 Found 86 hits with Last Name = 'yajima' and Initial = 'm'
Found 86 hits with Last Name = 'yajima' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549700
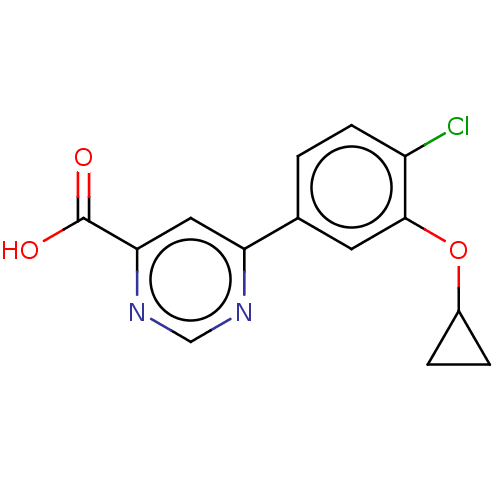
(CHEMBL4740126) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KMO (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549707
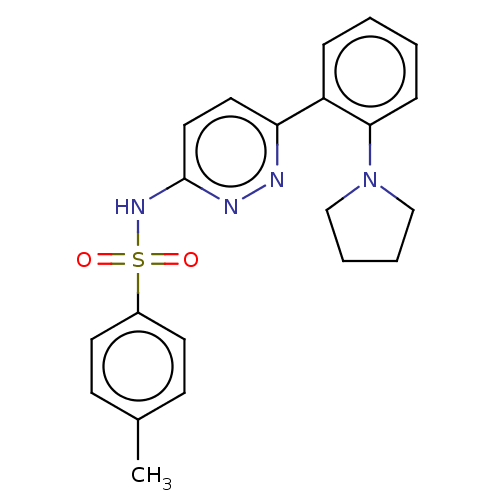
(CHEMBL4781856)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1ccccc1N1CCCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549704
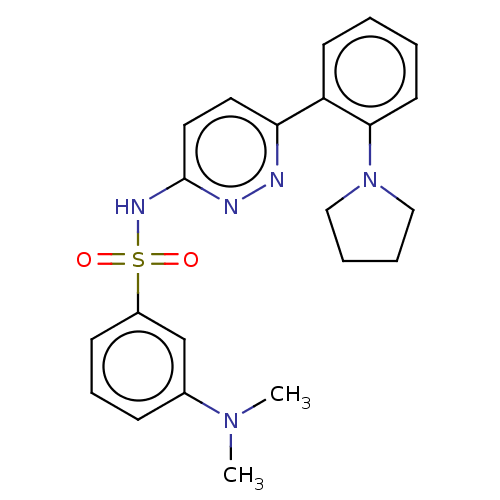
(CHEMBL4764931)Show SMILES CN(C)c1cccc(c1)S(=O)(=O)Nc1ccc(nn1)-c1ccccc1N1CCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549705
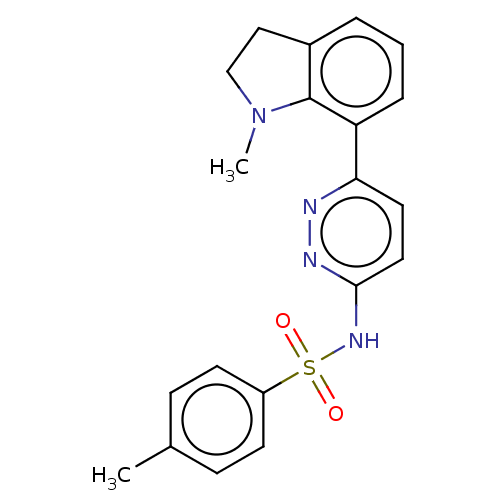
(CHEMBL4756311)Show SMILES CN1CCc2cccc(c12)-c1ccc(NS(=O)(=O)c2ccc(C)cc2)nn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50266003
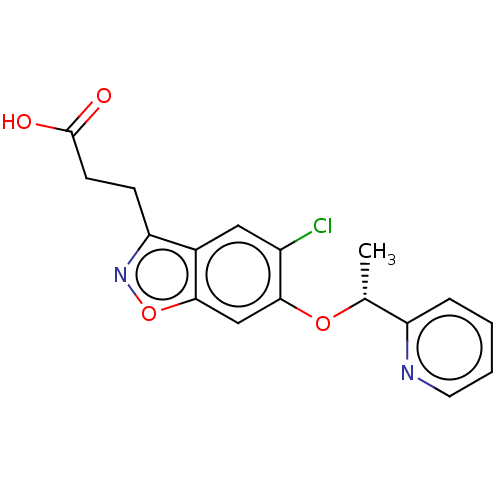
(CHEMBL4091152)Show SMILES C[C@@H](Oc1cc2onc(CCC(O)=O)c2cc1Cl)c1ccccn1 |r| Show InChI InChI=1S/C17H15ClN2O4/c1-10(13-4-2-3-7-19-13)23-16-9-15-11(8-12(16)18)14(20-24-15)5-6-17(21)22/h2-4,7-10H,5-6H2,1H3,(H,21,22)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KMO (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549706
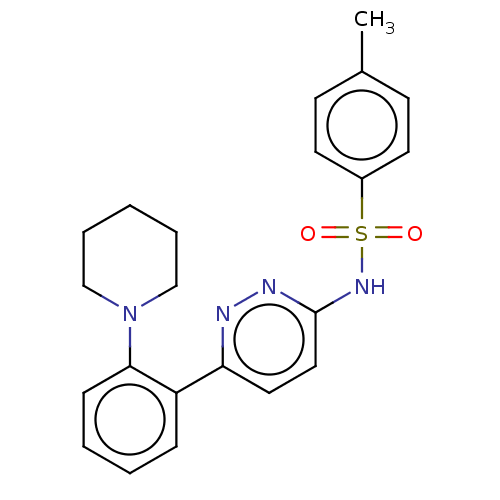
(CHEMBL4744083)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1ccccc1N1CCCCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549704

(CHEMBL4764931)Show SMILES CN(C)c1cccc(c1)S(=O)(=O)Nc1ccc(nn1)-c1ccccc1N1CCCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549706

(CHEMBL4744083)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1ccccc1N1CCCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549714
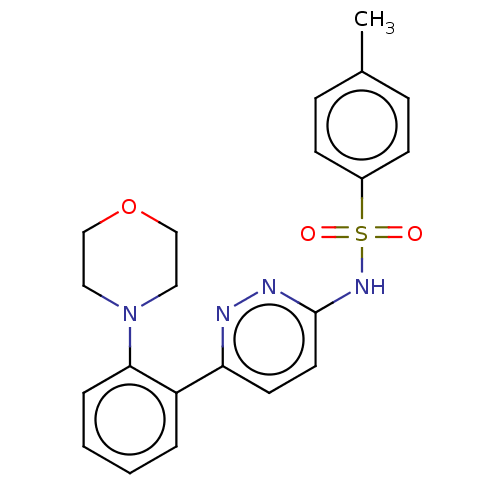
(CHEMBL4762572)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1ccccc1N1CCOCC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549707

(CHEMBL4781856)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1ccccc1N1CCCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549700

(CHEMBL4740126) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549715
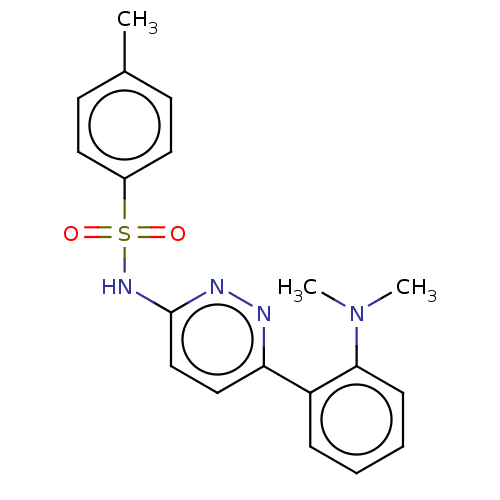
(CHEMBL4760680)Show SMILES CN(C)c1ccccc1-c1ccc(NS(=O)(=O)c2ccc(C)cc2)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549711
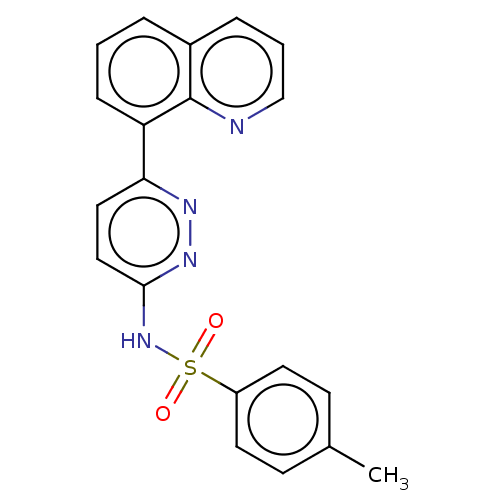
(CHEMBL4764672)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1cccc2cccnc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549700

(CHEMBL4740126) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549705

(CHEMBL4756311)Show SMILES CN1CCc2cccc(c12)-c1ccc(NS(=O)(=O)c2ccc(C)cc2)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549708

(CHEMBL4776854) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549712
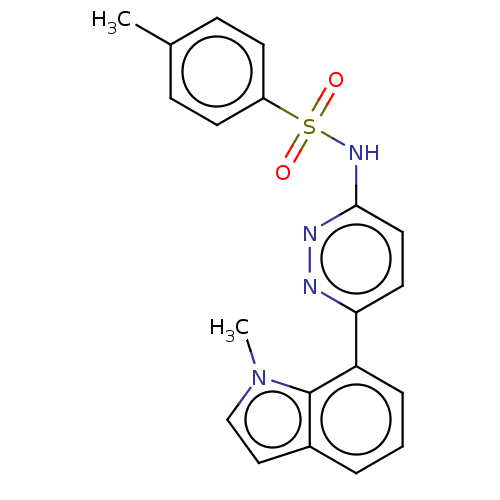
(CHEMBL4790147)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1cccc2ccn(C)c12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549719
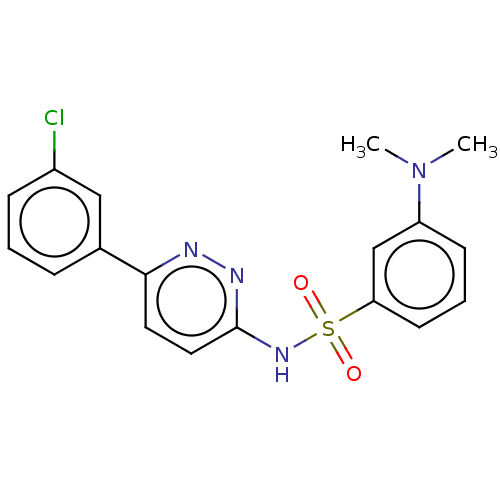
(CHEMBL4762842)Show SMILES CN(C)c1cccc(c1)S(=O)(=O)Nc1ccc(nn1)-c1cccc(Cl)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549701
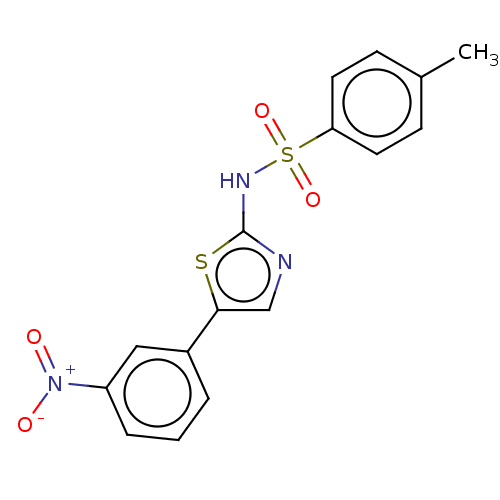
(CHEMBL4745247)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ncc(s1)-c1cccc(c1)[N+]([O-])=O | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549709
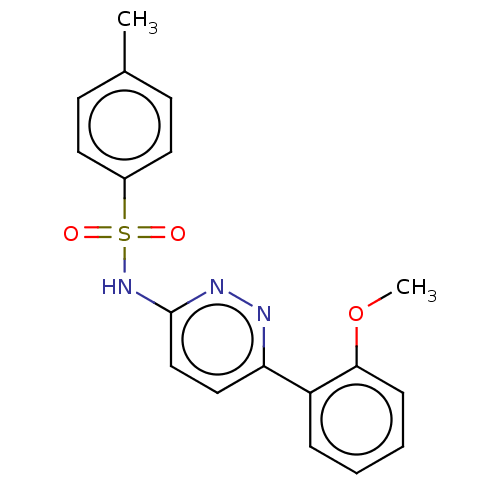
(CHEMBL4743950) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549701

(CHEMBL4745247)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ncc(s1)-c1cccc(c1)[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KMO (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50126147
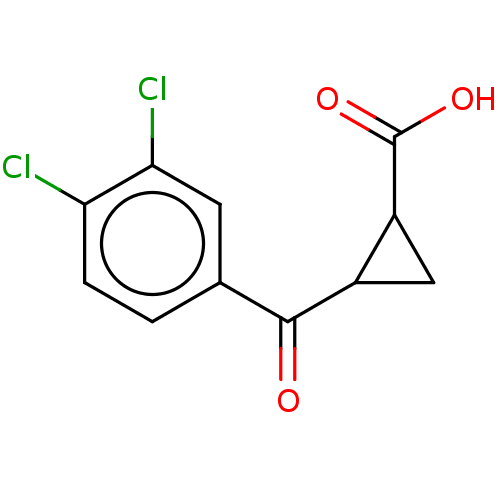
(CHEMBL3629571)Show InChI InChI=1S/C11H8Cl2O3/c12-8-2-1-5(3-9(8)13)10(14)6-4-7(6)11(15)16/h1-3,6-7H,4H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KMO (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549710
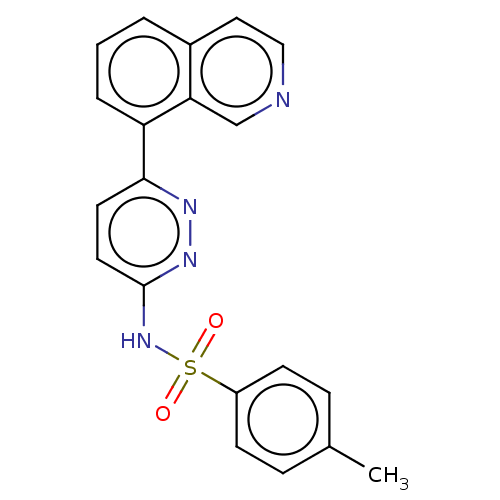
(CHEMBL4745015)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1cccc2ccncc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549701

(CHEMBL4745247)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1ncc(s1)-c1cccc(c1)[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549716
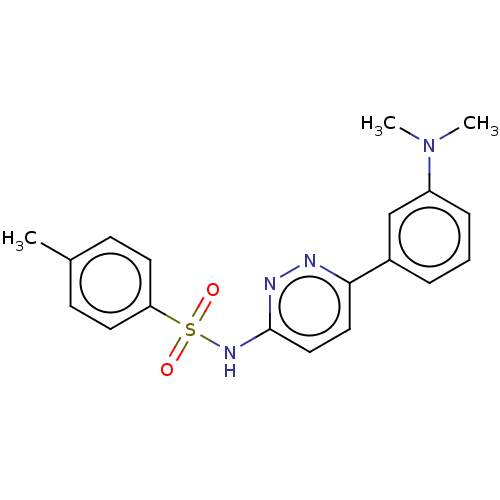
(CHEMBL4749459)Show SMILES CN(C)c1cccc(c1)-c1ccc(NS(=O)(=O)c2ccc(C)cc2)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549713
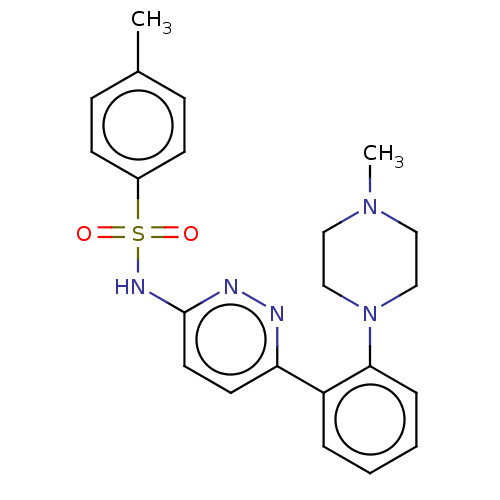
(CHEMBL4790669)Show SMILES CN1CCN(CC1)c1ccccc1-c1ccc(NS(=O)(=O)c2ccc(C)cc2)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549721
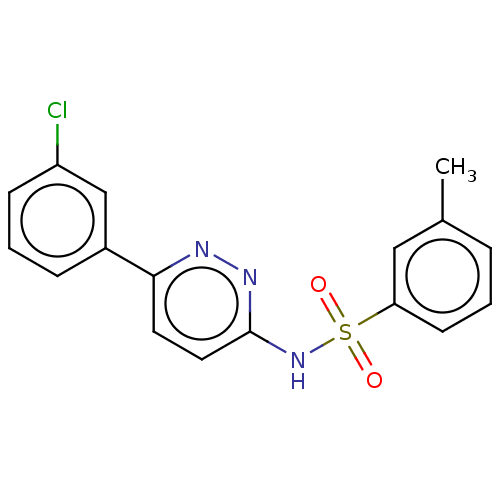
(CHEMBL4791546)Show SMILES Cc1cccc(c1)S(=O)(=O)Nc1ccc(nn1)-c1cccc(Cl)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549718
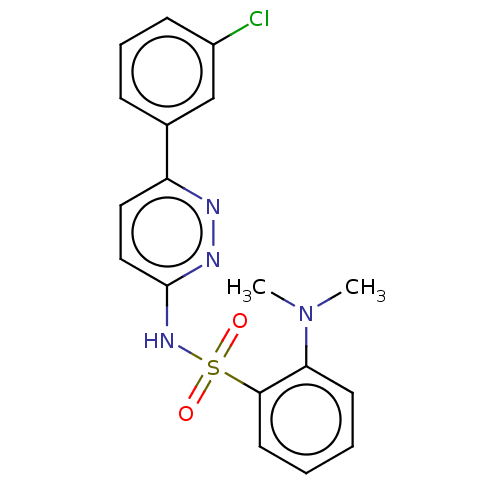
(CHEMBL4784999)Show SMILES CN(C)c1ccccc1S(=O)(=O)Nc1ccc(nn1)-c1cccc(Cl)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113633

(CHEMBL3604691)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3cc(C)c4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;4.42,14.95,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-17(31-25-24(16)36-14-21(34)32-25)12-30-26-7-9-27(10-8-26,37-15-26)6-5-18-19(28)13-29-20-3-4-22(35-2)33-23(18)20/h3-4,11,13,30H,5-10,12,14-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549720
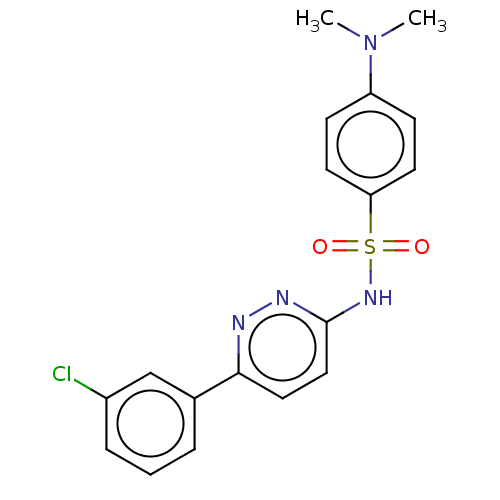
(CHEMBL4763938)Show SMILES CN(C)c1ccc(cc1)S(=O)(=O)Nc1ccc(nn1)-c1cccc(Cl)c1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113638

(CHEMBL3604797)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3C=C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.41,10.66,;8.05,9.48,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C29H32FN5O4/c1-4-18-17(2)26-27(34-23(36)15-38-26)33-22(18)14-32-28-9-11-29(12-10-28,39-16-28)8-7-19-20(30)13-31-21-5-6-24(37-3)35-25(19)21/h4-6,13,32H,1,7-12,14-16H2,2-3H3,(H,33,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113634

(CHEMBL3604692)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-16-17(2)25-26(33-22(35)14-37-25)32-21(16)13-31-27-8-10-28(11-9-27,38-15-27)7-6-18-19(29)12-30-20-4-5-23(36-3)34-24(18)20/h4-5,12,31H,6-11,13-15H2,1-3H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Mus musculus) | BDBM50549717
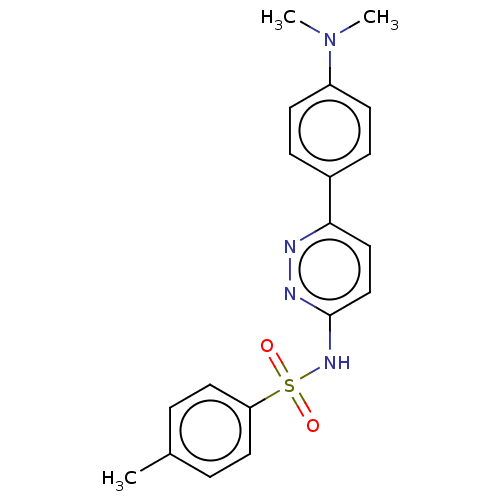
(CHEMBL4751196)Show SMILES CN(C)c1ccc(cc1)-c1ccc(NS(=O)(=O)c2ccc(C)cc2)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse liver mitochondrial KMO by measuring the 3-HK metabolite formation using L-kynurenine as substrate preincubated for 5 mins follow... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082303
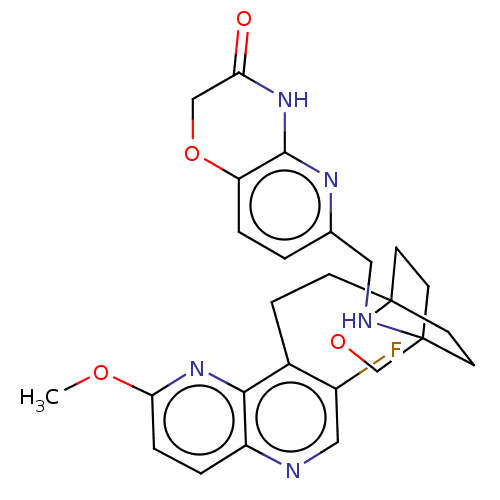
(CHEMBL3305005)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(5.32,-5.97,;5.32,-7.51,;6.66,-8.28,;6.65,-9.83,;7.99,-10.6,;9.32,-9.82,;10.66,-10.59,;11.99,-9.81,;11.98,-8.26,;11.97,-6.72,;10.65,-7.5,;10.64,-5.96,;11.97,-5.19,;13.3,-5.95,;13.3,-7.49,;14.63,-8.25,;15.97,-7.48,;15.28,-6.35,;14.06,-7.27,;15.96,-5.94,;14.62,-5.17,;17.29,-8.27,;17.28,-9.81,;18.6,-10.59,;19.93,-9.84,;21.25,-10.62,;21.24,-12.16,;22.57,-12.94,;22.54,-14.49,;21.2,-15.24,;21.18,-16.78,;19.88,-14.45,;19.9,-12.92,;18.57,-12.13,;9.32,-8.28,;7.98,-7.51,)| Show InChI InChI=1S/C26H28FN5O4/c1-34-22-5-3-19-23(32-22)17(18(27)13-28-19)6-7-26-10-8-25(9-11-26,15-36-26)29-12-16-2-4-20-24(30-16)31-21(33)14-35-20/h2-5,13,29H,6-12,14-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured for 5 mins by automated patch clamp assay |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113635
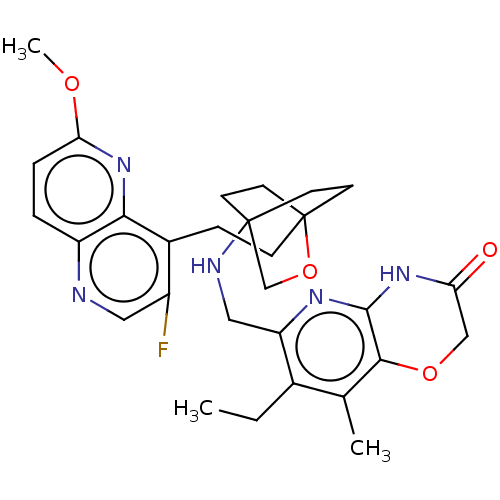
(CHEMBL3604693)Show SMILES CCc1c(C)c2OCC(=O)Nc2nc1CNC12CCC(CCc3c(F)cnc4ccc(OC)nc34)(CC1)OC2 |(8.05,9.48,;8.41,10.66,;7.36,11.79,;7.8,13.28,;9,13.57,;6.74,14.4,;7.18,15.88,;6.13,17,;4.63,16.64,;3.78,17.54,;4.19,15.17,;5.24,14.05,;4.8,12.57,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,;-1.33,1.54,;,.77,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,)| Show InChI InChI=1S/C29H34FN5O4/c1-4-18-17(2)26-27(34-23(36)15-38-26)33-22(18)14-32-28-9-11-29(12-10-28,39-16-28)8-7-19-20(30)13-31-21-5-6-24(37-3)35-25(19)21/h5-6,13,32H,4,7-12,14-16H2,1-3H3,(H,33,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113640

(CHEMBL3604799)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OC(C)C(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.07,17.4,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-25(34)33-24-21(37-16)5-3-17(31-24)13-30-26-9-11-27(12-10-26,36-15-26)8-7-18-19(28)14-29-20-4-6-22(35-2)32-23(18)20/h3-6,14,16,30H,7-13,15H2,1-2H3,(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113640

(CHEMBL3604799)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OC(C)C(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.07,17.4,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-25(34)33-24-21(37-16)5-3-17(31-24)13-30-26-9-11-27(12-10-26,36-15-26)8-7-18-19(28)14-29-20-4-6-22(35-2)32-23(18)20/h3-6,14,16,30H,7-13,15H2,1-2H3,(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113643

(CHEMBL3604802)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)N(C)c4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;10.16,12.72,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-33-23(34)15-36-21-5-3-17(31-25(21)33)13-30-26-9-11-27(12-10-26,37-16-26)8-7-18-19(28)14-29-20-4-6-22(35-2)32-24(18)20/h3-6,14,30H,7-13,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113639

(CHEMBL3604798)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3F)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H29F2N5O4/c1-15-22(29)19(32-25-24(15)37-13-20(35)33-25)12-31-26-7-9-27(10-8-26,38-14-26)6-5-16-17(28)11-30-18-3-4-21(36-2)34-23(16)18/h3-4,11,31H,5-10,12-14H2,1-2H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113632

(CHEMBL3604690)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4cc3C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-21-25(32-22(34)14-36-21)31-20(16)13-30-26-7-9-27(10-8-26,37-15-26)6-5-17-18(28)12-29-19-3-4-23(35-2)33-24(17)19/h3-4,11-12,30H,5-10,13-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113637

(CHEMBL3604694)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4c(C)c3C(C)C)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.8,13.28,;9,13.57,;7.36,11.79,;8.41,10.66,;8.05,9.48,;9.61,10.94,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C30H36FN5O4/c1-17(2)25-18(3)27-28(35-23(37)15-39-27)34-22(25)14-33-29-9-11-30(12-10-29,40-16-29)8-7-19-20(31)13-32-21-5-6-24(38-4)36-26(19)21/h5-6,13,17,33H,7-12,14-16H2,1-4H3,(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113644
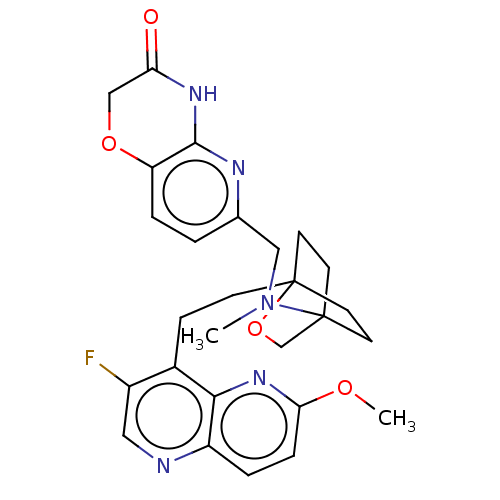
(CHEMBL3604803)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)N(C)Cc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;3.06,10.51,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-33(14-17-3-5-21-25(30-17)31-22(34)15-36-21)26-9-11-27(12-10-26,37-16-26)8-7-18-19(28)13-29-20-4-6-23(35-2)32-24(18)20/h3-6,13H,7-12,14-16H2,1-2H3,(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113631
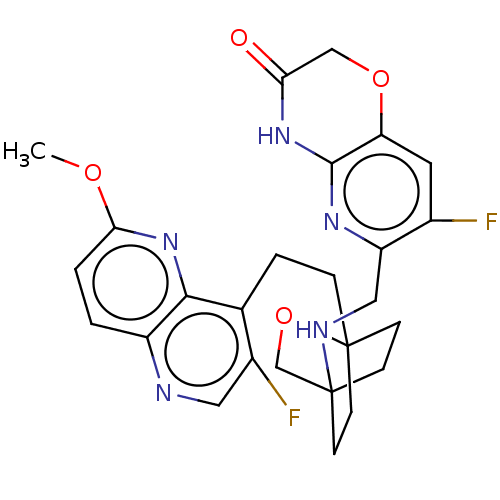
(CHEMBL3604688)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3nc4NC(=O)COc4cc3F)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.57,;5.24,14.05,;4.19,15.17,;4.63,16.64,;3.78,17.54,;6.13,17,;7.18,15.88,;6.74,14.4,;7.79,13.28,;7.36,11.79,;8.2,10.89,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H27F2N5O4/c1-35-22-3-2-18-23(33-22)15(17(28)11-29-18)4-5-26-8-6-25(7-9-26,14-37-26)30-12-19-16(27)10-20-24(31-19)32-21(34)13-36-20/h2-3,10-11,30H,4-9,12-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082303

(CHEMBL3305005)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(5.32,-5.97,;5.32,-7.51,;6.66,-8.28,;6.65,-9.83,;7.99,-10.6,;9.32,-9.82,;10.66,-10.59,;11.99,-9.81,;11.98,-8.26,;11.97,-6.72,;10.65,-7.5,;10.64,-5.96,;11.97,-5.19,;13.3,-5.95,;13.3,-7.49,;14.63,-8.25,;15.97,-7.48,;15.28,-6.35,;14.06,-7.27,;15.96,-5.94,;14.62,-5.17,;17.29,-8.27,;17.28,-9.81,;18.6,-10.59,;19.93,-9.84,;21.25,-10.62,;21.24,-12.16,;22.57,-12.94,;22.54,-14.49,;21.2,-15.24,;21.18,-16.78,;19.88,-14.45,;19.9,-12.92,;18.57,-12.13,;9.32,-8.28,;7.98,-7.51,)| Show InChI InChI=1S/C26H28FN5O4/c1-34-22-5-3-19-23(32-22)17(18(27)13-28-19)6-7-26-10-8-25(9-11-26,15-36-26)29-12-16-2-4-20-24(30-16)31-21(33)14-35-20/h2-5,13,29H,6-12,14-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113633

