 Found 59 hits with Last Name = 'makowski' and Initial = 'm'
Found 59 hits with Last Name = 'makowski' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380736
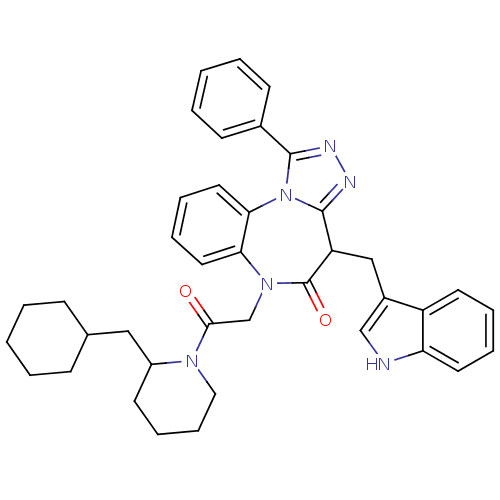
(CHEMBL2017828)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCCC1CC1CCCCC1 Show InChI InChI=1S/C39H42N6O2/c46-36(43-22-12-11-17-30(43)23-27-13-3-1-4-14-27)26-44-34-20-9-10-21-35(34)45-37(28-15-5-2-6-16-28)41-42-38(45)32(39(44)47)24-29-25-40-33-19-8-7-18-31(29)33/h2,5-10,15-16,18-21,25,27,30,32,40H,1,3-4,11-14,17,22-24,26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380725

(CHEMBL2017832)Show SMILES COCCC1CCCCN1C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2n[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C34H35N7O3/c1-44-20-18-24-13-9-10-19-39(24)31(42)22-40-29-16-7-8-17-30(29)41-32(23-11-3-2-4-12-23)37-38-33(41)26(34(40)43)21-28-25-14-5-6-15-27(25)35-36-28/h2-8,11-12,14-17,24,26H,9-10,13,18-22H2,1H3,(H,35,36)/t24?,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338523
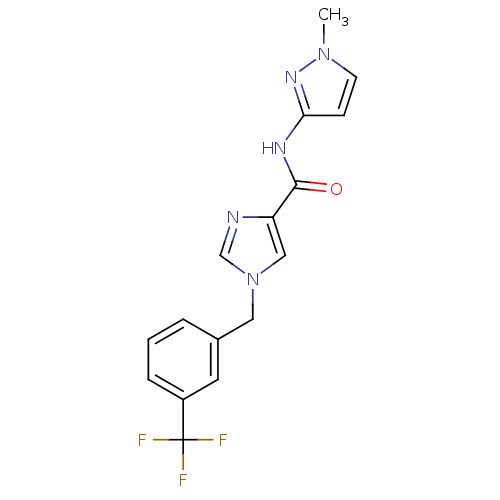
(CHEMBL1683578 | N-(1-methyl-1H-pyrazol-3-yl)-1-(3-...)Show SMILES Cn1ccc(NC(=O)c2cn(Cc3cccc(c3)C(F)(F)F)cn2)n1 Show InChI InChI=1S/C16H14F3N5O/c1-23-6-5-14(22-23)21-15(25)13-9-24(10-20-13)8-11-3-2-4-12(7-11)16(17,18)19/h2-7,9-10H,8H2,1H3,(H,21,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338522
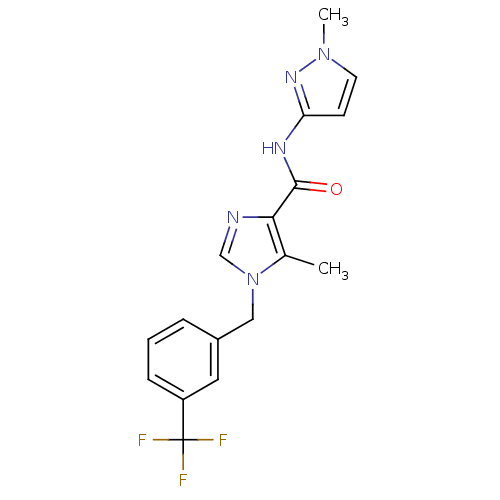
(5-methyl-N-(1-methyl-1H-pyrazol-3-yl)-1-(3-(triflu...)Show SMILES Cc1c(ncn1Cc1cccc(c1)C(F)(F)F)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C17H16F3N5O/c1-11-15(16(26)22-14-6-7-24(2)23-14)21-10-25(11)9-12-4-3-5-13(8-12)17(18,19)20/h3-8,10H,9H2,1-2H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50380728
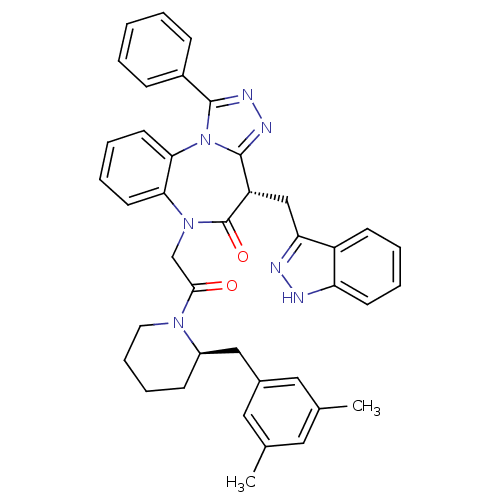
(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from rat CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338524
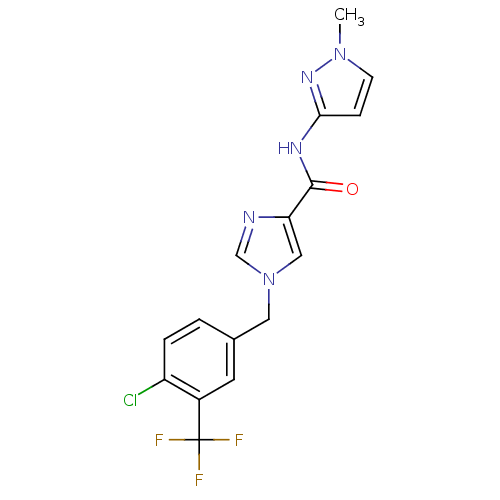
(1-(4-chloro-3-(trifluoromethyl)benzyl)-N-(1-methyl...)Show SMILES Cn1ccc(NC(=O)c2cn(Cc3ccc(Cl)c(c3)C(F)(F)F)cn2)n1 Show InChI InChI=1S/C16H13ClF3N5O/c1-24-5-4-14(23-24)22-15(26)13-8-25(9-21-13)7-10-2-3-12(17)11(6-10)16(18,19)20/h2-6,8-9H,7H2,1H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338525
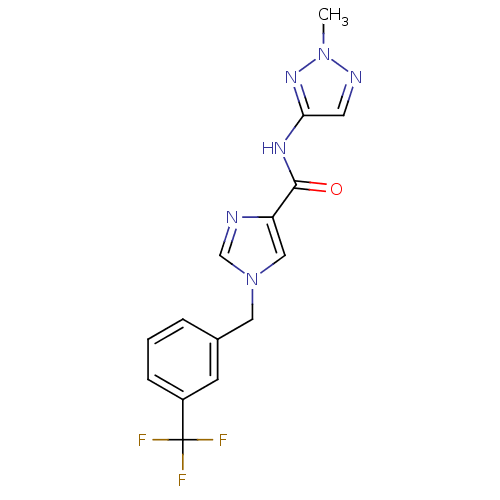
(CHEMBL1683580 | N-(2-methyl-2H-1,2,3-triazol-4-yl)...)Show SMILES Cn1ncc(NC(=O)c2cn(Cc3cccc(c3)C(F)(F)F)cn2)n1 Show InChI InChI=1S/C15H13F3N6O/c1-23-20-6-13(22-23)21-14(25)12-8-24(9-19-12)7-10-3-2-4-11(5-10)15(16,17)18/h2-6,8-9H,7H2,1H3,(H,21,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338521
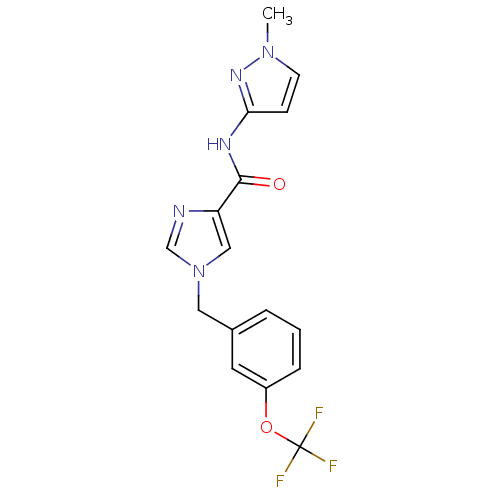
(CHEMBL1683576 | N-(1-methyl-1H-pyrazol-3-yl)-1-(3-...)Show SMILES Cn1ccc(NC(=O)c2cn(Cc3cccc(OC(F)(F)F)c3)cn2)n1 Show InChI InChI=1S/C16H14F3N5O2/c1-23-6-5-14(22-23)21-15(25)13-9-24(10-20-13)8-11-3-2-4-12(7-11)26-16(17,18)19/h2-7,9-10H,8H2,1H3,(H,21,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338532

(1-(4-chloro-3-(trifluoromethyl)benzyl)-N-(1-methyl...)Show SMILES Cn1ccc(NC(=O)c2cn(Cc3ccc(Cl)c(c3)C(F)(F)F)nn2)n1 Show InChI InChI=1S/C15H12ClF3N6O/c1-24-5-4-13(22-24)20-14(26)12-8-25(23-21-12)7-9-2-3-11(16)10(6-9)15(17,18)19/h2-6,8H,7H2,1H3,(H,20,22,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380726

(CHEMBL2017833)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2n[nH]c3ccccc23)C1=O)N1CCCCC1Cc1ccccc1 |r| Show InChI InChI=1S/C38H35N7O2/c46-35(43-22-12-11-17-28(43)23-26-13-3-1-4-14-26)25-44-33-20-9-10-21-34(33)45-36(27-15-5-2-6-16-27)41-42-37(45)30(38(44)47)24-32-29-18-7-8-19-31(29)39-40-32/h1-10,13-16,18-21,28,30H,11-12,17,22-25H2,(H,39,40)/t28?,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380730

(CHEMBL2017691)Show SMILES CCC1CCCCN1C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C34H34N6O2/c1-2-25-14-10-11-19-38(25)31(41)22-39-29-17-8-9-18-30(29)40-32(23-12-4-3-5-13-23)36-37-33(40)27(34(39)42)20-24-21-35-28-16-7-6-15-26(24)28/h3-9,12-13,15-18,21,25,27,35H,2,10-11,14,19-20,22H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380724
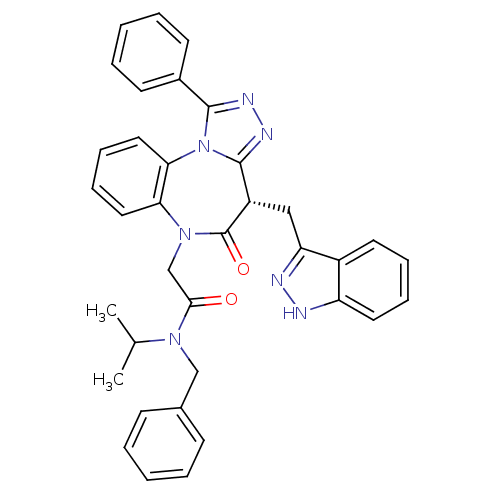
(CHEMBL2017831)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2n[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C36H33N7O2/c1-24(2)41(22-25-13-5-3-6-14-25)33(44)23-42-31-19-11-12-20-32(31)43-34(26-15-7-4-8-16-26)39-40-35(43)28(36(42)45)21-30-27-17-9-10-18-29(27)37-38-30/h3-20,24,28H,21-23H2,1-2H3,(H,37,38)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380735

(CHEMBL2017827)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCCC1Cc1ccccc1 Show InChI InChI=1S/C39H36N6O2/c46-36(43-22-12-11-17-30(43)23-27-13-3-1-4-14-27)26-44-34-20-9-10-21-35(34)45-37(28-15-5-2-6-16-28)41-42-38(45)32(39(44)47)24-29-25-40-33-19-8-7-18-31(29)33/h1-10,13-16,18-21,25,30,32,40H,11-12,17,22-24,26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380729
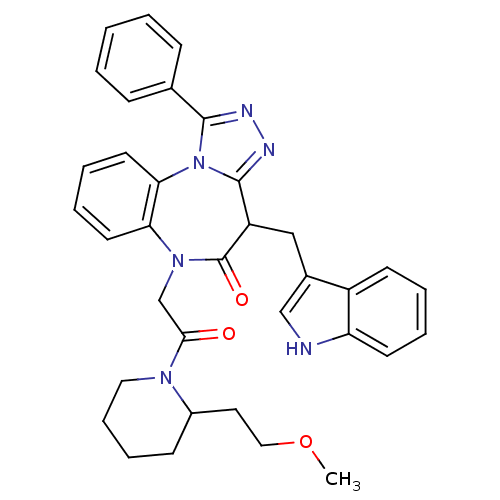
(CHEMBL2017690)Show SMILES COCCC1CCCCN1C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C35H36N6O3/c1-44-20-18-26-13-9-10-19-39(26)32(42)23-40-30-16-7-8-17-31(30)41-33(24-11-3-2-4-12-24)37-38-34(41)28(35(40)43)21-25-22-36-29-15-6-5-14-27(25)29/h2-8,11-12,14-17,22,26,28,36H,9-10,13,18-21,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338526
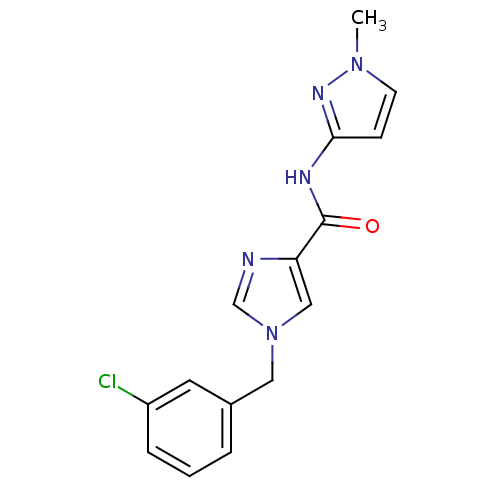
(1-(3-chlorobenzyl)-N-(1-methyl-1H-pyrazol-3-yl)-1H...)Show InChI InChI=1S/C15H14ClN5O/c1-20-6-5-14(19-20)18-15(22)13-9-21(10-17-13)8-11-3-2-4-12(16)7-11/h2-7,9-10H,8H2,1H3,(H,18,19,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338531
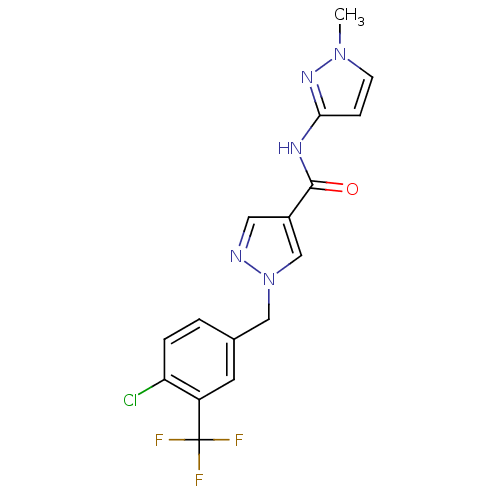
(1-(4-chloro-3-(trifluoromethyl)benzyl)-N-(1-methyl...)Show SMILES Cn1ccc(NC(=O)c2cnn(Cc3ccc(Cl)c(c3)C(F)(F)F)c2)n1 Show InChI InChI=1S/C16H13ClF3N5O/c1-24-5-4-14(23-24)22-15(26)11-7-21-25(9-11)8-10-2-3-13(17)12(6-10)16(18,19)20/h2-7,9H,8H2,1H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338527
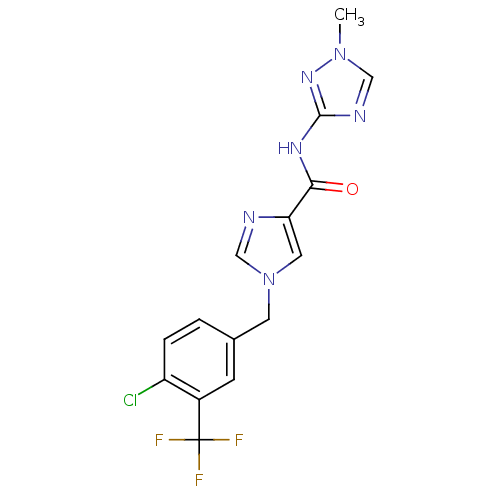
(1-(4-chloro-3-(trifluoromethyl)benzyl)-N-(1-methyl...)Show SMILES Cn1cnc(NC(=O)c2cn(Cc3ccc(Cl)c(c3)C(F)(F)F)cn2)n1 Show InChI InChI=1S/C15H12ClF3N6O/c1-24-7-21-14(23-24)22-13(26)12-6-25(8-20-12)5-9-2-3-11(16)10(4-9)15(17,18)19/h2-4,6-8H,5H2,1H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338520
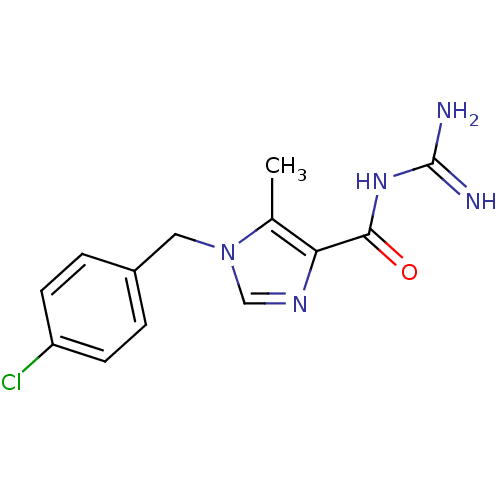
(1-(4-chlorobenzyl)-N-(diaminomethylene)-5-methyl-1...)Show InChI InChI=1S/C13H14ClN5O/c1-8-11(12(20)18-13(15)16)17-7-19(8)6-9-2-4-10(14)5-3-9/h2-5,7H,6H2,1H3,(H4,15,16,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 assessed as formation of deuterated oleoylCoA by HTMS assay |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380731
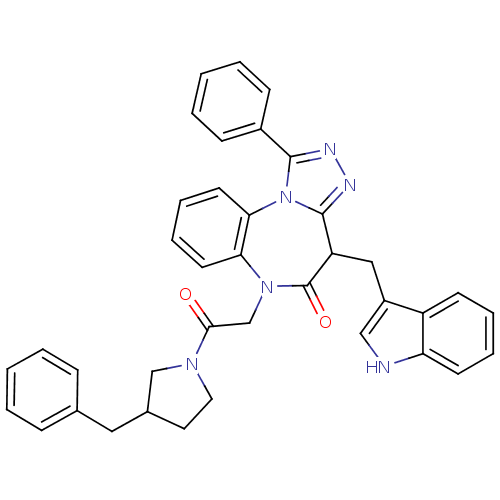
(CHEMBL2017692)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCC(Cc2ccccc2)C1 Show InChI InChI=1S/C38H34N6O2/c45-35(42-20-19-27(24-42)21-26-11-3-1-4-12-26)25-43-33-17-9-10-18-34(33)44-36(28-13-5-2-6-14-28)40-41-37(44)31(38(43)46)22-29-23-39-32-16-8-7-15-30(29)32/h1-18,23,27,31,39H,19-22,24-25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380734
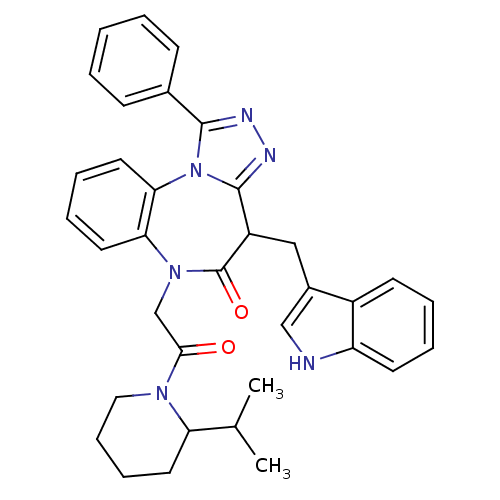
(CHEMBL2017826)Show SMILES CC(C)C1CCCCN1C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C35H36N6O2/c1-23(2)29-16-10-11-19-39(29)32(42)22-40-30-17-8-9-18-31(30)41-33(24-12-4-3-5-13-24)37-38-34(41)27(35(40)43)20-25-21-36-28-15-7-6-14-26(25)28/h3-9,12-15,17-18,21,23,27,29,36H,10-11,16,19-20,22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380727
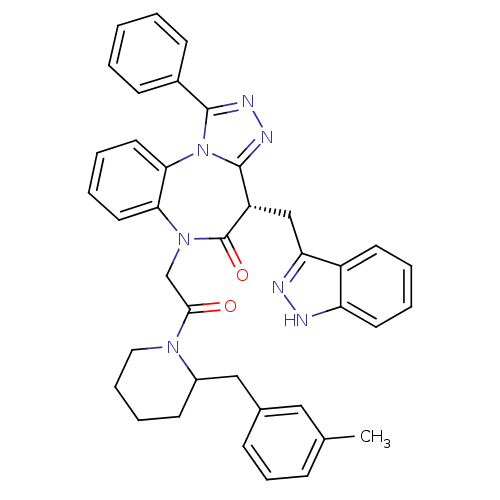
(CHEMBL2017834)Show SMILES Cc1cccc(CC2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C39H37N7O2/c1-26-12-11-13-27(22-26)23-29-16-9-10-21-44(29)36(47)25-45-34-19-7-8-20-35(34)46-37(28-14-3-2-4-15-28)42-43-38(46)31(39(45)48)24-33-30-17-5-6-18-32(30)40-41-33/h2-8,11-15,17-20,22,29,31H,9-10,16,21,23-25H2,1H3,(H,40,41)/t29?,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50338521

(CHEMBL1683576 | N-(1-methyl-1H-pyrazol-3-yl)-1-(3-...)Show SMILES Cn1ccc(NC(=O)c2cn(Cc3cccc(OC(F)(F)F)c3)cn2)n1 Show InChI InChI=1S/C16H14F3N5O2/c1-23-6-5-14(22-23)21-15(25)13-9-24(10-20-13)8-11-3-2-4-12(7-11)26-16(17,18)19/h2-7,9-10H,8H2,1H3,(H,21,22,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SCD1 |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380722

(CHEMBL2017829)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCCC1Cc1ccccn1 Show InChI InChI=1S/C38H35N7O2/c46-35(43-21-11-9-15-29(43)23-28-14-8-10-20-39-28)25-44-33-18-6-7-19-34(33)45-36(26-12-2-1-3-13-26)41-42-37(45)31(38(44)47)22-27-24-40-32-17-5-4-16-30(27)32/h1-8,10,12-14,16-20,24,29,31,40H,9,11,15,21-23,25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338520

(1-(4-chlorobenzyl)-N-(diaminomethylene)-5-methyl-1...)Show InChI InChI=1S/C13H14ClN5O/c1-8-11(12(20)18-13(15)16)17-7-19(8)6-9-2-4-10(14)5-3-9/h2-5,7H,6H2,1H3,(H4,15,16,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338528
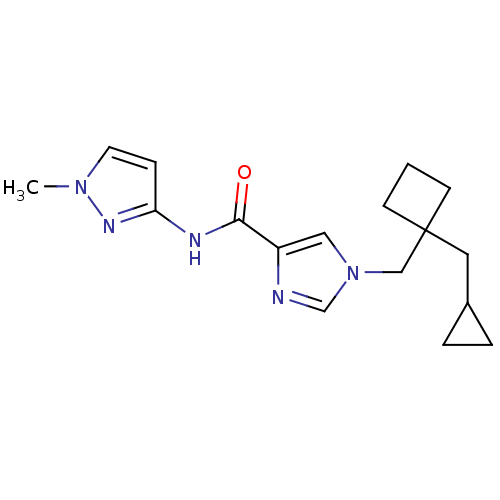
(1-((1-(cyclopropylmethyl)cyclobutyl)methyl)-N-(1-m...)Show InChI InChI=1S/C17H23N5O/c1-21-8-5-15(20-21)19-16(23)14-10-22(12-18-14)11-17(6-2-7-17)9-13-3-4-13/h5,8,10,12-13H,2-4,6-7,9,11H2,1H3,(H,19,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338533
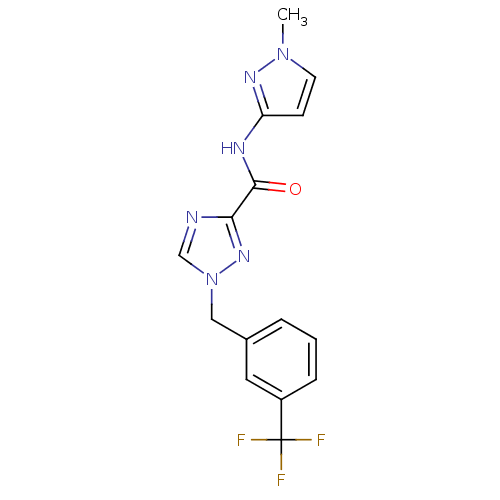
(CHEMBL1683588 | N-(1-methyl-1H-pyrazol-3-yl)-1-(3-...)Show SMILES Cn1ccc(NC(=O)c2ncn(Cc3cccc(c3)C(F)(F)F)n2)n1 Show InChI InChI=1S/C15H13F3N6O/c1-23-6-5-12(21-23)20-14(25)13-19-9-24(22-13)8-10-3-2-4-11(7-10)15(16,17)18/h2-7,9H,8H2,1H3,(H,20,21,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338529
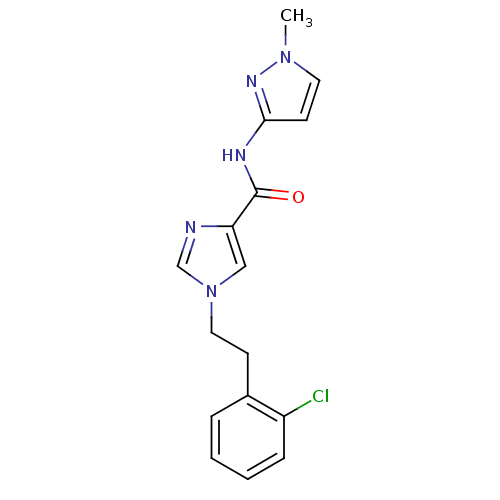
(1-(2-chlorophenethyl)-N-(1-methyl-1H-pyrazol-3-yl)...)Show InChI InChI=1S/C16H16ClN5O/c1-21-8-7-15(20-21)19-16(23)14-10-22(11-18-14)9-6-12-4-2-3-5-13(12)17/h2-5,7-8,10-11H,6,9H2,1H3,(H,19,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380721
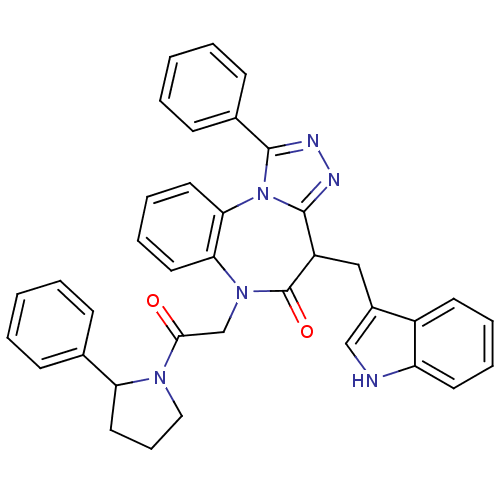
(CHEMBL2017825)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCC1c1ccccc1 Show InChI InChI=1S/C37H32N6O2/c44-34(41-21-11-20-31(41)25-12-3-1-4-13-25)24-42-32-18-9-10-19-33(32)43-35(26-14-5-2-6-15-26)39-40-36(43)29(37(42)45)22-27-23-38-30-17-8-7-16-28(27)30/h1-10,12-19,23,29,31,38H,11,20-22,24H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50338520

(1-(4-chlorobenzyl)-N-(diaminomethylene)-5-methyl-1...)Show InChI InChI=1S/C13H14ClN5O/c1-8-11(12(20)18-13(15)16)17-7-19(8)6-9-2-4-10(14)5-3-9/h2-5,7H,6H2,1H3,(H4,15,16,18,20) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SCD1 |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380733

(CHEMBL2017824)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCCC1c1ccccc1 Show InChI InChI=1S/C38H34N6O2/c45-35(42-22-12-11-19-32(42)26-13-3-1-4-14-26)25-43-33-20-9-10-21-34(33)44-36(27-15-5-2-6-16-27)40-41-37(44)30(38(43)46)23-28-24-39-31-18-8-7-17-29(28)31/h1-10,13-18,20-21,24,30,32,39H,11-12,19,22-23,25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380723

(CHEMBL2017830)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCCC1Cc1ccncc1 Show InChI InChI=1S/C38H35N7O2/c46-35(43-21-9-8-12-29(43)22-26-17-19-39-20-18-26)25-44-33-15-6-7-16-34(33)45-36(27-10-2-1-3-11-27)41-42-37(45)31(38(44)47)23-28-24-40-32-14-5-4-13-30(28)32/h1-7,10-11,13-20,24,29,31,40H,8-9,12,21-23,25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380732

(CHEMBL2017823)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCC1Cc1ccccn1 Show InChI InChI=1S/C37H33N7O2/c45-34(42-20-10-14-28(42)22-27-13-8-9-19-38-27)24-43-32-17-6-7-18-33(32)44-35(25-11-2-1-3-12-25)40-41-36(44)30(37(43)46)21-26-23-39-31-16-5-4-15-29(26)31/h1-9,11-13,15-19,23,28,30,39H,10,14,20-22,24H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-2 from human CCK1 receptor expressed in CHO cells after 90 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338534
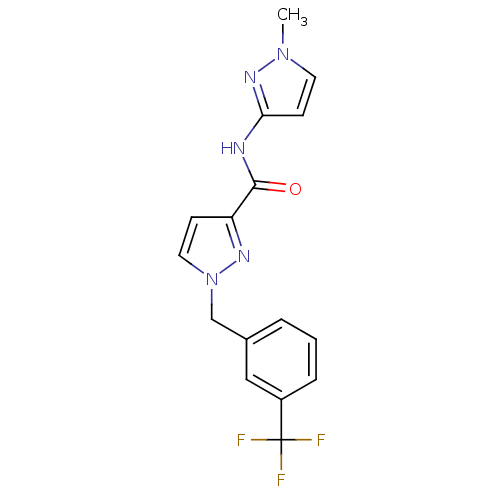
(CHEMBL1683589 | N-(1-methyl-1H-pyrazol-3-yl)-1-(3-...)Show SMILES Cn1ccc(NC(=O)c2ccn(Cc3cccc(c3)C(F)(F)F)n2)n1 Show InChI InChI=1S/C16H14F3N5O/c1-23-7-6-14(22-23)20-15(25)13-5-8-24(21-13)10-11-3-2-4-12(9-11)16(17,18)19/h2-9H,10H2,1H3,(H,20,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Rattus norvegicus (Rat)) | BDBM50338530
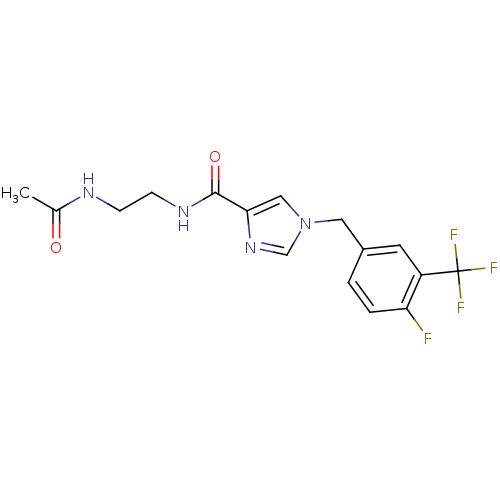
(CHEMBL1683585 | N-(2-acetamidoethyl)-1-(4-fluoro-3...)Show SMILES CC(=O)NCCNC(=O)c1cn(Cc2ccc(F)c(c2)C(F)(F)F)cn1 Show InChI InChI=1S/C16H16F4N4O2/c1-10(25)21-4-5-22-15(26)14-8-24(9-23-14)7-11-2-3-13(17)12(6-11)16(18,19)20/h2-3,6,8-9H,4-5,7H2,1H3,(H,21,25)(H,22,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver SCD1 using [3H]-stearoylCoA |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50338522

(5-methyl-N-(1-methyl-1H-pyrazol-3-yl)-1-(3-(triflu...)Show SMILES Cc1c(ncn1Cc1cccc(c1)C(F)(F)F)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C17H16F3N5O/c1-11-15(16(26)22-14-6-7-24(2)23-14)21-10-25(11)9-12-4-3-5-13(8-12)17(18,19)20/h3-8,10H,9H2,1-2H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50338522

(5-methyl-N-(1-methyl-1H-pyrazol-3-yl)-1-(3-(triflu...)Show SMILES Cc1c(ncn1Cc1cccc(c1)C(F)(F)F)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C17H16F3N5O/c1-11-15(16(26)22-14-6-7-24(2)23-14)21-10-25(11)9-12-4-3-5-13(8-12)17(18,19)20/h3-8,10H,9H2,1-2H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338522

(5-methyl-N-(1-methyl-1H-pyrazol-3-yl)-1-(3-(triflu...)Show SMILES Cc1c(ncn1Cc1cccc(c1)C(F)(F)F)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C17H16F3N5O/c1-11-15(16(26)22-14-6-7-24(2)23-14)21-10-25(11)9-12-4-3-5-13(8-12)17(18,19)20/h3-8,10H,9H2,1-2H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50338522

(5-methyl-N-(1-methyl-1H-pyrazol-3-yl)-1-(3-(triflu...)Show SMILES Cc1c(ncn1Cc1cccc(c1)C(F)(F)F)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C17H16F3N5O/c1-11-15(16(26)22-14-6-7-24(2)23-14)21-10-25(11)9-12-4-3-5-13(8-12)17(18,19)20/h3-8,10H,9H2,1-2H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50338522

(5-methyl-N-(1-methyl-1H-pyrazol-3-yl)-1-(3-(triflu...)Show SMILES Cc1c(ncn1Cc1cccc(c1)C(F)(F)F)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C17H16F3N5O/c1-11-15(16(26)22-14-6-7-24(2)23-14)21-10-25(11)9-12-4-3-5-13(8-12)17(18,19)20/h3-8,10H,9H2,1-2H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50338522

(5-methyl-N-(1-methyl-1H-pyrazol-3-yl)-1-(3-(triflu...)Show SMILES Cc1c(ncn1Cc1cccc(c1)C(F)(F)F)C(=O)Nc1ccn(C)n1 Show InChI InChI=1S/C17H16F3N5O/c1-11-15(16(26)22-14-6-7-24(2)23-14)21-10-25(11)9-12-4-3-5-13(8-12)17(18,19)20/h3-8,10H,9H2,1-2H3,(H,22,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem Lett 21: 1621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.113
BindingDB Entry DOI: 10.7270/Q2571C9J |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380736

(CHEMBL2017828)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCCC1CC1CCCCC1 Show InChI InChI=1S/C39H42N6O2/c46-36(43-22-12-11-17-30(43)23-27-13-3-1-4-14-27)26-44-34-20-9-10-21-35(34)45-37(28-15-5-2-6-16-28)41-42-38(45)32(39(44)47)24-29-25-40-33-19-8-7-18-31(29)33/h2,5-10,15-16,18-21,25,27,30,32,40H,1,3-4,11-14,17,22-24,26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 256 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati... |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380735

(CHEMBL2017827)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCCC1Cc1ccccc1 Show InChI InChI=1S/C39H36N6O2/c46-36(43-22-12-11-17-30(43)23-27-13-3-1-4-14-27)26-44-34-20-9-10-21-35(34)45-37(28-15-5-2-6-16-28)41-42-38(45)32(39(44)47)24-29-25-40-33-19-8-7-18-31(29)33/h1-10,13-16,18-21,25,30,32,40H,11-12,17,22-24,26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 84.2 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati... |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380734

(CHEMBL2017826)Show SMILES CC(C)C1CCCCN1C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C35H36N6O2/c1-23(2)29-16-10-11-19-39(29)32(42)22-40-30-17-8-9-18-31(30)41-33(24-12-4-3-5-13-24)37-38-34(41)27(35(40)43)20-25-21-36-28-15-7-6-14-26(25)28/h3-9,12-15,17-18,21,23,27,29,36H,10-11,16,19-20,22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 494 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati... |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380733

(CHEMBL2017824)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCCC1c1ccccc1 Show InChI InChI=1S/C38H34N6O2/c45-35(42-22-12-11-19-32(42)26-13-3-1-4-14-26)25-43-33-20-9-10-21-34(33)44-36(27-15-5-2-6-16-27)40-41-37(44)30(38(43)46)23-28-24-39-31-18-8-7-17-29(28)31/h1-10,13-18,20-21,24,30,32,39H,11-12,19,22-23,25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati... |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380732

(CHEMBL2017823)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCCC1Cc1ccccn1 Show InChI InChI=1S/C37H33N7O2/c45-34(42-20-10-14-28(42)22-27-13-8-9-19-38-27)24-43-32-17-6-7-18-33(32)44-35(25-11-2-1-3-12-25)40-41-36(44)30(37(43)46)21-26-23-39-31-16-5-4-15-29(26)31/h1-9,11-13,15-19,23,28,30,39H,10,14,20-22,24H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati... |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380731

(CHEMBL2017692)Show SMILES O=C(CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O)N1CCC(Cc2ccccc2)C1 Show InChI InChI=1S/C38H34N6O2/c45-35(42-20-19-27(24-42)21-26-11-3-1-4-12-26)25-43-33-17-9-10-18-34(33)44-36(28-13-5-2-6-14-28)40-41-37(44)31(38(43)46)22-29-23-39-32-16-8-7-15-30(29)32/h1-18,23,27,31,39H,19-22,24-25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati... |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380730

(CHEMBL2017691)Show SMILES CCC1CCCCN1C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C34H34N6O2/c1-2-25-14-10-11-19-38(25)31(41)22-39-29-17-8-9-18-30(29)40-32(23-12-4-3-5-13-23)36-37-33(40)27(34(39)42)20-24-21-35-28-16-7-6-15-26(24)28/h3-9,12-13,15-18,21,25,27,35H,2,10-11,14,19-20,22H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati... |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380729

(CHEMBL2017690)Show SMILES COCCC1CCCCN1C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)C(Cc2c[nH]c3ccccc23)C1=O Show InChI InChI=1S/C35H36N6O3/c1-44-20-18-26-13-9-10-19-39(26)32(42)23-40-30-16-7-8-17-31(30)41-33(24-11-3-2-4-12-24)37-38-34(41)28(35(40)43)21-25-22-36-29-15-6-5-14-27(25)29/h2-8,11-12,14-17,22,26,28,36H,9-10,13,18-21,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 52.5 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor assessed as increase in CCK8-induced calcium release incubated in dark for 30 mins followed by light incubati... |
Bioorg Med Chem Lett 22: 2943-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.049
BindingDB Entry DOI: 10.7270/Q2K35VP4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data