

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50234795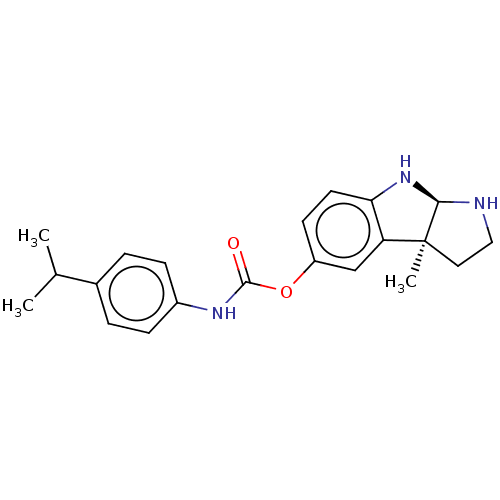 (CHEMBL4089082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234798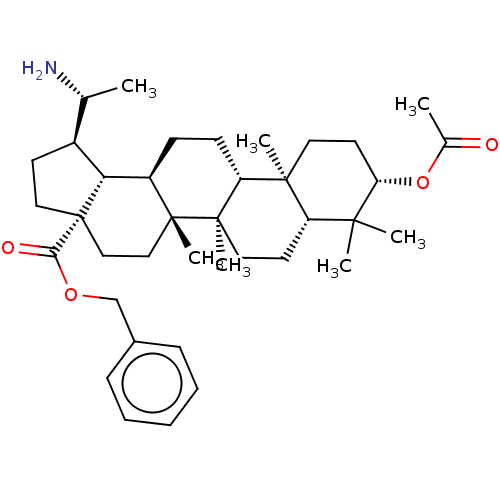 (CHEMBL4091899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234782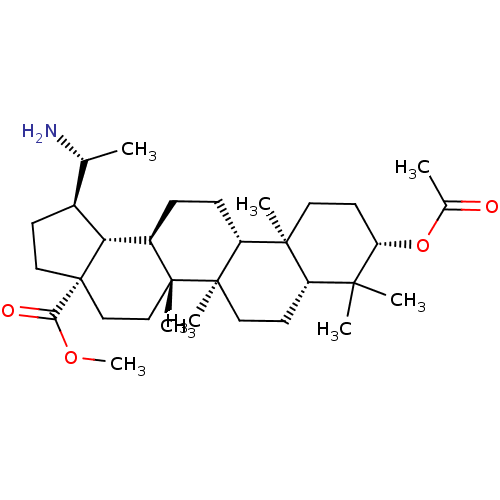 (CHEMBL4086293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234803 (CHEMBL4099629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234800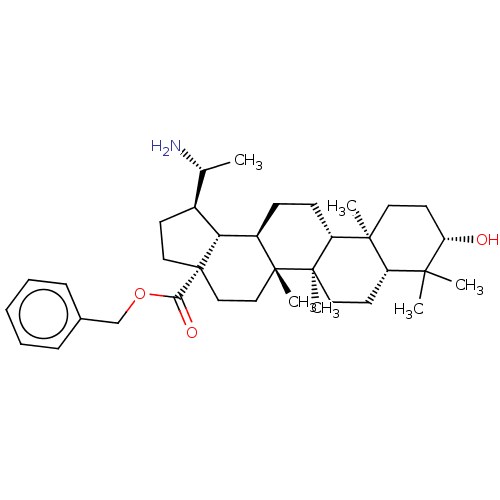 (CHEMBL4084140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234796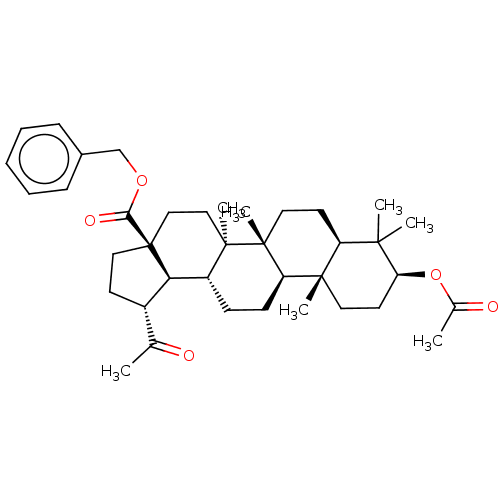 (CHEMBL4071069) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234782 (CHEMBL4086293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234799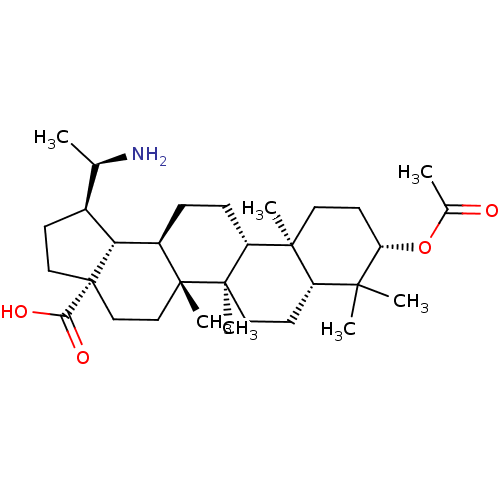 (CHEMBL4096275) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234780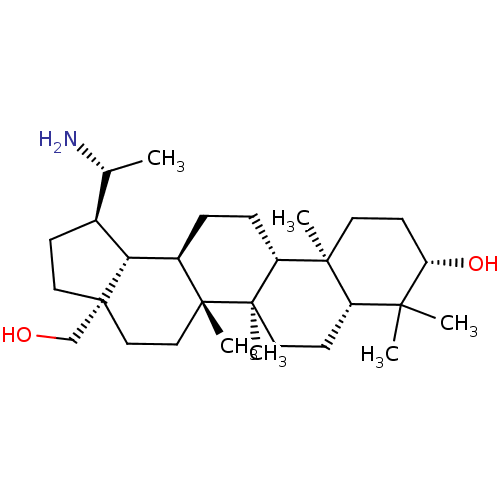 (CHEMBL4081627) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234800 (CHEMBL4084140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234796 (CHEMBL4071069) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition mea... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234783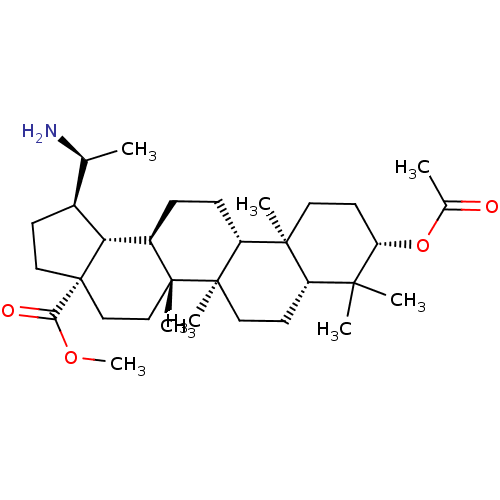 (CHEMBL4104205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234803 (CHEMBL4099629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234797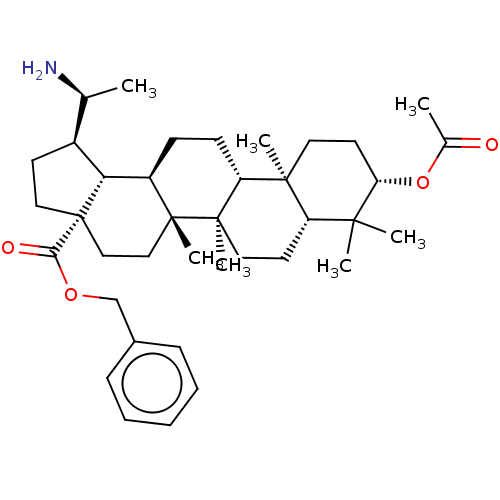 (CHEMBL4062429) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234798 (CHEMBL4091899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234787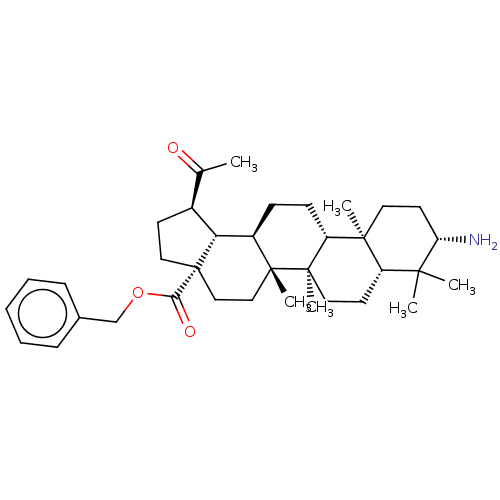 (CHEMBL4072365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234789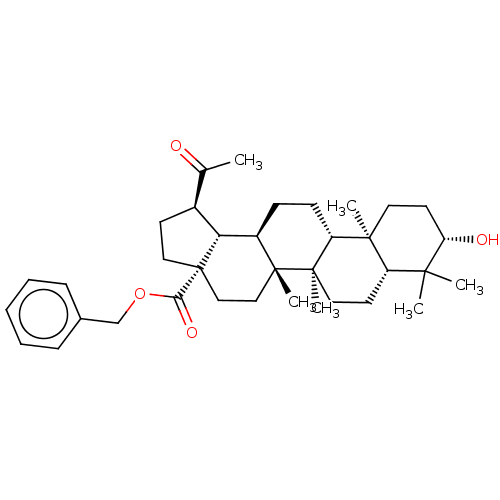 (CHEMBL4063629) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50058819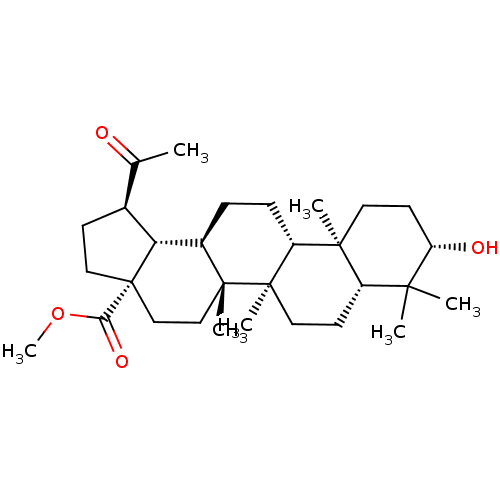 (CHEMBL3325723) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234788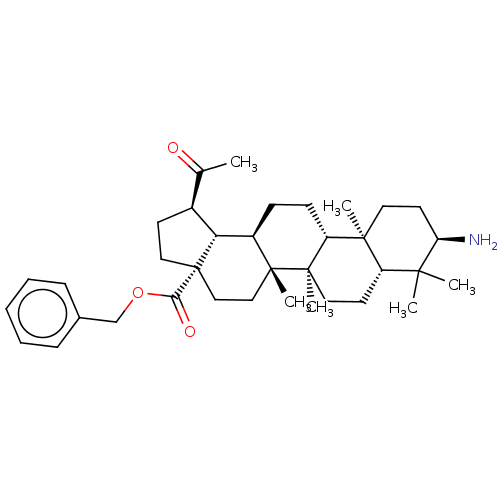 (CHEMBL4093124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234780 (CHEMBL4081627) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234802 (CHEMBL4085251) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234799 (CHEMBL4096275) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234792 (CHEMBL4100709) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234787 (CHEMBL4072365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234783 (CHEMBL4104205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50058819 (CHEMBL3325723) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234781 (CHEMBL4061347) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234782 (CHEMBL4086293) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234781 (CHEMBL4061347) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234789 (CHEMBL4063629) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition mea... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234803 (CHEMBL4099629) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234802 (CHEMBL4085251) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition mea... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234788 (CHEMBL4093124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234791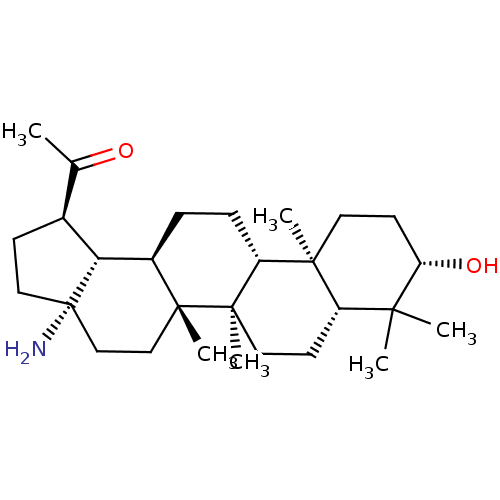 (CHEMBL4062563) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234786 (CHEMBL4075135) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234797 (CHEMBL4062429) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition me... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234792 (CHEMBL4100709) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition mea... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234805 (CHEMBL4063777) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234779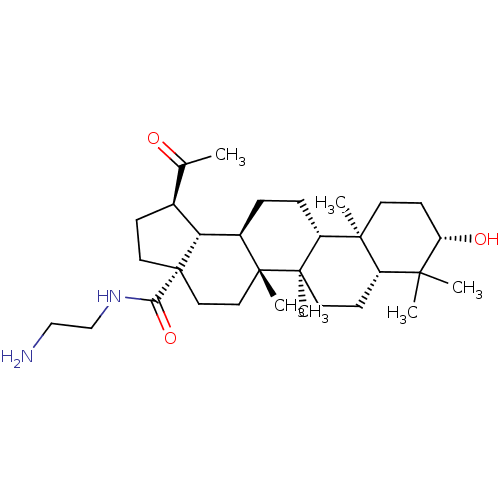 (CHEMBL4090532) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234793 (CHEMBL4082718) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234779 (CHEMBL4090532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234798 (CHEMBL4091899) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234794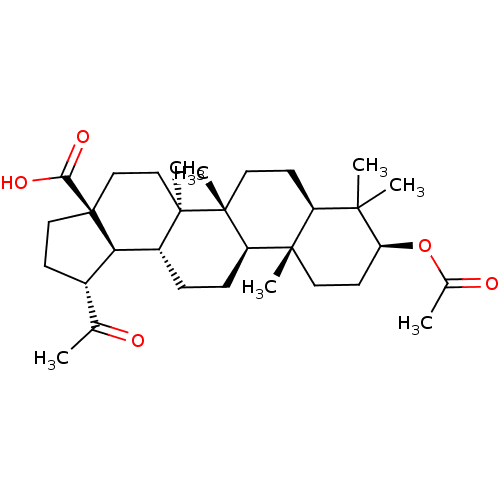 (CHEMBL4081749) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measu... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50234779 (CHEMBL4090532) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Uncompetitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition mea... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234805 (CHEMBL4063777) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234793 (CHEMBL4082718) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234789 (CHEMBL4063629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234802 (CHEMBL4085251) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |