

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50336345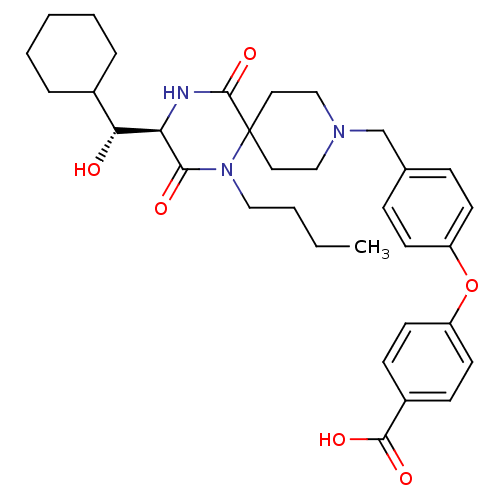 (4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced chemotaxis | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50336345 (4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of MIP-1alpha from human CCR5 expressed in CHO cells | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116666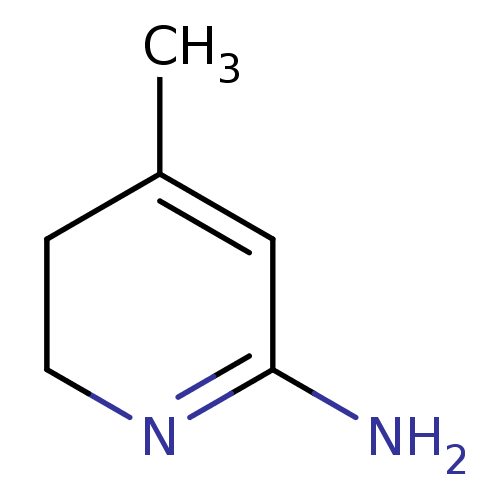 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50336345 (4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 assessed as inhibition of RANTES-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116668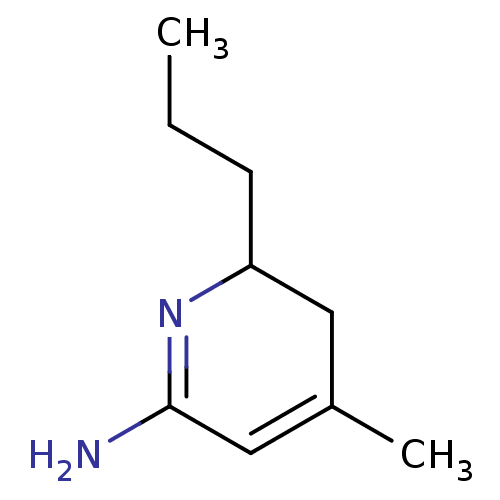 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116667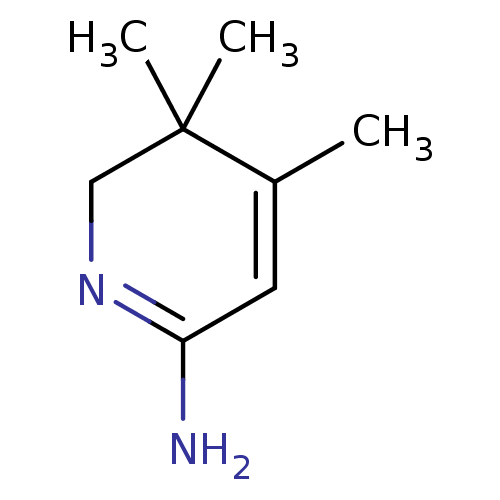 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116668 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116670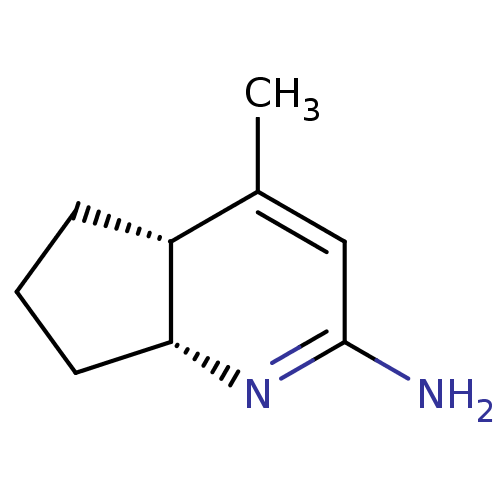 ((4aR,7aR)-4-Methyl-1,4a,5,6,7,7a-hexahydro-[1]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116676 (4,6-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116676 (4,6-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116673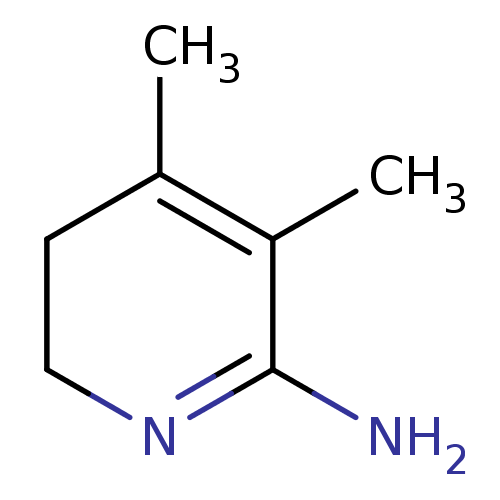 (3,4-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116673 (3,4-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116667 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116670 ((4aR,7aR)-4-Methyl-1,4a,5,6,7,7a-hexahydro-[1]pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116675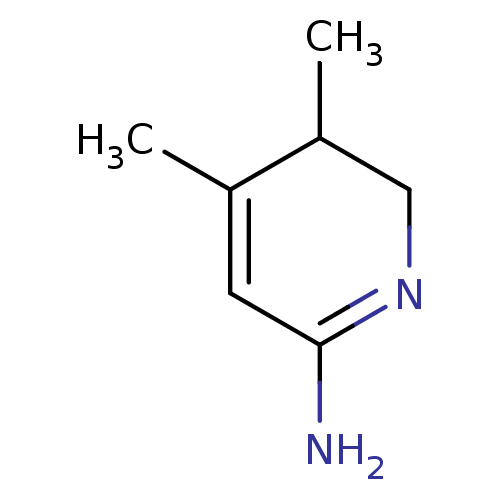 (4,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116675 (4,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116668 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50237936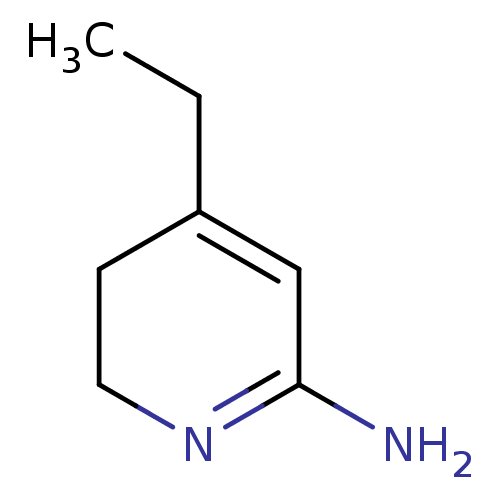 (4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350064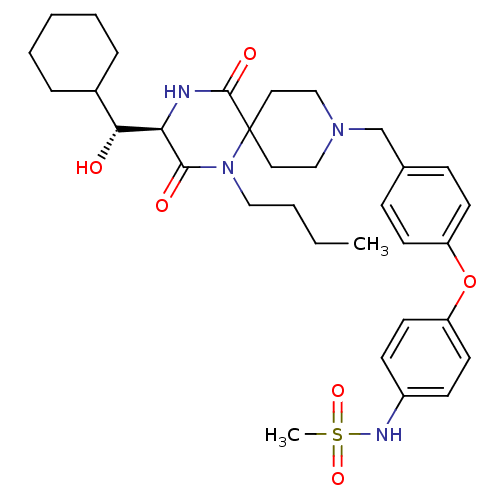 (CHEMBL1813435) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116677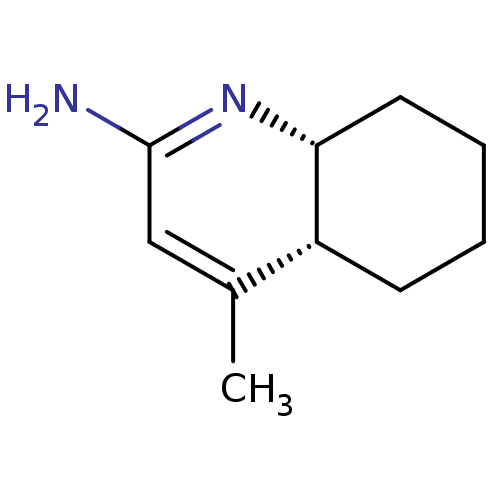 ((4aR,8aR)-4-Methyl-4a,5,6,7,8,8a-hexahydro-1H-quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50237936 (4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116667 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350045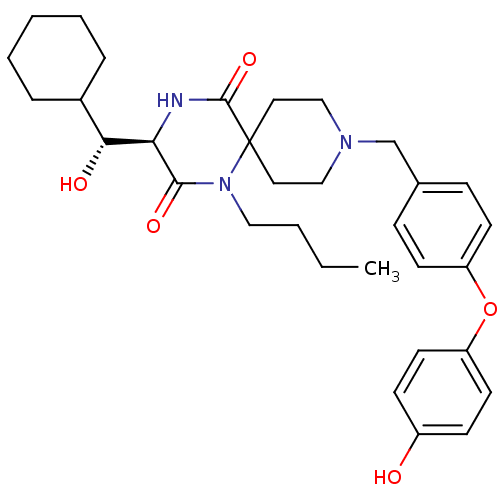 (CHEMBL1813270) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116669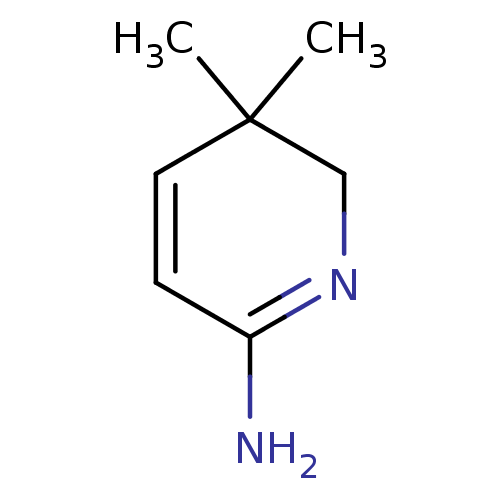 (5,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350060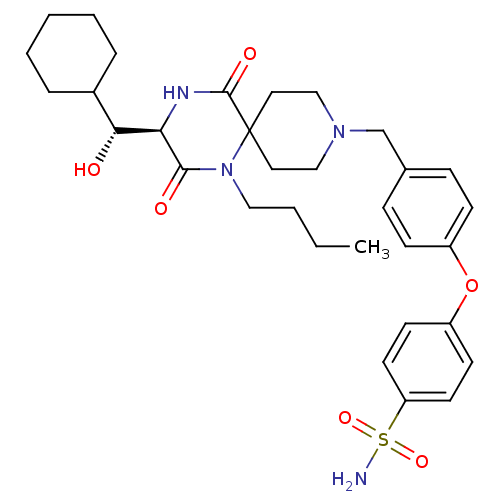 (CHEMBL1813433) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350042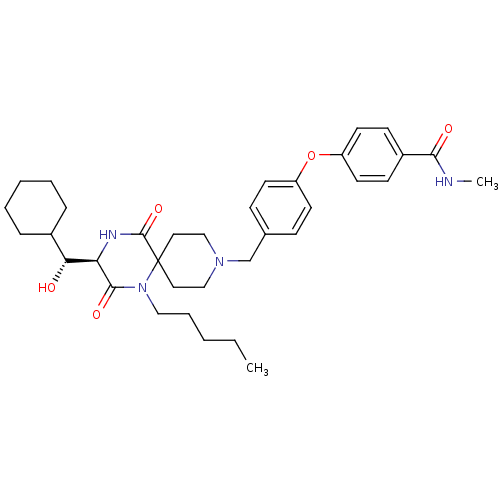 (CHEMBL1813450) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350054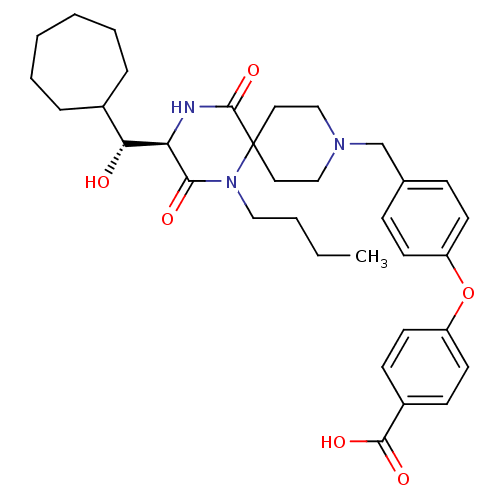 (CHEMBL1813440) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50336345 (4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350046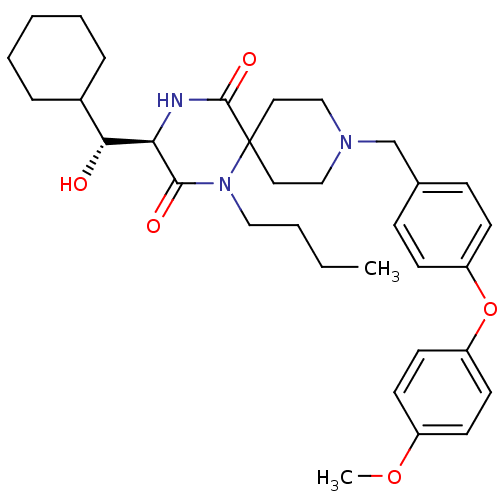 (CHEMBL1813269) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350061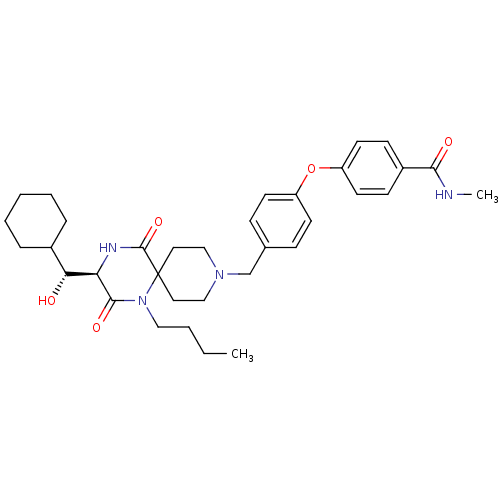 (CHEMBL1813432) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116677 ((4aR,8aR)-4-Methyl-4a,5,6,7,8,8a-hexahydro-1H-quin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50198936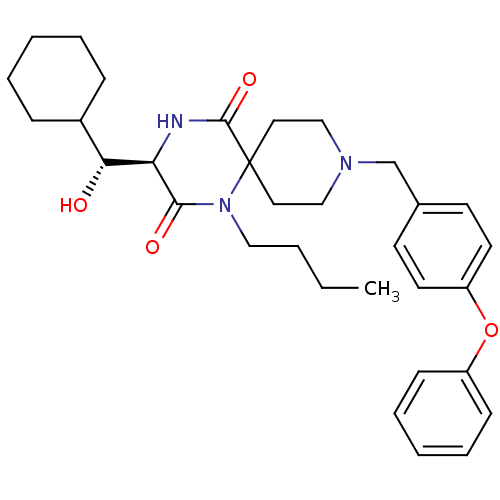 ((R)-1-butyl-3-((R)-cyclohexyl-hydroxy-methyl)-9-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50350047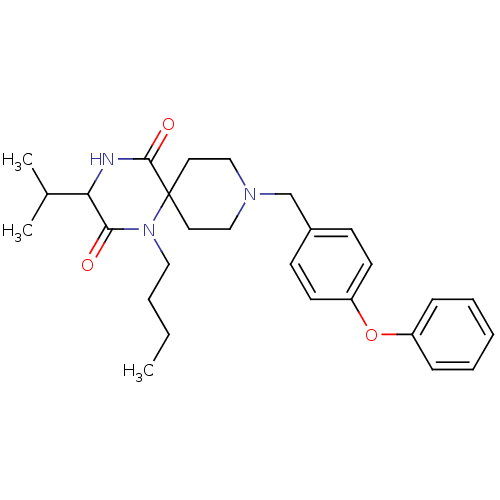 (CHEMBL1813459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350055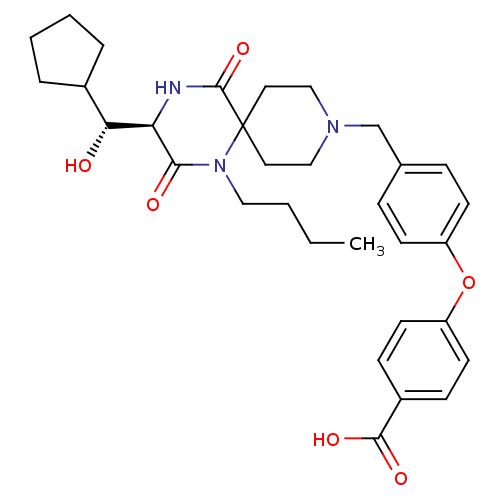 (CHEMBL1813439) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350062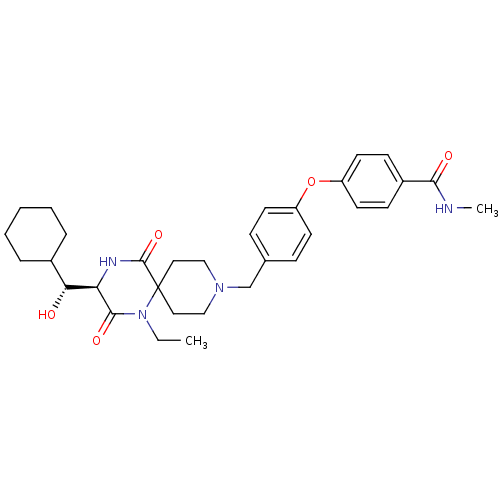 (CHEMBL1813447) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350043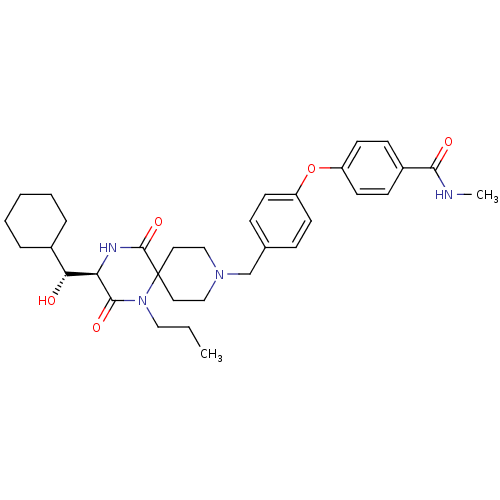 (CHEMBL1813449) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human CCR5 receptor assessed as inhibition of HIV1 gp120-induced cell-cell fusion between viral envolop protein expressin... | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350037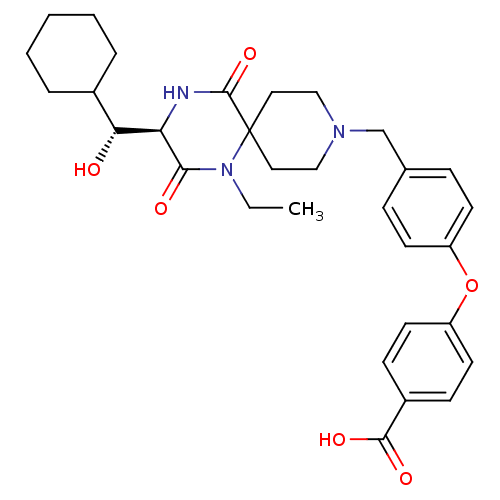 (CHEMBL1813446) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350051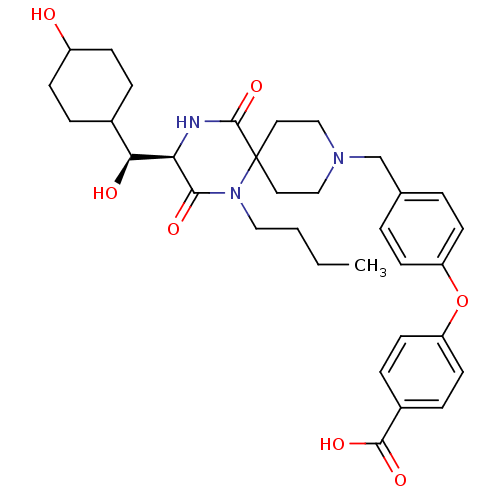 (CHEMBL1813443) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350056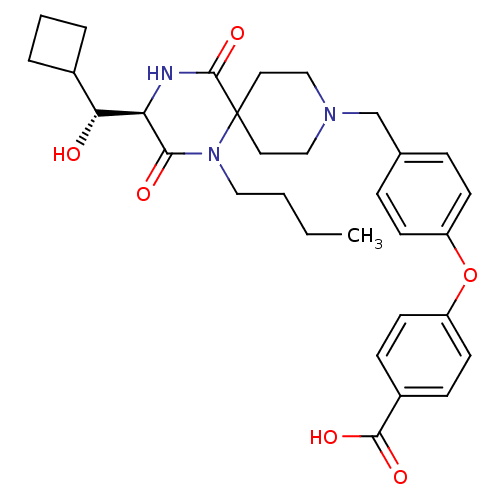 (CHEMBL1813438) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350064 (CHEMBL1813435) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350043 (CHEMBL1813449) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Rattus norvegicus) | BDBM50336345 (4-(4-(((R)-1-butyl-3-((R)-cyclohexyl(hydroxy)methy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat CCR5 assessed as inhibition of RANTES-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50350041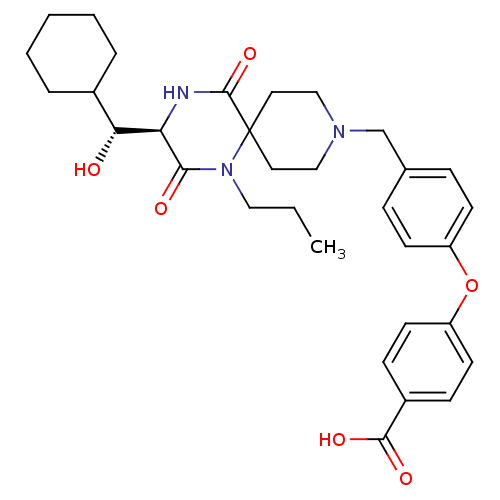 (CHEMBL1813448) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 expressed in CHO cells assessed as inhibition of MIP-1alpha-induced calcium mobilization | Bioorg Med Chem 19: 4028-42 (2011) Article DOI: 10.1016/j.bmc.2011.05.022 BindingDB Entry DOI: 10.7270/Q2H70G5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |