

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36717 (1,25(OH)2D3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001092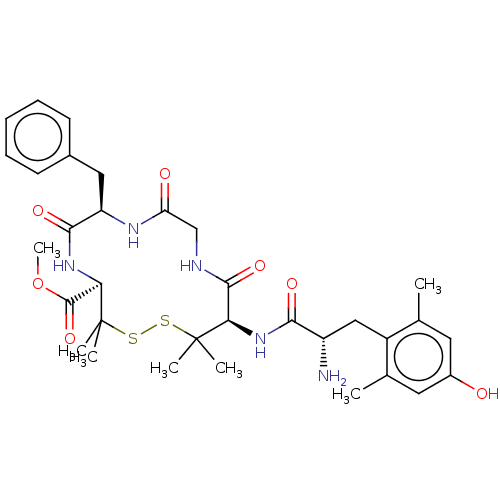 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement of [3H]- DAMPGO from opioid receptor mu by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001092 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000335 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In Vitro evaluation for the binding affinity in homogenates of rat brain at Opioid receptor delta 1 by displacing [3H]- DSLET | J Med Chem 35: 684-7 (1992) BindingDB Entry DOI: 10.7270/Q2FB53JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001090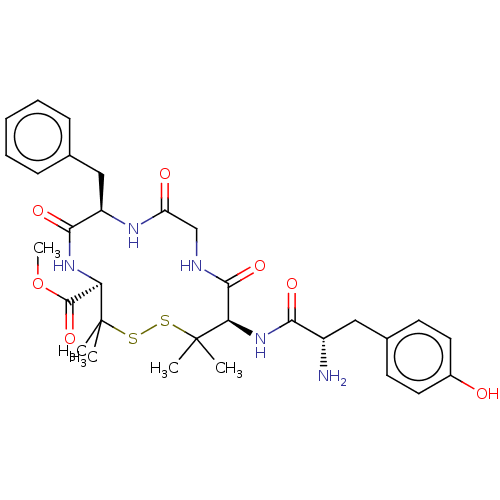 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001088 ((DPDPE)13-[2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001090 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement of [3H]- DAMPGO from opioid receptor mu by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001093 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement of [3H]- DAMPGO from opioid receptor mu by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001093 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In Vitro evaluation for the binding affinity in homogenates of rat brain at Opioid receptor delta 1 by displacing [3H]- DSLET | J Med Chem 35: 684-7 (1992) BindingDB Entry DOI: 10.7270/Q2FB53JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001093 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001093 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001089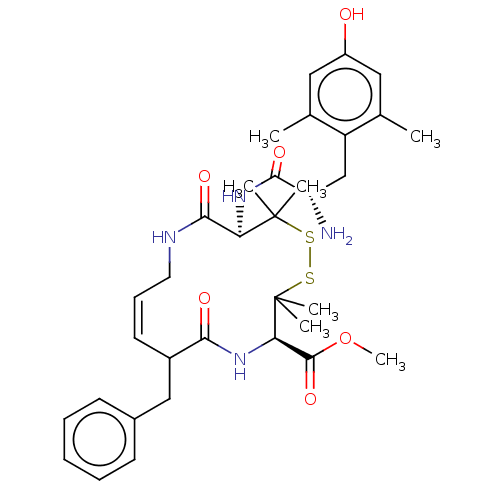 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Dissociation rate constant of compound for mutant T46S Escherichia coli dihydrofolate reductase | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001089 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement of [3H]- DAMPGO from opioid receptor mu by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001089 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001089 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000335 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In Vitro evaluation for the binding affinity in homogenates of rat brain at opioid receptor mu by displacing [3H]- DAMGO | J Med Chem 35: 684-7 (1992) BindingDB Entry DOI: 10.7270/Q2FB53JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001091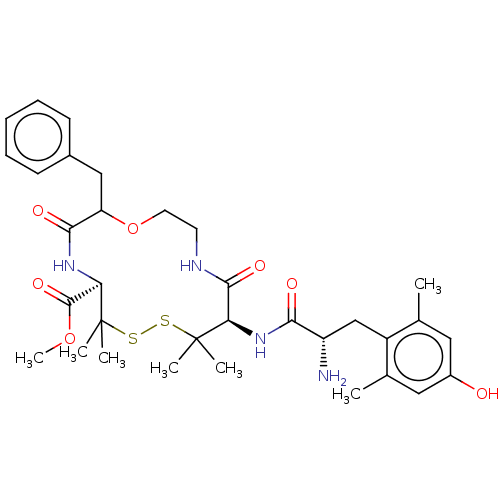 (10-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Dissociation rate constant of compound for mutant T46A Escherichia coli dihydrofolate reductase | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001091 (10-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement [3H]- DSLET from Opioid receptor delta 1 by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36722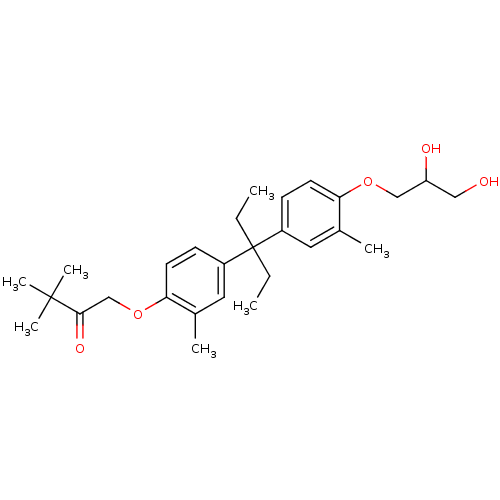 (LG190178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001091 (10-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement of [3H]- DAMPGO from opioid receptor mu by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001091 (10-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement of [3H]- DAMPGO from opioid receptor mu by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001088 ((DPDPE)13-[2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacement of [3H]- DAMPGO from opioid receptor mu by using opioid radioligand binding assay | J Med Chem 35: 2928-38 (1992) BindingDB Entry DOI: 10.7270/Q2ST7QF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36721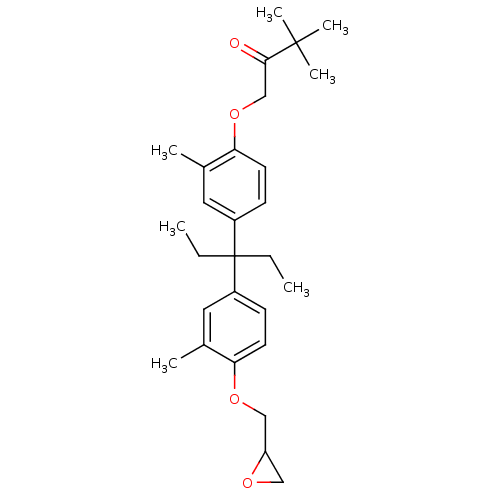 (LG190176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36719 (LG190119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36720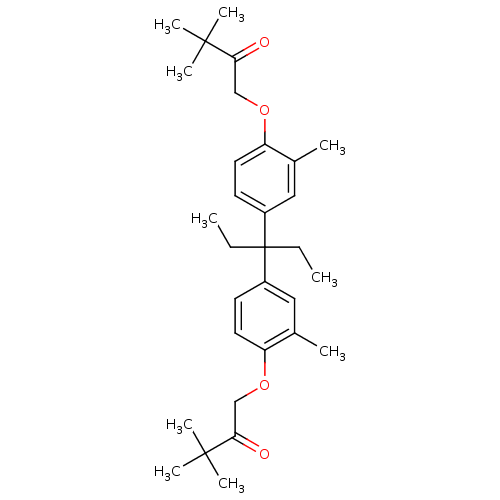 (LG190155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36718 (LG190090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In Vitro evaluation for the binding affinity in homogenates of rat brain at opioid receptor mu by displacing [3H]- DAMGO | J Med Chem 35: 684-7 (1992) BindingDB Entry DOI: 10.7270/Q2FB53JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D-binding protein (Rattus norvegicus) | BDBM36717 (1,25(OH)2D3) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description DBP competition binding assay were done using human serum (Scantibodies) or rat serum (Gibco-BRL). | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D-binding protein (Homo sapiens (Human)) | BDBM36717 (1,25(OH)2D3) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description DBP competition binding assay were done using human serum (Scantibodies) or rat serum (Gibco-BRL). | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D-binding protein (Homo sapiens (Human)) | BDBM36719 (LG190119) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description DBP competition binding assay were done using human serum (Scantibodies) or rat serum (Gibco-BRL). | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D-binding protein (Homo sapiens (Human)) | BDBM36720 (LG190155) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description DBP competition binding assay were done using human serum (Scantibodies) or rat serum (Gibco-BRL). | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D-binding protein (Homo sapiens (Human)) | BDBM36721 (LG190176) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description DBP competition binding assay were done using human serum (Scantibodies) or rat serum (Gibco-BRL). | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D-binding protein (Homo sapiens (Human)) | BDBM36722 (LG190178) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description DBP competition binding assay were done using human serum (Scantibodies) or rat serum (Gibco-BRL). | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D-binding protein (Homo sapiens (Human)) | BDBM36718 (LG190090) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description DBP competition binding assay were done using human serum (Scantibodies) or rat serum (Gibco-BRL). | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||