

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485494 (CHEMBL2063089) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485491 (CHEMBL2063088) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485490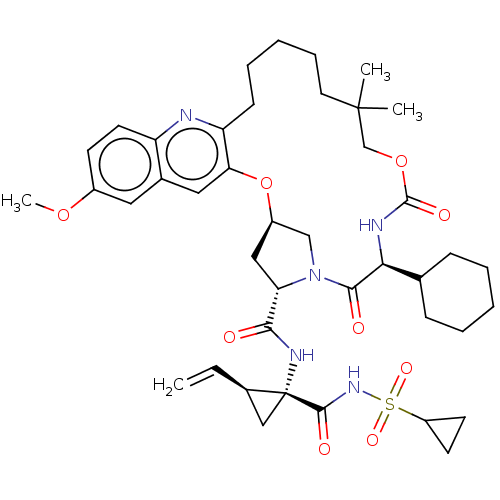 (CHEMBL2063087) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485495 (CHEMBL2063085) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485493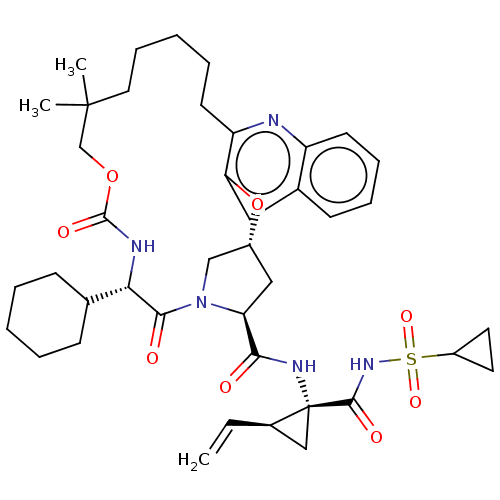 (CHEMBL2063086) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485490 (CHEMBL2063087) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485495 (CHEMBL2063085) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485493 (CHEMBL2063086) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156T mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50485489 (CHEMBL1672609 | MK-1220) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease A156V mutant expressed in Escherichia coli by time-resolved fluorescence ana... | ACS Med Chem Lett 3: 332-6 (2012) Article DOI: 10.1021/ml300017p BindingDB Entry DOI: 10.7270/Q2KH0R6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278725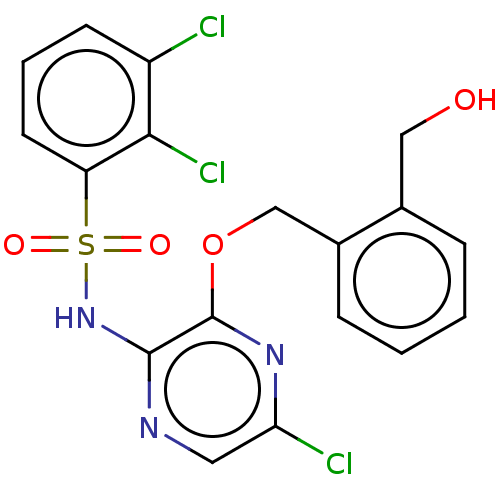 (CHEMBL4172769) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278758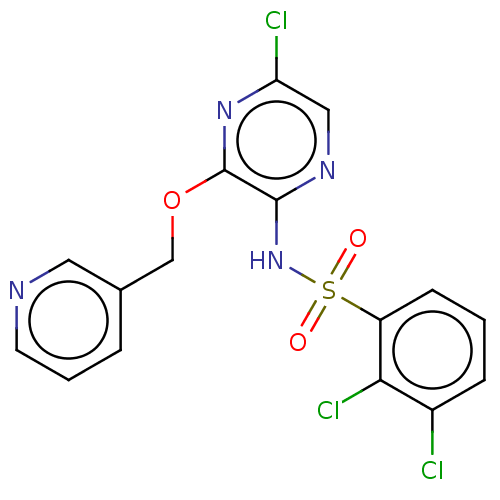 (CHEMBL4167445) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50278798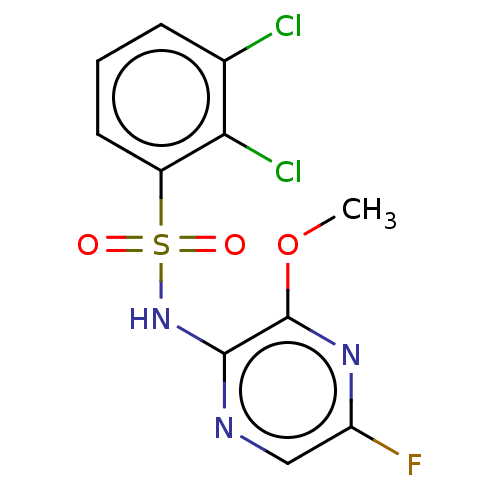 (CHEMBL4172635) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278799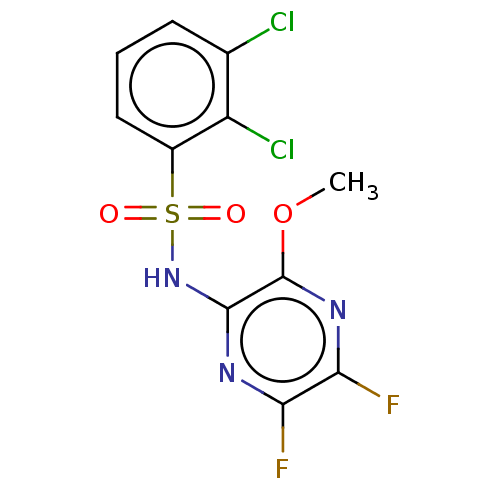 (CHEMBL4162364) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278798 (CHEMBL4172635) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278793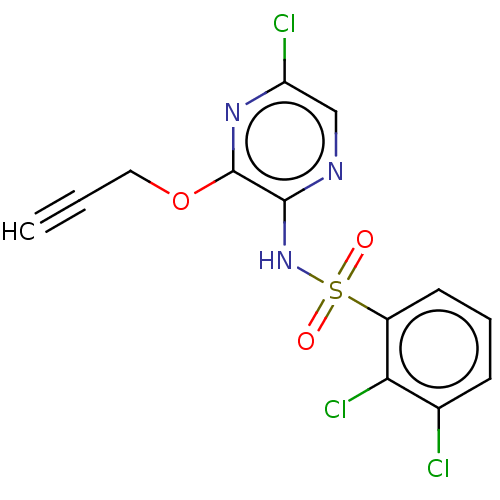 (CHEMBL4160218) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Canis lupus familiaris) | BDBM50278798 (CHEMBL4172635) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278727 (CHEMBL4173046) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278798 (CHEMBL4172635) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278794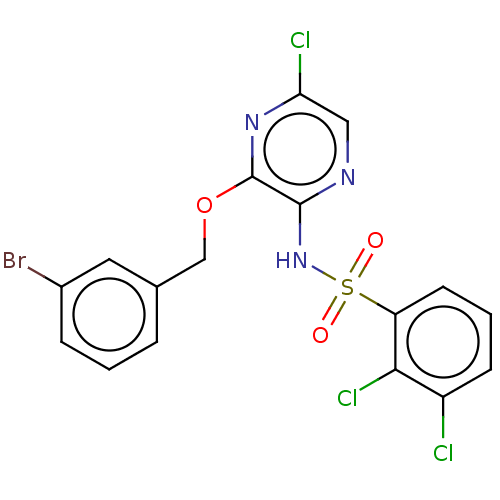 (CHEMBL4175323) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278797 (CHEMBL4160846) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro Binding affinity towards Nicotinic acetylcholine receptor alpha4-beta2 was determined | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50378979 (CHEMBL2011441) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at mouse CCR4 | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278787 (CHEMBL4173861) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278801 (CHEMBL4165356) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278785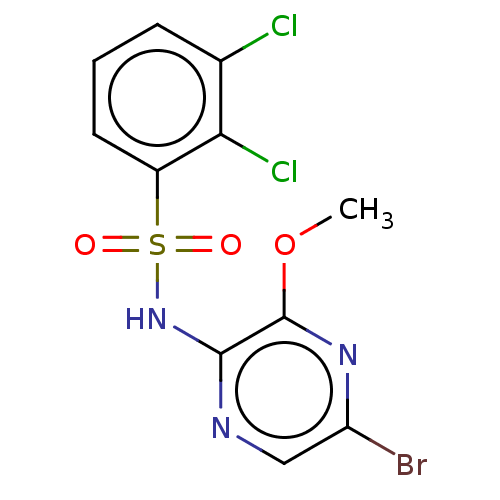 (CHEMBL4170431) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50278724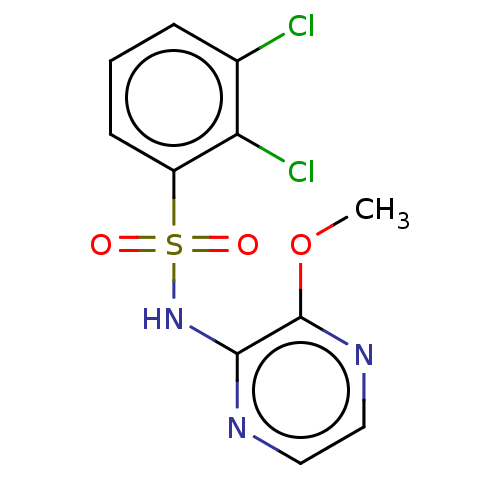 (CHEMBL4169247) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278800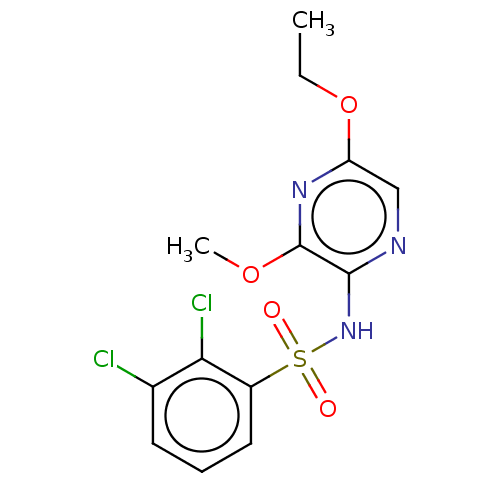 (CHEMBL4175972) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278723 (CHEMBL4162097) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278786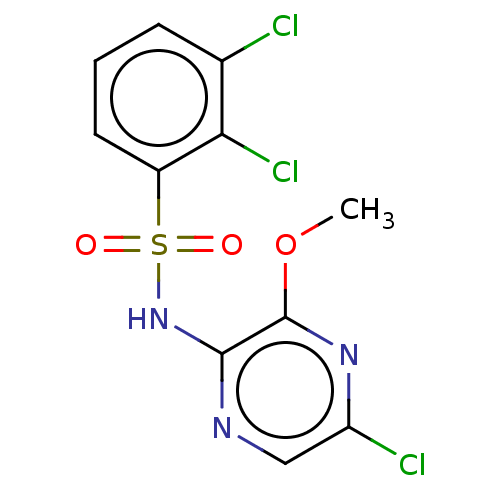 (CHEMBL4165939) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Effective concentration required to inhibit HIV-1 induced cytopathicity by 50% in CEM cells | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278724 (CHEMBL4169247) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278749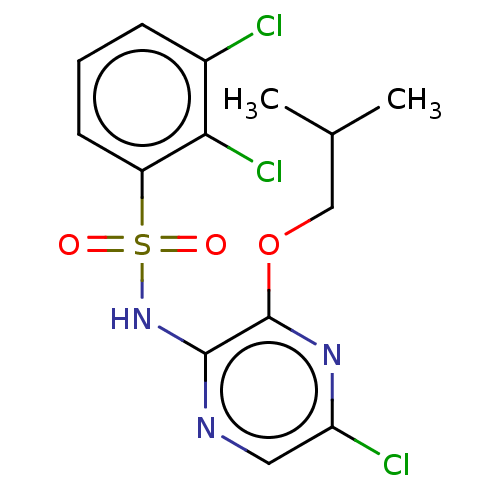 (CHEMBL4170833) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278738 (CHEMBL4162555) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278802 (CHEMBL4164738) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278761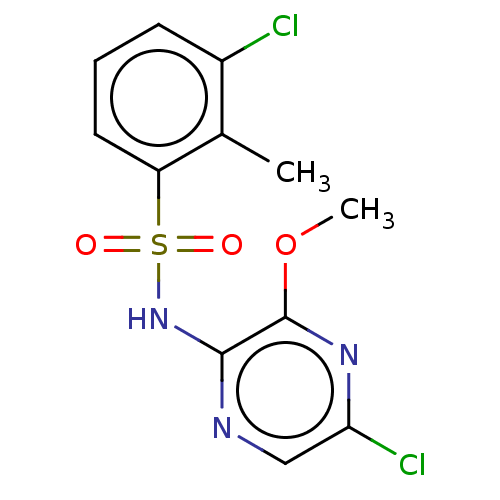 (CHEMBL4168237) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278759 (CHEMBL4168775) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Canis lupus familiaris) | BDBM50278724 (CHEMBL4169247) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of NADH oxidase activity in sub-mitochondrial particles from bovine heart | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278724 (CHEMBL4169247) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in CHO cells coexpressing CD4+ assessed as inhibition of CCL22 induced Ca2+ mobilization | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278750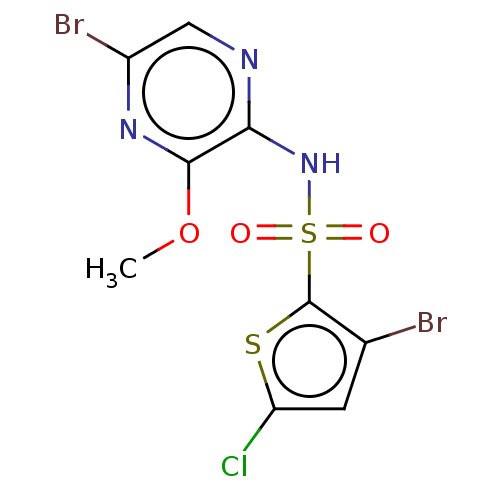 (CHEMBL4160139) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at recombinant human CCR4 expressed in CHO-K1 cells assessed as inhibition of CCL22 induced Ca2+ mobilization after 2 hrs by FMAT... | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50278726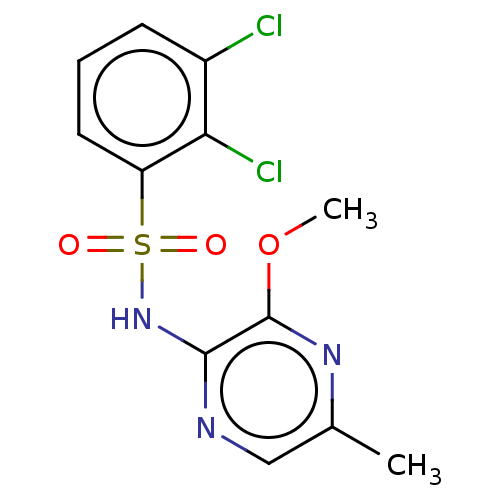 (CHEMBL4172568) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) | ACS Med Chem Lett 8: 981-986 (2017) Article DOI: 10.1021/acsmedchemlett.7b00315 BindingDB Entry DOI: 10.7270/Q2KD21DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |