 Found 616 hits with Last Name = 'mogemark' and Initial = 'm'
Found 616 hits with Last Name = 'mogemark' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50274638
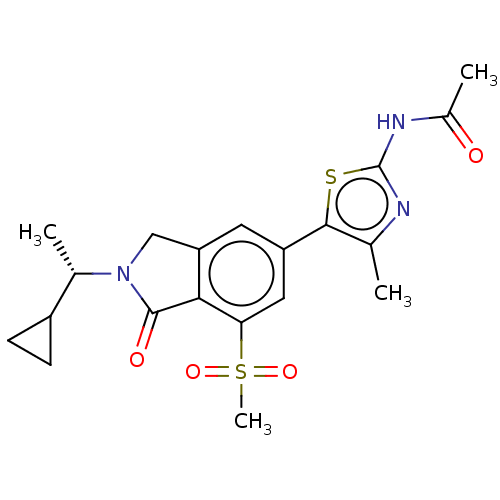
(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human P2Y1 receptor |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512885
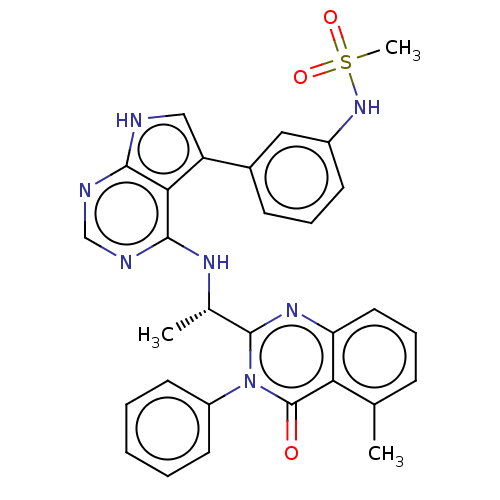
(CHEMBL4441003)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cccc(NS(C)(=O)=O)c3)c12)c1nc2cccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H27N7O3S/c1-18-9-7-14-24-25(18)30(38)37(22-12-5-4-6-13-22)29(35-24)19(2)34-28-26-23(16-31-27(26)32-17-33-28)20-10-8-11-21(15-20)36-41(3,39)40/h4-17,19,36H,1-3H3,(H2,31,32,33,34)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) preincubated for 30 mins followed by insulin stimulation for 5 mins by Western blot analysis |
J Med Chem 62: 4783-4814 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01298
BindingDB Entry DOI: 10.7270/Q25D8W6S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512862

(CHEMBL4541570)Show SMILES CC(Nc1ncnc2[nH]cnc12)c1cc2ccccn2c(=O)c1-c1ccccc1 Show InChI InChI=1S/C22H18N6O/c1-14(27-21-19-20(24-12-23-19)25-13-26-21)17-11-16-9-5-6-10-28(16)22(29)18(17)15-7-3-2-4-8-15/h2-14H,1H3,(H2,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 62: 4783-4814 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01298
BindingDB Entry DOI: 10.7270/Q25D8W6S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512880
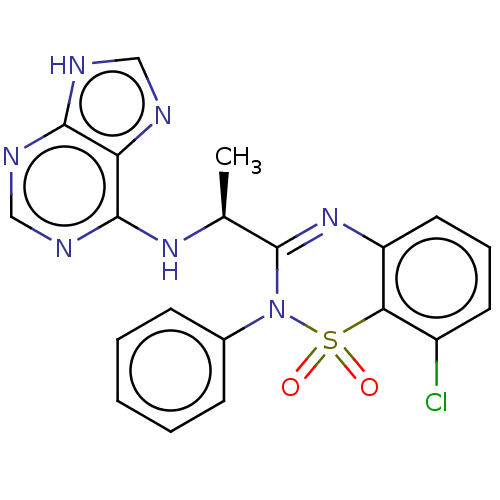
(CHEMBL4468379)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)C1=Nc2cccc(Cl)c2S(=O)(=O)N1c1ccccc1 |r,t:14| Show InChI InChI=1S/C20H16ClN7O2S/c1-12(26-19-16-18(23-10-22-16)24-11-25-19)20-27-15-9-5-8-14(21)17(15)31(29,30)28(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,22,23,24,25,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta in human SUDHL6 cells |
J Med Chem 62: 4783-4814 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01298
BindingDB Entry DOI: 10.7270/Q25D8W6S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM236918
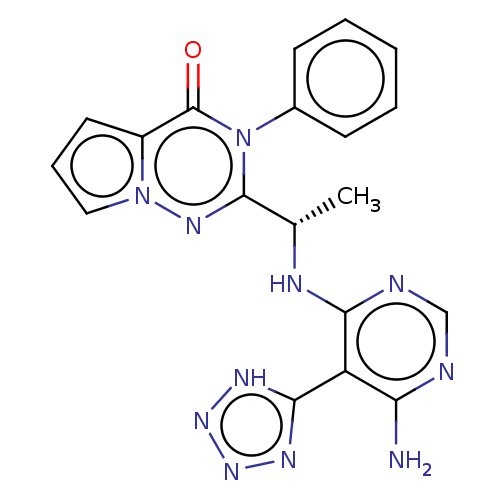
(US9388189, 1)Show SMILES C[C@H](Nc1ncnc(N)c1-c1nnn[nH]1)c1nn2cccc2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C19H17N11O/c1-11(23-16-14(15(20)21-10-22-16)17-24-27-28-25-17)18-26-29-9-5-8-13(29)19(31)30(18)12-6-3-2-4-7-12/h2-11H,1H3,(H3,20,21,22,23)(H,24,25,27,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) preincubated for 30 mins followed by insulin stimulation for 5 mins by Western blot analysis |
J Med Chem 62: 4783-4814 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01298
BindingDB Entry DOI: 10.7270/Q25D8W6S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512872
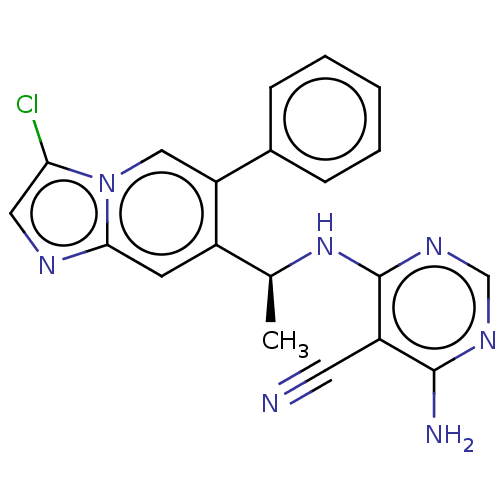
(CHEMBL4470131)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2ncc(Cl)n2cc1-c1ccccc1 |r| Show InChI InChI=1S/C20H16ClN7/c1-12(27-20-15(8-22)19(23)25-11-26-20)14-7-18-24-9-17(21)28(18)10-16(14)13-5-3-2-4-6-13/h2-7,9-12H,1H3,(H3,23,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 62: 4783-4814 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01298
BindingDB Entry DOI: 10.7270/Q25D8W6S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489244

(N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(methylsul...)Show SMILES CCOCC(=O)N(C)c1cccc(Nc2nc(C)c(s2)-c2cc3CN([C@@H](C)C4CC4)C(=O)c3c(c2)S(=O)(=O)NC)n1 |r| Show InChI InChI=1S/C28H34N6O5S2/c1-6-39-15-24(35)33(5)23-9-7-8-22(31-23)32-28-30-16(2)26(40-28)19-12-20-14-34(17(3)18-10-11-18)27(36)25(20)21(13-19)41(37,38)29-4/h7-9,12-13,17-18,29H,6,10-11,14-15H2,1-5H3,(H,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489245

(N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(methylsul...)Show SMILES CCN(C(=O)COC)c1cccc(Nc2nc(C)c(s2)-c2cc3CN([C@@H](C)C4CC4)C(=O)c3c(c2)S(=O)(=O)NC)n1 |r| Show InChI InChI=1S/C28H34N6O5S2/c1-6-33(24(35)15-39-5)23-9-7-8-22(31-23)32-28-30-16(2)26(40-28)19-12-20-14-34(17(3)18-10-11-18)27(36)25(20)21(13-19)41(37,38)29-4/h7-9,12-13,17-18,29H,6,10-11,14-15H2,1-5H3,(H,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM350391

((S)-4-amino-6-((1-(3-chloro-6-phenylimidazo[1,2-b]...)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2ncc(Cl)n2nc1-c1ccccc1 |r| Show InChI InChI=1S/C19H15ClN8/c1-11(26-19-14(8-21)18(22)24-10-25-19)13-7-16-23-9-15(20)28(16)27-17(13)12-5-3-2-4-6-12/h2-7,9-11H,1H3,(H3,22,24,25,26)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant full length human p110delta/untagged recombinant full length human p85alpha expressed in baculovirus... |
J Med Chem 62: 4783-4814 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01298
BindingDB Entry DOI: 10.7270/Q25D8W6S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474007

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-(ethylsulfamoy...)Show SMILES CCNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H26N4O4S2/c1-5-22-31(28,29)17-9-15(19-11(2)23-21(30-19)24-13(4)26)8-16-10-25(20(27)18(16)17)12(3)14-6-7-14/h8-9,12,14,22H,5-7,10H2,1-4H3,(H,23,24,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474011

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-({[1-(fluorome...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NCC1(CCF)CC1)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C25H31FN4O4S2/c1-14-22(35-24(28-14)29-16(3)31)18-10-19-12-30(15(2)17-4-5-17)23(32)21(19)20(11-18)36(33,34)27-13-25(6-7-25)8-9-26/h10-11,15,17,27H,4-9,12-13H2,1-3H3,(H,28,29,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489241
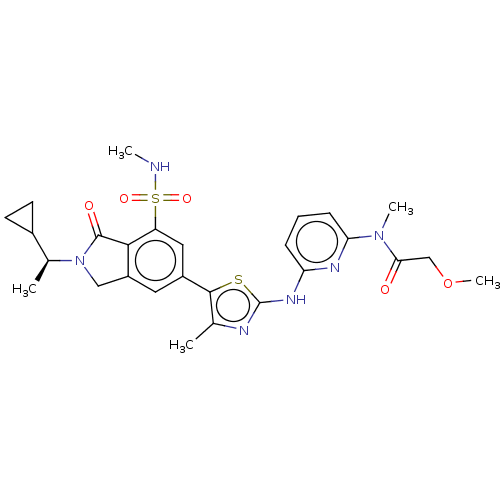
(N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(methylsul...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N(C)C(=O)COC)nc1C |r| Show InChI InChI=1S/C27H32N6O5S2/c1-15-25(39-27(29-15)31-21-7-6-8-22(30-21)32(4)23(34)14-38-5)18-11-19-13-33(16(2)17-9-10-17)26(35)24(19)20(12-18)40(36,37)28-3/h6-8,11-12,16-17,28H,9-10,13-14H2,1-5H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489251
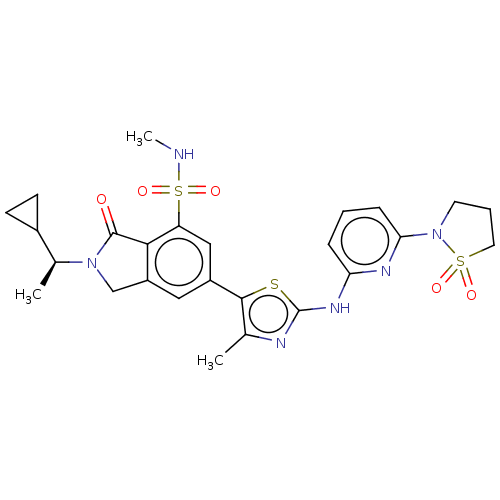
(2-[(1S)-1-Cyclopropylethyl]-6-(2-{[6-(1,1-dioxido-...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCCS2(=O)=O)nc1C |r| Show InChI InChI=1S/C26H30N6O5S3/c1-15-24(38-26(28-15)30-21-6-4-7-22(29-21)32-10-5-11-39(32,34)35)18-12-19-14-31(16(2)17-8-9-17)25(33)23(19)20(13-18)40(36,37)27-3/h4,6-7,12-13,16-17,27H,5,8-11,14H2,1-3H3,(H,28,29,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512875
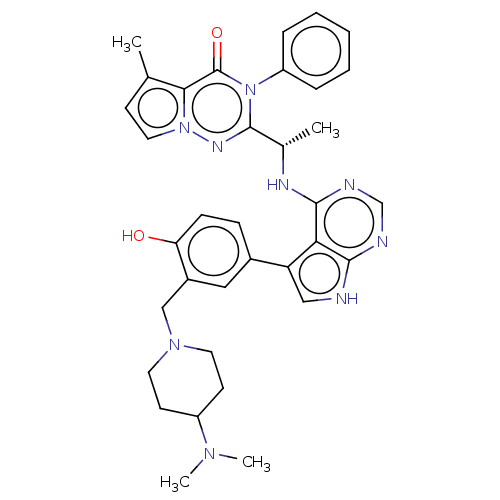
(CHEMBL4567071)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3ccc(O)c(CN4CCC(CC4)N(C)C)c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C35H39N9O2/c1-22-12-17-43-31(22)35(46)44(27-8-6-5-7-9-27)34(40-43)23(2)39-33-30-28(19-36-32(30)37-21-38-33)24-10-11-29(45)25(18-24)20-42-15-13-26(14-16-42)41(3)4/h5-12,17-19,21,23,26,45H,13-16,20H2,1-4H3,(H2,36,37,38,39)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) preincubated for 30 mins followed by insulin stimulation for 5 mins by Western blot analysis |
J Med Chem 62: 4783-4814 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01298
BindingDB Entry DOI: 10.7270/Q25D8W6S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489287
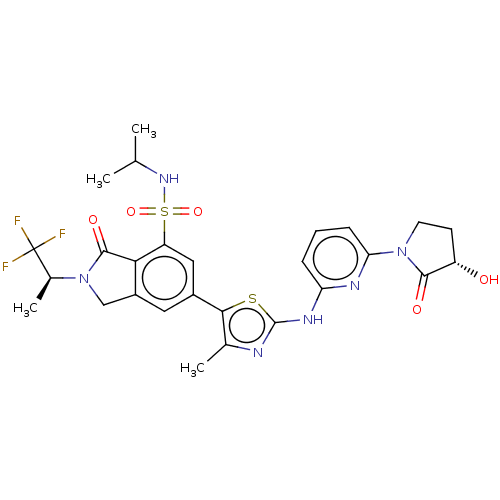
(6-[2-({6-[(3S)-3-Hydroxy-2-oxopyrrolidin-1-yl]pyri...)Show SMILES CC(C)NS(=O)(=O)c1cc(cc2CN([C@@H](C)C(F)(F)F)C(=O)c12)-c1sc(Nc2cccc(n2)N2CC[C@H](O)C2=O)nc1C |r| Show InChI InChI=1S/C27H29F3N6O5S2/c1-13(2)34-43(40,41)19-11-16(10-17-12-36(25(39)22(17)19)15(4)27(28,29)30)23-14(3)31-26(42-23)33-20-6-5-7-21(32-20)35-9-8-18(37)24(35)38/h5-7,10-11,13,15,18,34,37H,8-9,12H2,1-4H3,(H,31,32,33)/t15-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489281

(2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(3R)-3-hydro...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CC[C@@H](O)C2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-14-24(39-27(29-14)31-21-5-4-6-22(30-21)32-10-9-19(34)25(32)35)17-11-18-13-33(15(2)16-7-8-16)26(36)23(18)20(12-17)40(37,38)28-3/h4-6,11-12,15-16,19,28,34H,7-10,13H2,1-3H3,(H,29,30,31)/t15-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489261
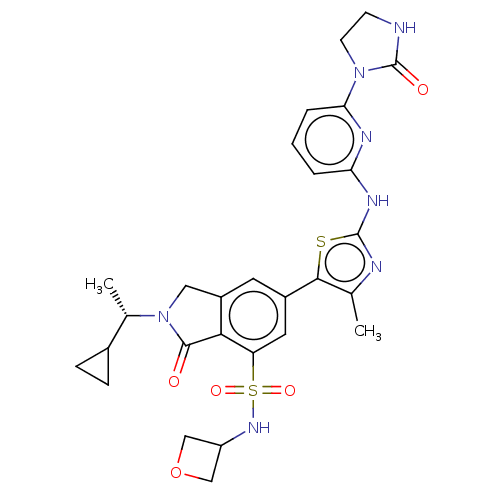
(2-[(1S)-1-Cyclopropylethyl]-6-(4-methyl-2-{[6-(2-o...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NC1COC1)-c1sc(Nc2cccc(n2)N2CCNC2=O)nc1C |r| Show InChI InChI=1S/C28H31N7O5S2/c1-15-25(41-27(30-15)32-22-4-3-5-23(31-22)34-9-8-29-28(34)37)18-10-19-12-35(16(2)17-6-7-17)26(36)24(19)21(11-18)42(38,39)33-20-13-40-14-20/h3-5,10-11,16-17,20,33H,6-9,12-14H2,1-2H3,(H,29,37)(H,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489257
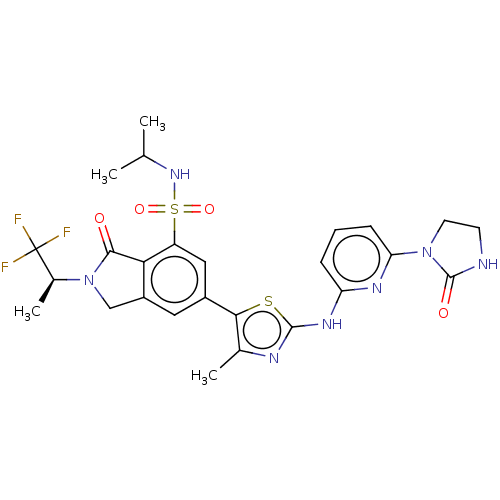
(6-(4-Methyl-2-{[6-(2-oxoimidazolidin-1-yl)pyridin-...)Show SMILES CC(C)NS(=O)(=O)c1cc(cc2CN([C@@H](C)C(F)(F)F)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCNC2=O)nc1C |r| Show InChI InChI=1S/C26H28F3N7O4S2/c1-13(2)34-42(39,40)18-11-16(10-17-12-36(23(37)21(17)18)15(4)26(27,28)29)22-14(3)31-24(41-22)33-19-6-5-7-20(32-19)35-9-8-30-25(35)38/h5-7,10-11,13,15,34H,8-9,12H2,1-4H3,(H,30,38)(H,31,32,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489283
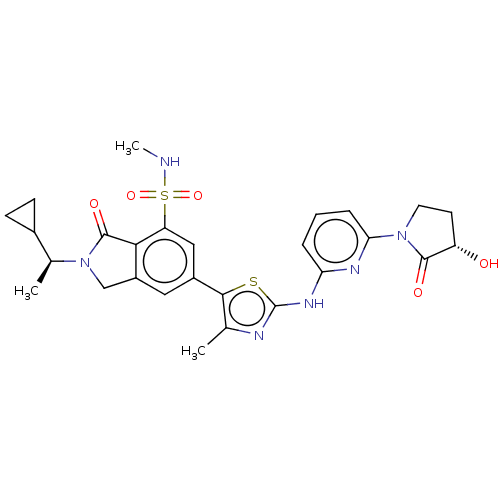
(2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(3S)-3-hydro...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CC[C@H](O)C2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-14-24(39-27(29-14)31-21-5-4-6-22(30-21)32-10-9-19(34)25(32)35)17-11-18-13-33(15(2)16-7-8-16)26(36)23(18)20(12-17)40(37,38)28-3/h4-6,11-12,15-16,19,28,34H,7-10,13H2,1-3H3,(H,29,30,31)/t15-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489256
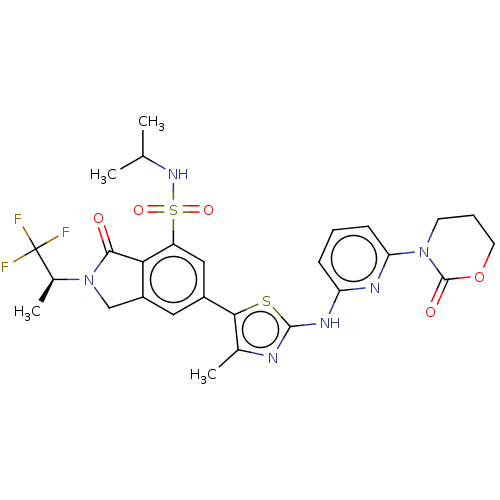
(6-(4-Methyl-2-{[6-(2-oxo-1,3-oxazinan-3-yl)pyridin...)Show SMILES CC(C)NS(=O)(=O)c1cc(cc2CN([C@@H](C)C(F)(F)F)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCCOC2=O)nc1C |r| Show InChI InChI=1S/C27H29F3N6O5S2/c1-14(2)34-43(39,40)19-12-17(11-18-13-36(24(37)22(18)19)16(4)27(28,29)30)23-15(3)31-25(42-23)33-20-7-5-8-21(32-20)35-9-6-10-41-26(35)38/h5,7-8,11-12,14,16,34H,6,9-10,13H2,1-4H3,(H,31,32,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489253
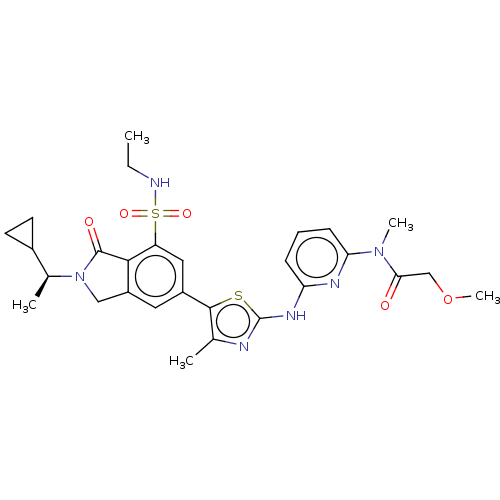
(N-{6-[(5-{2-[(1S)-1-Cyclopropylethyl]-7-(ethylsulf...)Show SMILES CCNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N(C)C(=O)COC)nc1C |r| Show InChI InChI=1S/C28H34N6O5S2/c1-6-29-41(37,38)21-13-19(12-20-14-34(27(36)25(20)21)17(3)18-10-11-18)26-16(2)30-28(40-26)32-22-8-7-9-23(31-22)33(4)24(35)15-39-5/h7-9,12-13,17-18,29H,6,10-11,14-15H2,1-5H3,(H,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474028

(N-(5-{7-[(3-Cyanophenyl)sulfamoyl]-2-[(1S)-1-cyclo...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)Nc1cccc(c1)C#N)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C26H25N5O4S2/c1-14-24(36-26(28-14)29-16(3)32)19-10-20-13-31(15(2)18-7-8-18)25(33)23(20)22(11-19)37(34,35)30-21-6-4-5-17(9-21)12-27/h4-6,9-11,15,18,30H,7-8,13H2,1-3H3,(H,28,29,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50579665

(CHEMBL5082066)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCCCCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489281

(2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(3R)-3-hydro...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CC[C@@H](O)C2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-14-24(39-27(29-14)31-21-5-4-6-22(30-21)32-10-9-19(34)25(32)35)17-11-18-13-33(15(2)16-7-8-16)26(36)23(18)20(12-17)40(37,38)28-3/h4-6,11-12,15-16,19,28,34H,7-10,13H2,1-3H3,(H,29,30,31)/t15-,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50579666
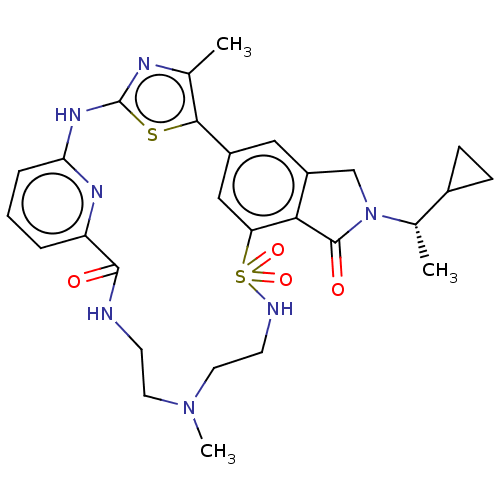
(CHEMBL5081964)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCN(C)CCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512883
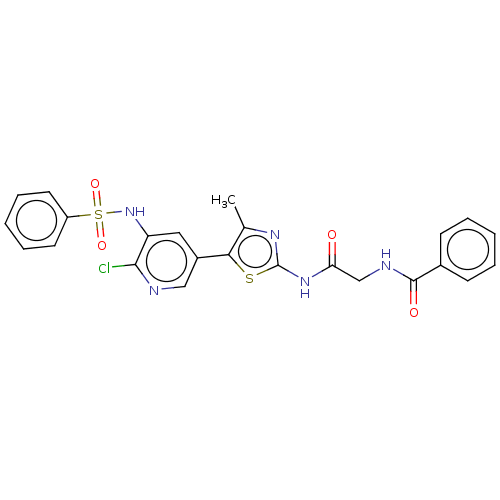
(CHEMBL4460140)Show SMILES Cc1nc(NC(=O)CNC(=O)c2ccccc2)sc1-c1cnc(Cl)c(NS(=O)(=O)c2ccccc2)c1 Show InChI InChI=1S/C24H20ClN5O4S2/c1-15-21(35-24(28-15)29-20(31)14-27-23(32)16-8-4-2-5-9-16)17-12-19(22(25)26-13-17)30-36(33,34)18-10-6-3-7-11-18/h2-13,30H,14H2,1H3,(H,27,32)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 62: 4783-4814 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01298
BindingDB Entry DOI: 10.7270/Q25D8W6S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489264
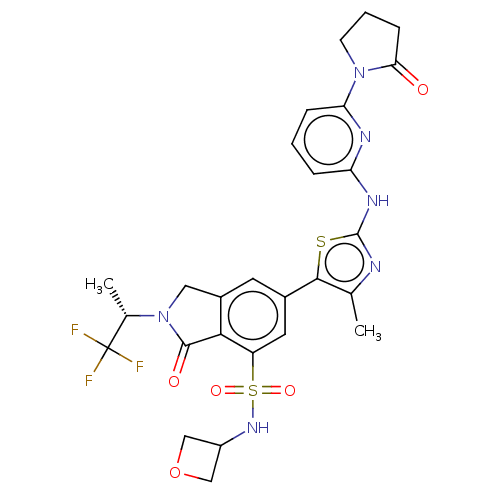
(6-(4-Methyl-2-{[6-(2-oxopyrrolidin-1-yl)pyridin-2-...)Show SMILES C[C@H](N1Cc2cc(cc(c2C1=O)S(=O)(=O)NC1COC1)-c1sc(Nc2cccc(n2)N2CCCC2=O)nc1C)C(F)(F)F |r| Show InChI InChI=1S/C27H27F3N6O5S2/c1-14-24(42-26(31-14)33-20-5-3-6-21(32-20)35-8-4-7-22(35)37)16-9-17-11-36(15(2)27(28,29)30)25(38)23(17)19(10-16)43(39,40)34-18-12-41-13-18/h3,5-6,9-10,15,18,34H,4,7-8,11-13H2,1-2H3,(H,31,32,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489237

(5-(4-Methyl-2-{[6-(2-oxopyrrolidin-1-yl)pyridin-2-...)Show SMILES C[C@H](N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)N2CCCC2=O)nc1C)C(F)(F)F |r| Show InChI InChI=1S/C25H24F3N5O4S2/c1-13-22(38-24(29-13)31-18-6-4-7-19(30-18)32-9-5-8-20(32)34)15-10-16-12-33(14(2)25(26,27)28)23(35)21(16)17(11-15)39(3,36)37/h4,6-7,10-11,14H,5,8-9,12H2,1-3H3,(H,29,30,31)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489248
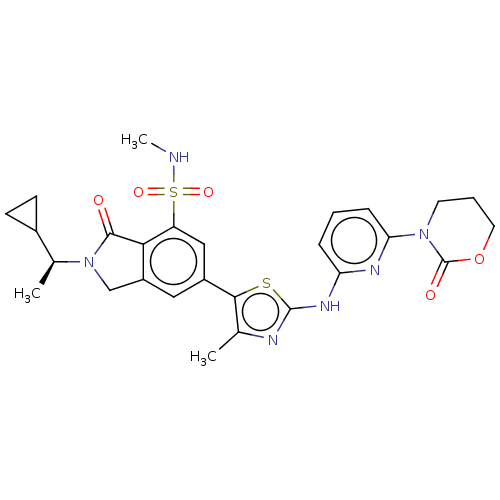
(2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCCOC2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-15-24(39-26(29-15)31-21-6-4-7-22(30-21)32-10-5-11-38-27(32)35)18-12-19-14-33(16(2)17-8-9-17)25(34)23(19)20(13-18)40(36,37)28-3/h4,6-7,12-13,16-17,28H,5,8-11,14H2,1-3H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50579671
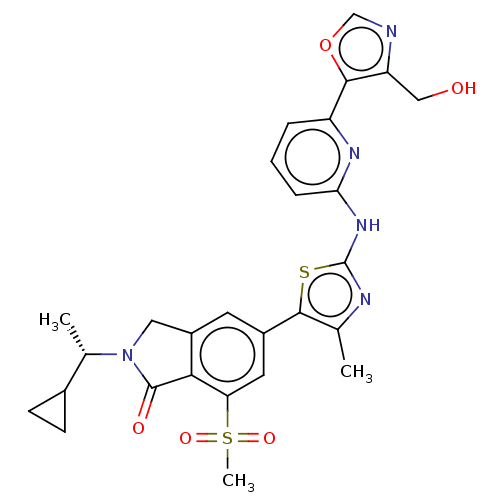
(CHEMBL5090959)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)-c2ocnc2CO)nc1C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kgamma assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50512861
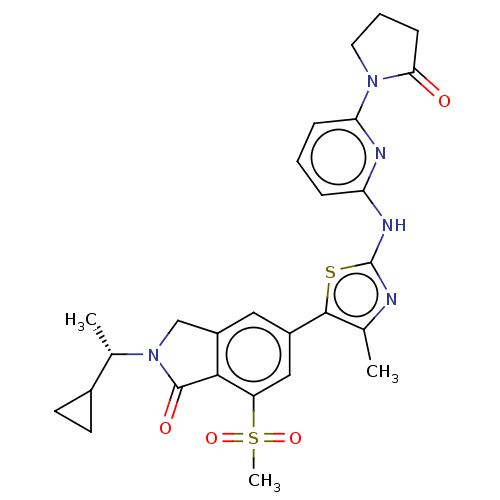
(CHEMBL4558527)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)N2CCCC2=O)nc1C |r| Show InChI InChI=1S/C27H29N5O4S2/c1-15-25(37-27(28-15)30-21-6-4-7-22(29-21)31-11-5-8-23(31)33)18-12-19-14-32(16(2)17-9-10-17)26(34)24(19)20(13-18)38(3,35)36/h4,6-7,12-13,16-17H,5,8-11,14H2,1-3H3,(H,28,29,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489261

(2-[(1S)-1-Cyclopropylethyl]-6-(4-methyl-2-{[6-(2-o...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NC1COC1)-c1sc(Nc2cccc(n2)N2CCNC2=O)nc1C |r| Show InChI InChI=1S/C28H31N7O5S2/c1-15-25(41-27(30-15)32-22-4-3-5-23(31-22)34-9-8-29-28(34)37)18-10-19-12-35(16(2)17-6-7-17)26(36)24(19)21(11-18)42(38,39)33-20-13-40-14-20/h3-5,10-11,16-17,20,33H,6-9,12-14H2,1-2H3,(H,29,37)(H,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489247
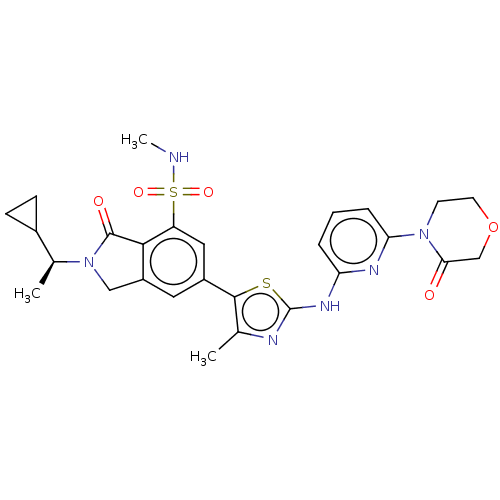
(2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCOCC2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-15-25(39-27(29-15)31-21-5-4-6-22(30-21)32-9-10-38-14-23(32)34)18-11-19-13-33(16(2)17-7-8-17)26(35)24(19)20(12-18)40(36,37)28-3/h4-6,11-12,16-17,28H,7-10,13-14H2,1-3H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489248

(2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCCOC2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O5S2/c1-15-24(39-26(29-15)31-21-6-4-7-22(30-21)32-10-5-11-38-27(32)35)18-12-19-14-33(16(2)17-8-9-17)25(34)23(19)20(13-18)40(36,37)28-3/h4,6-7,12-13,16-17,28H,5,8-11,14H2,1-3H3,(H,29,30,31)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648
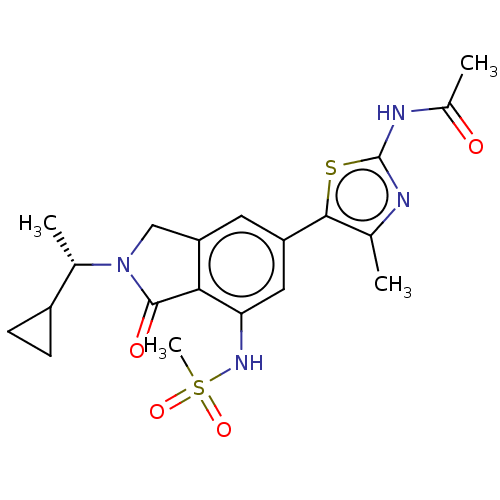
(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274660
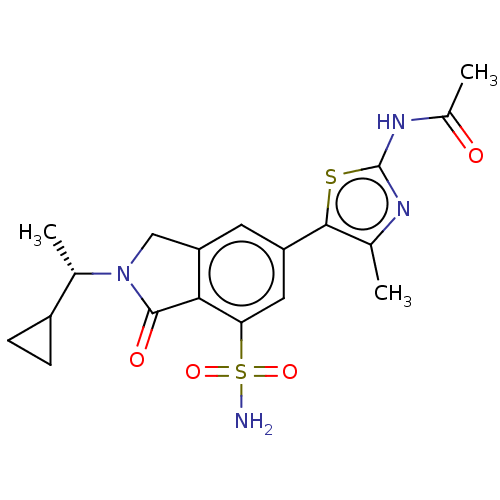
(CHEMBL4128537 | US10858355, Example 12)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(N)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C19H22N4O4S2/c1-9-17(28-19(21-9)22-11(3)24)13-6-14-8-23(10(2)12-4-5-12)18(25)16(14)15(7-13)29(20,26)27/h6-7,10,12H,4-5,8H2,1-3H3,(H2,20,26,27)(H,21,22,24)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274640
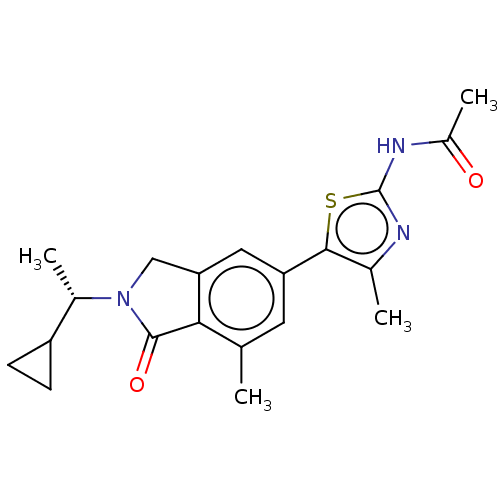
(CHEMBL4126445)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O2S/c1-10-7-15(18-11(2)21-20(26-18)22-13(4)24)8-16-9-23(19(25)17(10)16)12(3)14-5-6-14/h7-8,12,14H,5-6,9H2,1-4H3,(H,21,22,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... |
J Med Chem 61: 5435-5441 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00447
BindingDB Entry DOI: 10.7270/Q27M0BFZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50579668
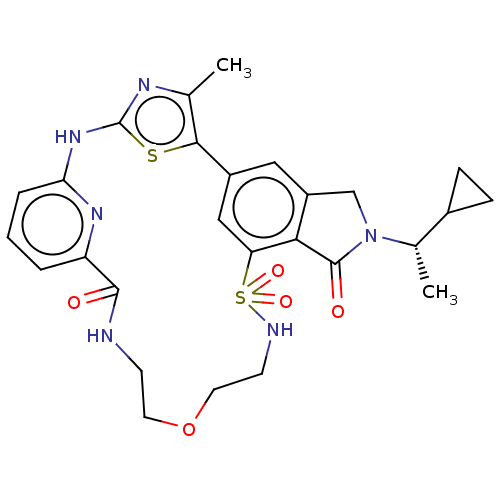
(CHEMBL5091438)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCOCCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
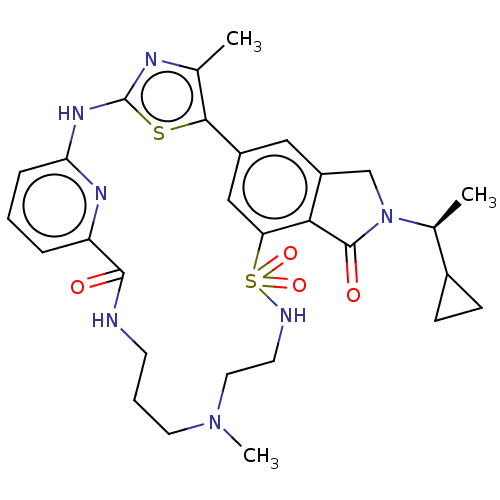
(Homo sapiens (Human)) | BDBM50579667
(CHEMBL5090799)Show SMILES C[C@@H](C1CC1)N1Cc2cc-3cc(c2C1=O)S(=O)(=O)NCCN(C)CCCNC(=O)c1cccc(Nc2nc(C)c-3s2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human PI3Kdelta assessed as reduction in ADP production using Dic8-PIP2 as substrate pre-treated for 15 mins followed by su... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00434
BindingDB Entry DOI: 10.7270/Q24Q7ZVJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474012

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-1-oxo-7-[(2,2,2-...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NCC(F)(F)F)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C21H23F3N4O4S2/c1-10-18(33-20(26-10)27-12(3)29)14-6-15-8-28(11(2)13-4-5-13)19(30)17(15)16(7-14)34(31,32)25-9-21(22,23)24/h6-7,11,13,25H,4-5,8-9H2,1-3H3,(H,26,27,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274648

(CHEMBL4127396 | US10858355, Example 22)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H24N4O4S2/c1-10-18(29-20(21-10)22-12(3)25)14-7-15-9-24(11(2)13-5-6-13)19(26)17(15)16(8-14)23-30(4,27)28/h7-8,11,13,23H,5-6,9H2,1-4H3,(H,21,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274638

(CHEMBL4126156 | US10858355, Example 4)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C20H23N3O4S2/c1-10-18(28-20(21-10)22-12(3)24)14-7-15-9-23(11(2)13-5-6-13)19(25)17(15)16(8-14)29(4,26)27/h7-8,11,13H,5-6,9H2,1-4H3,(H,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474008

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-(oxetan-3-ylsu...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)NC1COC1)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H26N4O5S2/c1-11-20(32-22(23-11)24-13(3)27)15-6-16-8-26(12(2)14-4-5-14)21(28)19(16)18(7-15)33(29,30)25-17-9-31-10-17/h6-7,12,14,17,25H,4-5,8-10H2,1-3H3,(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM474015

(N-(5-{2-[(1S)-1-Cyclopropylethyl]-7-[(cyclopropyls...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(=O)(=O)C3CC3)c2C1=O)-c1sc(NC(C)=O)nc1C |r| Show InChI InChI=1S/C22H26N4O4S2/c1-11-20(31-22(23-11)24-13(3)27)15-8-16-10-26(12(2)14-4-5-14)21(28)19(16)18(9-15)25-32(29,30)17-6-7-17/h8-9,12,14,17,25H,4-7,10H2,1-3H3,(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ (aa144-1102)-6His was determined by measuring the ADP level after phosphorylation of DiC8-PIP2 using a c... |
US Patent US10858355 (2020)
BindingDB Entry DOI: 10.7270/Q2BZ6945 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489280
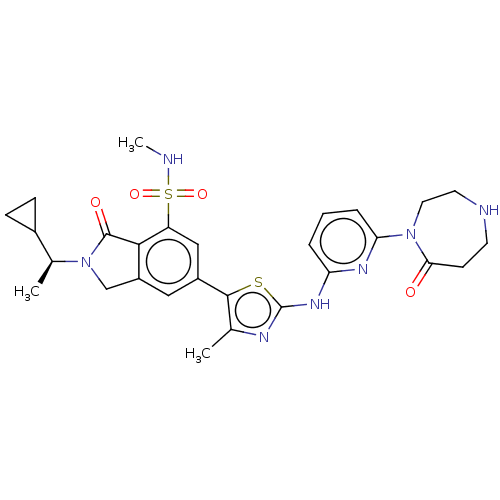
(2-[(1S)-1-Cyclopropylethyl]-N-methyl-6-(4-methyl-2...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCNCCC2=O)nc1C |r| Show InChI InChI=1S/C28H33N7O4S2/c1-16-26(40-28(31-16)33-22-5-4-6-23(32-22)34-12-11-30-10-9-24(34)36)19-13-20-15-35(17(2)18-7-8-18)27(37)25(20)21(14-19)41(38,39)29-3/h4-6,13-14,17-18,29-30H,7-12,15H2,1-3H3,(H,31,32,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489227
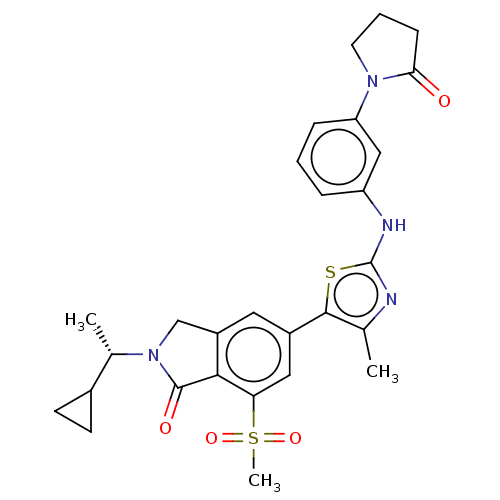
(2-[(1S)-1-Cyclopropylethyl]-5-(4-methyl-2-{[6-(2-o...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(c2)N2CCCC2=O)nc1C |r| Show InChI InChI=1S/C28H30N4O4S2/c1-16-26(37-28(29-16)30-21-6-4-7-22(14-21)31-11-5-8-24(31)33)19-12-20-15-32(17(2)18-9-10-18)27(34)25(20)23(13-19)38(3,35)36/h4,6-7,12-14,17-18H,5,8-11,15H2,1-3H3,(H,29,30)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489231

(2-[(1S)-1-Cyclopropylethyl]-5-(4-methyl-2-{[6-(3-m...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)N2CCN(C)C2=O)nc1C |r| Show InChI InChI=1S/C27H30N6O4S2/c1-15-24(38-26(28-15)30-21-6-5-7-22(29-21)32-11-10-31(3)27(32)35)18-12-19-14-33(16(2)17-8-9-17)25(34)23(19)20(13-18)39(4,36)37/h5-7,12-13,16-17H,8-11,14H2,1-4H3,(H,28,29,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489286
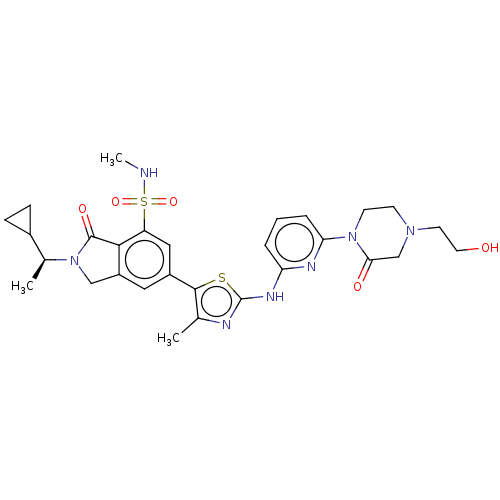
(2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[4-(2-hydroxy...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2CCN(CCO)CC2=O)nc1C |r| Show InChI InChI=1S/C29H35N7O5S2/c1-17-27(20-13-21-15-36(18(2)19-7-8-19)28(39)26(21)22(14-20)43(40,41)30-3)42-29(31-17)33-23-5-4-6-24(32-23)35-10-9-34(11-12-37)16-25(35)38/h4-6,13-14,18-19,30,37H,7-12,15-16H2,1-3H3,(H,31,32,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489284

(2-[(1S)-1-Cyclopropylethyl]-6-[2-({6-[(2S)-2-(hydr...)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1sc(Nc2cccc(n2)N2[C@H](CO)CCC2=O)nc1C |r| Show InChI InChI=1S/C28H32N6O5S2/c1-15-26(40-28(30-15)32-22-5-4-6-23(31-22)34-20(14-35)9-10-24(34)36)18-11-19-13-33(16(2)17-7-8-17)27(37)25(19)21(12-18)41(38,39)29-3/h4-6,11-12,16-17,20,29,35H,7-10,13-14H2,1-3H3,(H,30,31,32)/t16-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM489232
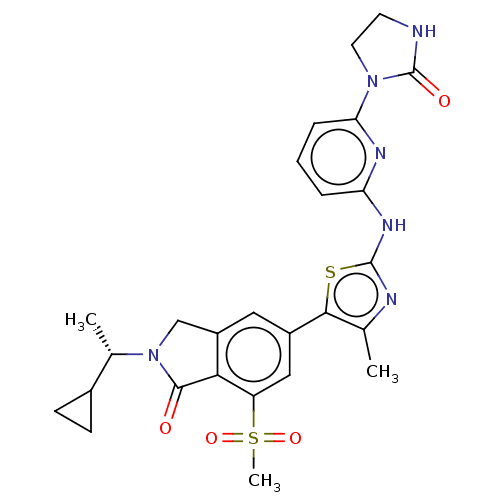
(2-[(1S)-1-Cyclopropylethyl]-5-(4-methyl-2-{[6-(2-o...)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1sc(Nc2cccc(n2)N2CCNC2=O)nc1C |r| Show InChI InChI=1S/C26H28N6O4S2/c1-14-23(37-25(28-14)30-20-5-4-6-21(29-20)31-10-9-27-26(31)34)17-11-18-13-32(15(2)16-7-8-16)24(33)22(18)19(12-17)38(3,35)36/h4-6,11-12,15-16H,7-10,13H2,1-3H3,(H,27,34)(H,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB
US Patent
| Assay Description
The activity of recombinant human PI3Kγ ((aa144-1102)-6His) and PI3Kα, β, δ (6-His(p110-p85α)) was determined by measuring t... |
US Patent US10961236 (2021)
BindingDB Entry DOI: 10.7270/Q2M90CS5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data