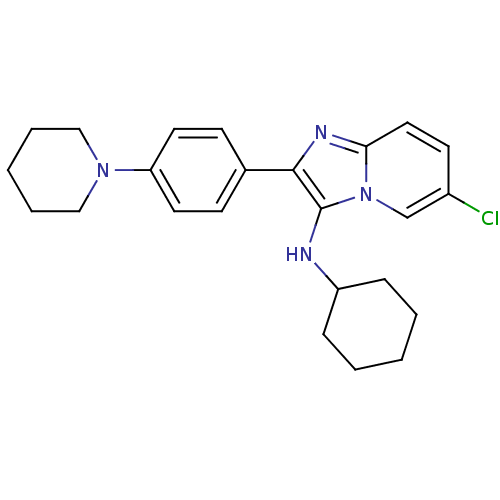
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365637
(CHEMBL1957976)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1ccc(cc1)-c1nc2ccc(Cl)cn2c1NC1CCCCC1 Show InChI InChI=1S/C28H36ClN5O2/c1-28(2,3)36-27(35)33-17-15-32(16-18-33)23-12-9-20(10-13-23)25-26(30-22-7-5-4-6-8-22)34-19-21(29)11-14-24(34)31-25/h9-14,19,22,30H,4-8,15-18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
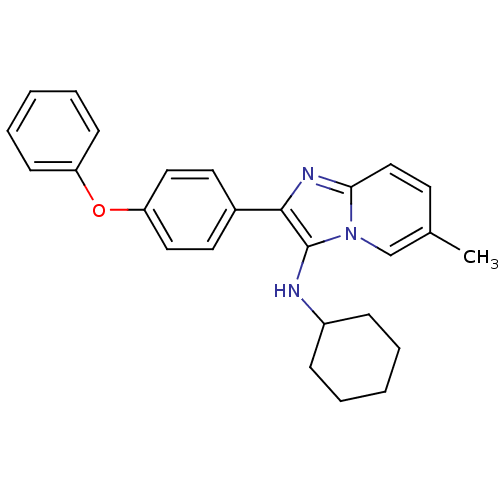
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365636
(CHEMBL1957975)Show SMILES Clc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C24H29ClN4/c25-19-11-14-22-27-23(24(29(22)17-19)26-20-7-3-1-4-8-20)18-9-12-21(13-10-18)28-15-5-2-6-16-28/h9-14,17,20,26H,1-8,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365631

(CHEMBL1957970)Show InChI InChI=1S/C21H25ClN4/c1-25(2)18-11-8-15(9-12-18)20-21(23-17-6-4-3-5-7-17)26-14-16(22)10-13-19(26)24-20/h8-14,17,23H,3-7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365617
(CHEMBL1958142)Show SMILES Cc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O/c1-19-12-17-24-28-25(26(29(24)18-19)27-21-8-4-2-5-9-21)20-13-15-23(16-14-20)30-22-10-6-3-7-11-22/h3,6-7,10-18,21,27H,2,4-5,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase-mediated 5(S)-H(p)ETE formation in fMLP-stimulated human PMNL incubated 10 mins prior to fMLP challenge |
J Med Chem 54: 4490-507 (2011)
Article DOI: 10.1021/jm200092b
BindingDB Entry DOI: 10.7270/Q2XG9S8B |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase-mediated 5(S)-H(p)ETE formation in fMLP-stimulated human PMNL incubated 10 mins prior to fMLP challenge |
J Med Chem 54: 4490-507 (2011)
Article DOI: 10.1021/jm200092b
BindingDB Entry DOI: 10.7270/Q2XG9S8B |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365632
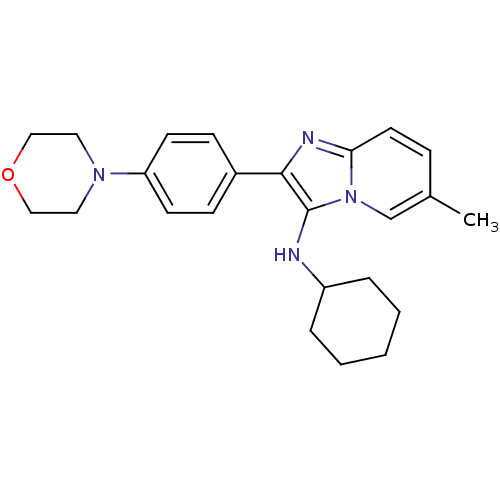
(CHEMBL1957971)Show SMILES Cc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C24H30N4O/c1-18-7-12-22-26-23(24(28(22)17-18)25-20-5-3-2-4-6-20)19-8-10-21(11-9-19)27-13-15-29-16-14-27/h7-12,17,20,25H,2-6,13-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50097346
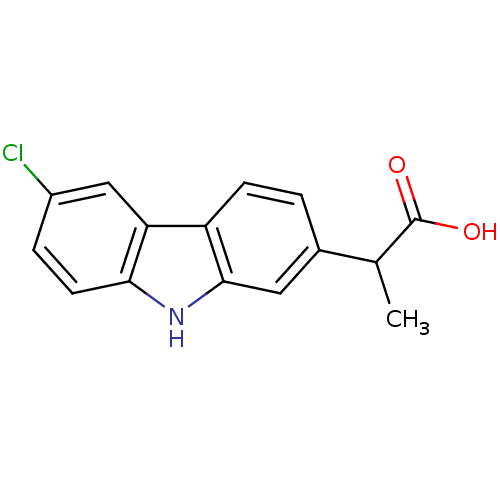
((+/-)-2-(3-chloro-9H-carbazol-7-yl)propanoic acid ...)Show InChI InChI=1S/C15H12ClNO2/c1-8(15(18)19)9-2-4-11-12-7-10(16)3-5-13(12)17-14(11)6-9/h2-8,17H,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365631

(CHEMBL1957970)Show InChI InChI=1S/C21H25ClN4/c1-25(2)18-11-8-15(9-12-18)20-21(23-17-6-4-3-5-7-17)26-14-16(22)10-13-19(26)24-20/h8-14,17,23H,3-7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50110164

((Z)-2-(3-(4-(methylthio)benzylidene)-6-fluoro-2-me...)Show SMILES CSc1ccc(\C=C2\C(C)=C(CC(O)=O)c3cc(F)ccc23)cc1 |t:9| Show InChI InChI=1S/C20H17FO2S/c1-12-17(9-13-3-6-15(24-2)7-4-13)16-8-5-14(21)10-19(16)18(12)11-20(22)23/h3-10H,11H2,1-2H3,(H,22,23)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337806
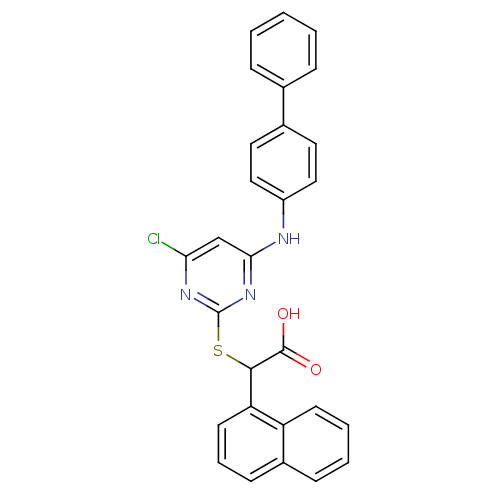
(2-(4-(biphenyl-4-ylamino)-6-chloropyrimidin-2-ylth...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccccc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O2S/c29-24-17-25(30-21-15-13-19(14-16-21)18-7-2-1-3-8-18)32-28(31-24)35-26(27(33)34)23-12-6-10-20-9-4-5-11-22(20)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50097346

((+/-)-2-(3-chloro-9H-carbazol-7-yl)propanoic acid ...)Show InChI InChI=1S/C15H12ClNO2/c1-8(15(18)19)9-2-4-11-12-7-10(16)3-5-13(12)17-14(11)6-9/h2-8,17H,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as residual activity by measuring formation of 12-HHT from arachidonic acid by HPLC analysis |
Bioorg Med Chem 19: 5372-82 (2011)
Article DOI: 10.1016/j.bmc.2011.08.003
BindingDB Entry DOI: 10.7270/Q2JQ11D0 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365636

(CHEMBL1957975)Show SMILES Clc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C24H29ClN4/c25-19-11-14-22-27-23(24(29(22)17-19)26-20-7-3-1-4-8-20)18-9-12-21(13-10-18)28-15-5-2-6-16-28/h9-14,17,20,26H,1-8,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365634
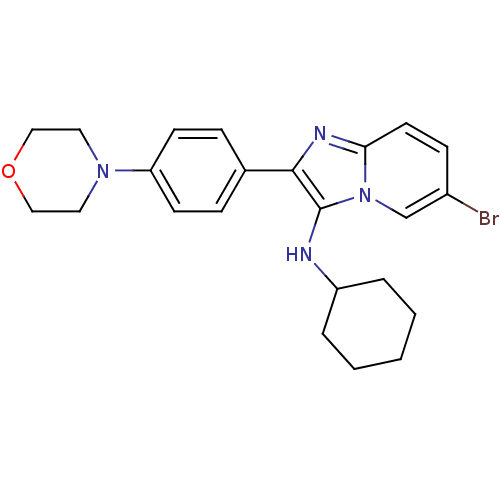
(CHEMBL1957973)Show SMILES Brc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C23H27BrN4O/c24-18-8-11-21-26-22(23(28(21)16-18)25-19-4-2-1-3-5-19)17-6-9-20(10-7-17)27-12-14-29-15-13-27/h6-11,16,19,25H,1-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase-mediated product formation from arachidonic acid after 5 to 10 mins by HPLC analysis |
J Med Chem 54: 4490-507 (2011)
Article DOI: 10.1021/jm200092b
BindingDB Entry DOI: 10.7270/Q2XG9S8B |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365632

(CHEMBL1957971)Show SMILES Cc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C24H30N4O/c1-18-7-12-22-26-23(24(28(22)17-18)25-20-5-3-2-4-6-20)19-8-10-21(11-9-19)27-13-15-29-16-14-27/h7-12,17,20,25H,2-6,13-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337808
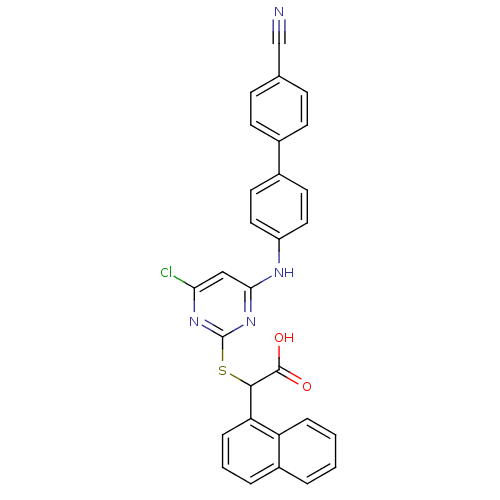
(2-(4-chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H19ClN4O2S/c30-25-16-26(32-22-14-12-20(13-15-22)19-10-8-18(17-31)9-11-19)34-29(33-25)37-27(28(35)36)24-7-3-5-21-4-1-2-6-23(21)24/h1-16,27H,(H,35,36)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365633
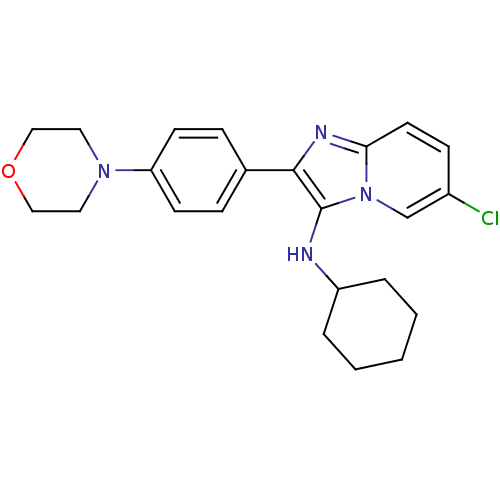
(CHEMBL1957972)Show SMILES Clc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C23H27ClN4O/c24-18-8-11-21-26-22(23(28(21)16-18)25-19-4-2-1-3-5-19)17-6-9-20(10-7-17)27-12-14-29-15-13-27/h6-11,16,19,25H,1-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50396066
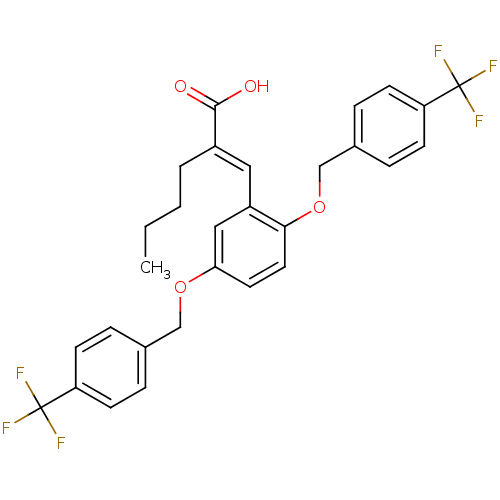
(CHEMBL1819479)Show SMILES CCCC\C(=C/c1cc(OCc2ccc(cc2)C(F)(F)F)ccc1OCc1ccc(cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C29H26F6O4/c1-2-3-4-21(27(36)37)15-22-16-25(38-17-19-5-9-23(10-6-19)28(30,31)32)13-14-26(22)39-18-20-7-11-24(12-8-20)29(33,34)35/h5-16H,2-4,17-18H2,1H3,(H,36,37)/b21-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase-mediated 5(S)-H(p)ETE formation in fMLP-stimulated human PMNL incubated 10 mins prior to fMLP challenge |
J Med Chem 54: 4490-507 (2011)
Article DOI: 10.1021/jm200092b
BindingDB Entry DOI: 10.7270/Q2XG9S8B |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50062724
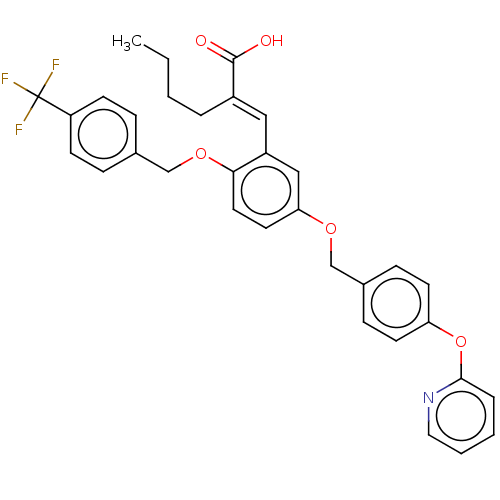
(CHEMBL3397723)Show SMILES CCCC\C(=C/c1cc(OCc2ccc(Oc3ccccn3)cc2)ccc1OCc1ccc(cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C33H30F3NO5/c1-2-3-6-25(32(38)39)19-26-20-29(16-17-30(26)41-22-23-8-12-27(13-9-23)33(34,35)36)40-21-24-10-14-28(15-11-24)42-31-7-4-5-18-37-31/h4-5,7-20H,2-3,6,21-22H2,1H3,(H,38,39)/b25-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... |
Bioorg Med Chem Lett 25: 841-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.073
BindingDB Entry DOI: 10.7270/Q2280989 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50062727

(CHEMBL3397721)Show SMILES CCCC\C(=C/c1cc(OCc2ccc3nc(cc(OC)c3c2)C(F)(F)F)ccc1OCc1ccc(cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C33H29F6NO5/c1-3-4-5-22(31(41)42)15-23-16-25(11-13-28(23)45-18-20-6-9-24(10-7-20)32(34,35)36)44-19-21-8-12-27-26(14-21)29(43-2)17-30(40-27)33(37,38)39/h6-17H,3-5,18-19H2,1-2H3,(H,41,42)/b22-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... |
Bioorg Med Chem Lett 25: 841-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.073
BindingDB Entry DOI: 10.7270/Q2280989 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50396066

(CHEMBL1819479)Show SMILES CCCC\C(=C/c1cc(OCc2ccc(cc2)C(F)(F)F)ccc1OCc1ccc(cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C29H26F6O4/c1-2-3-4-21(27(36)37)15-22-16-25(38-17-19-5-9-23(10-6-19)28(30,31)32)13-14-26(22)39-18-20-7-11-24(12-8-20)29(33,34)35/h5-16H,2-4,17-18H2,1H3,(H,36,37)/b21-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... |
Bioorg Med Chem Lett 25: 841-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.073
BindingDB Entry DOI: 10.7270/Q2280989 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337813
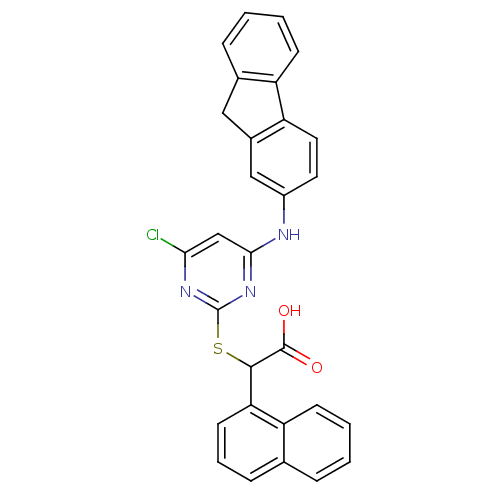
(2-(4-(9H-fluoren-2-ylamino)-6-chloropyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc-3c(Cc4ccccc-34)c2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H20ClN3O2S/c30-25-16-26(31-20-12-13-23-19(15-20)14-18-7-2-4-10-22(18)23)33-29(32-25)36-27(28(34)35)24-11-5-8-17-6-1-3-9-21(17)24/h1-13,15-16,27H,14H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50062722
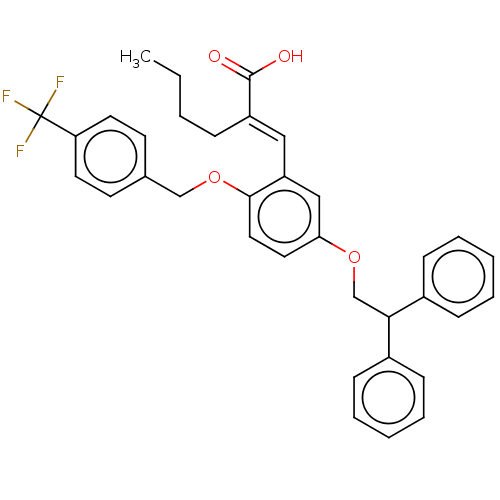
(CHEMBL3397726)Show SMILES CCCC\C(=C/c1cc(OCC(c2ccccc2)c2ccccc2)ccc1OCc1ccc(cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C35H33F3O4/c1-2-3-10-28(34(39)40)21-29-22-31(19-20-33(29)42-23-25-15-17-30(18-16-25)35(36,37)38)41-24-32(26-11-6-4-7-12-26)27-13-8-5-9-14-27/h4-9,11-22,32H,2-3,10,23-24H2,1H3,(H,39,40)/b28-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... |
Bioorg Med Chem Lett 25: 841-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.073
BindingDB Entry DOI: 10.7270/Q2280989 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365617

(CHEMBL1958142)Show SMILES Cc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H27N3O/c1-19-12-17-24-28-25(26(29(24)18-19)27-21-8-4-2-5-9-21)20-13-15-23(16-14-20)30-22-10-6-3-7-11-22/h3,6-7,10-18,21,27H,2,4-5,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50315660
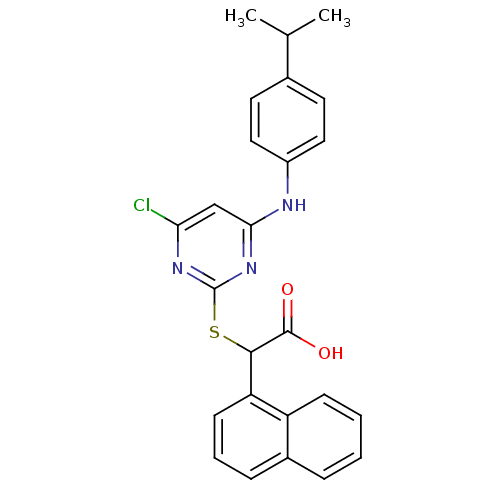
(2-(4-chloro-6-(4-isopropylphenylamino)pyrimidin-2-...)Show SMILES CC(C)c1ccc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)cc1 Show InChI InChI=1S/C25H22ClN3O2S/c1-15(2)16-10-12-18(13-11-16)27-22-14-21(26)28-25(29-22)32-23(24(30)31)20-9-5-7-17-6-3-4-8-19(17)20/h3-15,23H,1-2H3,(H,30,31)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337805
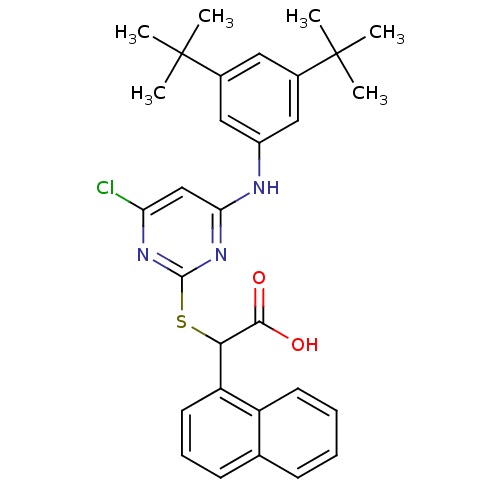
(2-(4-chloro-6-(3,5-di-tert-butylphenylamino)pyrimi...)Show SMILES CC(C)(C)c1cc(Nc2cc(Cl)nc(SC(C(O)=O)c3cccc4ccccc34)n2)cc(c1)C(C)(C)C Show InChI InChI=1S/C30H32ClN3O2S/c1-29(2,3)19-14-20(30(4,5)6)16-21(15-19)32-25-17-24(31)33-28(34-25)37-26(27(35)36)23-13-9-11-18-10-7-8-12-22(18)23/h7-17,26H,1-6H3,(H,35,36)(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365633

(CHEMBL1957972)Show SMILES Clc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C23H27ClN4O/c24-18-8-11-21-26-22(23(28(21)16-18)25-19-4-2-1-3-5-19)17-6-9-20(10-7-17)27-12-14-29-15-13-27/h6-11,16,19,25H,1-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365616

(CHEMBL1958141)Show SMILES Cc1ccc2nc(c(NC3CCCCC3)n2c1)-c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C27H29N3O/c1-20-12-17-25-29-26(27(30(25)18-20)28-23-10-6-3-7-11-23)22-13-15-24(16-14-22)31-19-21-8-4-2-5-9-21/h2,4-5,8-9,12-18,23,28H,3,6-7,10-11,19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50396061
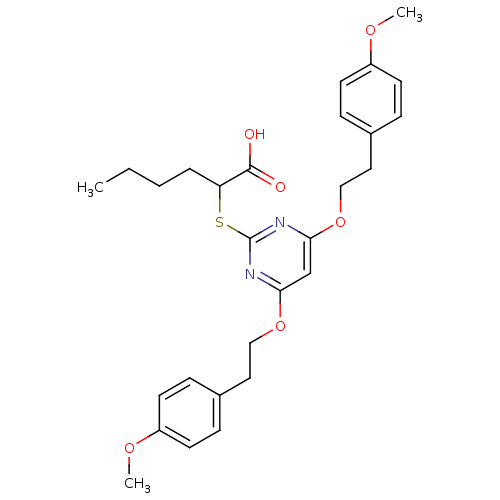
(CHEMBL1173121)Show SMILES CCCCC(Sc1nc(OCCc2ccc(OC)cc2)cc(OCCc2ccc(OC)cc2)n1)C(O)=O Show InChI InChI=1S/C28H34N2O6S/c1-4-5-6-24(27(31)32)37-28-29-25(35-17-15-20-7-11-22(33-2)12-8-20)19-26(30-28)36-18-16-21-9-13-23(34-3)14-10-21/h7-14,19,24H,4-6,15-18H2,1-3H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase-mediated 5(S)-H(p)ETE formation in fMLP-stimulated human PMNL incubated 10 mins prior to fMLP challenge |
J Med Chem 54: 4490-507 (2011)
Article DOI: 10.1021/jm200092b
BindingDB Entry DOI: 10.7270/Q2XG9S8B |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50351425
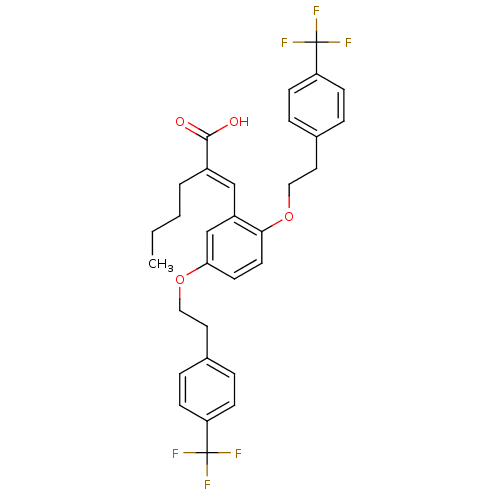
(CHEMBL1819478)Show SMILES CCCC\C(=C/c1cc(OCCc2ccc(cc2)C(F)(F)F)ccc1OCCc1ccc(cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C31H30F6O4/c1-2-3-4-23(29(38)39)19-24-20-27(40-17-15-21-5-9-25(10-6-21)30(32,33)34)13-14-28(24)41-18-16-22-7-11-26(12-8-22)31(35,36)37/h5-14,19-20H,2-4,15-18H2,1H3,(H,38,39)/b23-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase-mediated 5(S)-H(p)ETE formation in fMLP-stimulated human PMNL incubated 10 mins prior to fMLP challenge |
J Med Chem 54: 4490-507 (2011)
Article DOI: 10.1021/jm200092b
BindingDB Entry DOI: 10.7270/Q2XG9S8B |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50062731
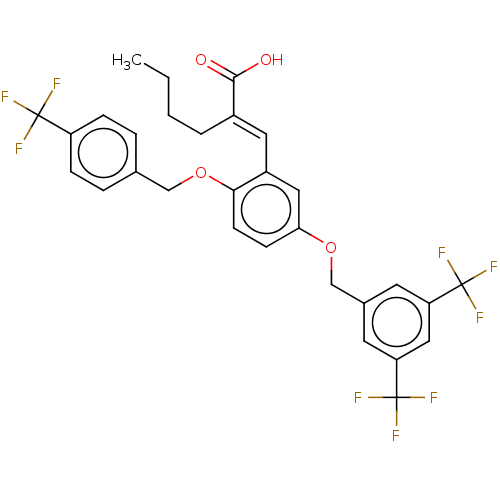
(CHEMBL3397717)Show SMILES CCCC\C(=C/c1cc(OCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)ccc1OCc1ccc(cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C30H25F9O4/c1-2-3-4-20(27(40)41)13-21-14-25(9-10-26(21)43-16-18-5-7-22(8-6-18)28(31,32)33)42-17-19-11-23(29(34,35)36)15-24(12-19)30(37,38)39/h5-15H,2-4,16-17H2,1H3,(H,40,41)/b20-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... |
Bioorg Med Chem Lett 25: 841-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.073
BindingDB Entry DOI: 10.7270/Q2280989 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50062730
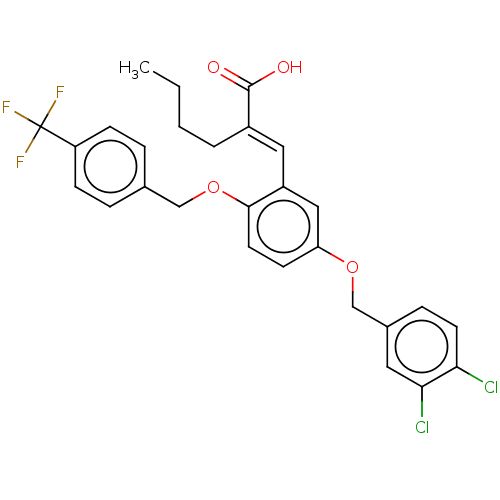
(CHEMBL3397716)Show SMILES CCCC\C(=C/c1cc(OCc2ccc(Cl)c(Cl)c2)ccc1OCc1ccc(cc1)C(F)(F)F)C(O)=O Show InChI InChI=1S/C28H25Cl2F3O4/c1-2-3-4-20(27(34)35)14-21-15-23(36-17-19-7-11-24(29)25(30)13-19)10-12-26(21)37-16-18-5-8-22(9-6-18)28(31,32)33/h5-15H,2-4,16-17H2,1H3,(H,34,35)/b20-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human human PMNL assessed as reduction in 5-LO product formation pre-incubated for 15 mins before addition of arachidonic acid ... |
Bioorg Med Chem Lett 25: 841-6 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.073
BindingDB Entry DOI: 10.7270/Q2280989 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337812
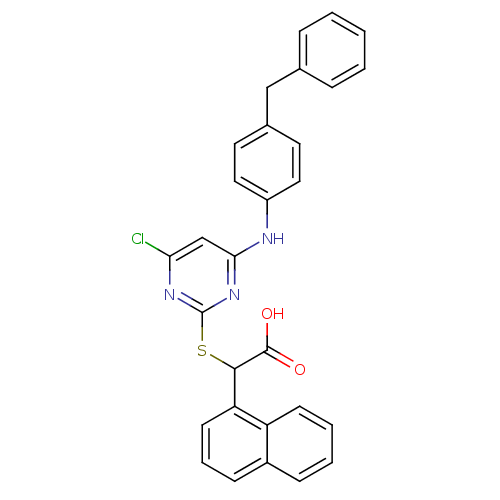
(2-(4-(4-benzylphenylamino)-6-chloropyrimidin-2-ylt...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Cc3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C29H22ClN3O2S/c30-25-18-26(31-22-15-13-20(14-16-22)17-19-7-2-1-3-8-19)33-29(32-25)36-27(28(34)35)24-12-6-10-21-9-4-5-11-23(21)24/h1-16,18,27H,17H2,(H,34,35)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337810
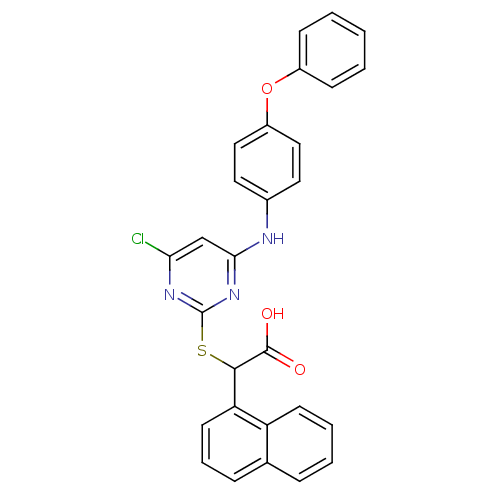
(2-(4-chloro-6-(4-phenoxyphenylamino)pyrimidin-2-yl...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(Oc3ccccc3)cc2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H20ClN3O3S/c29-24-17-25(30-19-13-15-21(16-14-19)35-20-9-2-1-3-10-20)32-28(31-24)36-26(27(33)34)23-12-6-8-18-7-4-5-11-22(18)23/h1-17,26H,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365629

(CHEMBL1957968)Show SMILES O=C(Nc1ccc(cc1)-c1nc2ccccn2c1NC1CCCCC1)c1cccs1 Show InChI InChI=1S/C24H24N4OS/c29-24(20-9-6-16-30-20)26-19-13-11-17(12-14-19)22-23(25-18-7-2-1-3-8-18)28-15-5-4-10-21(28)27-22/h4-6,9-16,18,25H,1-3,7-8H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human polymorphonuclear leukocytes assessed as reduction in A23187 and AA-stimulated 5-LO product formation preincuba... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50365629

(CHEMBL1957968)Show SMILES O=C(Nc1ccc(cc1)-c1nc2ccccn2c1NC1CCCCC1)c1cccs1 Show InChI InChI=1S/C24H24N4OS/c29-24(20-9-6-16-30-20)26-19-13-11-17(12-14-19)22-23(25-18-7-2-1-3-8-18)28-15-5-4-10-21(28)27-22/h4-6,9-16,18,25H,1-3,7-8H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/OSF Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in S100 cell free fraction of human polymorphonuclear leukocytes assessed as reduction in 5-LO product formation preincu... |
Bioorg Med Chem Lett 22: 1969-75 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.038
BindingDB Entry DOI: 10.7270/Q2P84CCK |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50337809
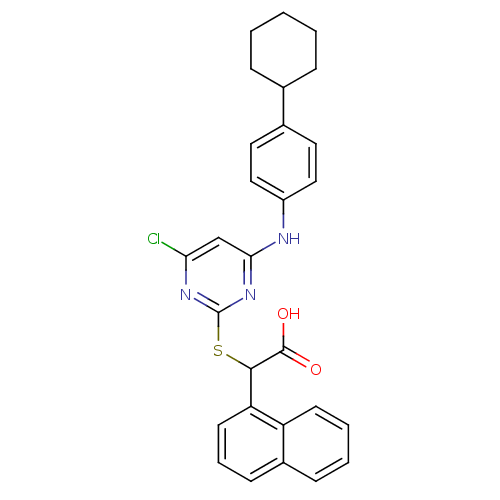
(2-(4-chloro-6-(4-cyclohexylphenylamino)pyrimidin-2...)Show SMILES OC(=O)C(Sc1nc(Cl)cc(Nc2ccc(cc2)C2CCCCC2)n1)c1cccc2ccccc12 Show InChI InChI=1S/C28H26ClN3O2S/c29-24-17-25(30-21-15-13-19(14-16-21)18-7-2-1-3-8-18)32-28(31-24)35-26(27(33)34)23-12-6-10-20-9-4-5-11-22(20)23/h4-6,9-18,26H,1-3,7-8H2,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human PMNL |
Bioorg Med Chem Lett 21: 1329-33 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.049
BindingDB Entry DOI: 10.7270/Q2WS8TJR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50322464
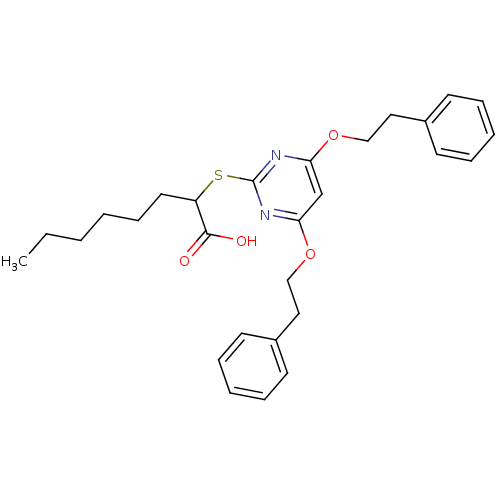
(2-(4,6-diphenethoxypyrimidin-2-ylthio)octanoic aci...)Show SMILES CCCCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C28H34N2O4S/c1-2-3-4-11-16-24(27(31)32)35-28-29-25(33-19-17-22-12-7-5-8-13-22)21-26(30-28)34-20-18-23-14-9-6-10-15-23/h5-10,12-15,21,24H,2-4,11,16-20H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase-mediated 5(S)-H(p)ETE formation in fMLP-stimulated human PMNL incubated 10 mins prior to fMLP challenge |
J Med Chem 54: 4490-507 (2011)
Article DOI: 10.1021/jm200092b
BindingDB Entry DOI: 10.7270/Q2XG9S8B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data