(CHEMBL3604691)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3cc(C)c4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;4.42,14.95,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-17(31-25-24(16)36-14-21(34)32-25)12-30-26-7-9-27(10-8-26,37-15-26)6-5-18-19(28)13-29-20-3-4-22(35-2)33-23(18)20/h3-4,11,13,30H,5-10,12,14-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells measured for 5 mins by automated patch clamp assay |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113642
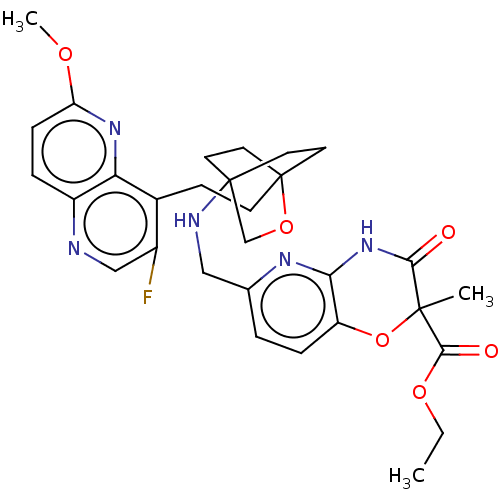
(CHEMBL3604801)Show SMILES CCOC(=O)C1(C)Oc2ccc(CNC34CCC(CCc5c(F)cnc6ccc(OC)nc56)(CC3)OC4)nc2NC1=O |(3.88,17.8,;5.08,18.08,;6.14,16.95,;7.64,17.3,;8,18.48,;8.71,16.22,;9.92,16.47,;7.21,15.87,;6.76,14.39,;5.26,14.05,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,;-1.33,1.54,;,.77,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,;9.76,15.09,;10.96,15.37,)| Show InChI InChI=1S/C30H34FN5O6/c1-4-40-27(38)28(2)26(37)36-25-22(42-28)7-5-18(34-25)15-33-29-11-13-30(14-12-29,41-17-29)10-9-19-20(31)16-32-21-6-8-23(39-3)35-24(19)21/h5-8,16,33H,4,9-15,17H2,1-3H3,(H,34,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113642

(CHEMBL3604801)Show SMILES CCOC(=O)C1(C)Oc2ccc(CNC34CCC(CCc5c(F)cnc6ccc(OC)nc56)(CC3)OC4)nc2NC1=O |(3.88,17.8,;5.08,18.08,;6.14,16.95,;7.64,17.3,;8,18.48,;8.71,16.22,;9.92,16.47,;7.21,15.87,;6.76,14.39,;5.26,14.05,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,;-1.33,1.54,;,.77,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,;9.76,15.09,;10.96,15.37,)| Show InChI InChI=1S/C30H34FN5O6/c1-4-40-27(38)28(2)26(37)36-25-22(42-28)7-5-18(34-25)15-33-29-11-13-30(14-12-29,41-17-29)10-9-19-20(31)16-32-21-6-8-23(39-3)35-24(19)21/h5-8,16,33H,4,9-15,17H2,1-3H3,(H,34,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113651

(CHEMBL3604807)Show SMILES COc1ccc2ncc(F)c(C[C@H](O)C3CCC(CO3)NCc3nc4NC(=O)COc4cc3F)c2n1 |r| Show InChI InChI=1S/C24H25F2N5O5/c1-34-22-5-3-16-23(31-22)13(15(26)8-28-16)6-18(32)19-4-2-12(10-35-19)27-9-17-14(25)7-20-24(29-17)30-21(33)11-36-20/h3,5,7-8,12,18-19,27,32H,2,4,6,9-11H2,1H3,(H,29,30,33)/t12?,18-,19?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |
Kynurenine 3-monooxygenase
(Homo sapiens (Human)) | BDBM50549703
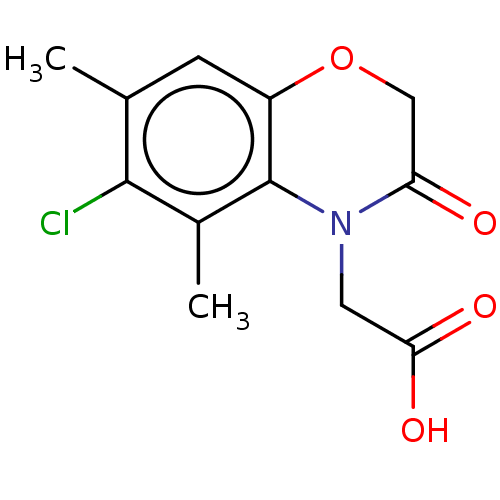
(CHEMBL4750377) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human KMO using L-kynurenine as substrate incubated for 25 mins in presence of NADPH by microplate reader based assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127753
BindingDB Entry DOI: 10.7270/Q2XK8K55 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50113641
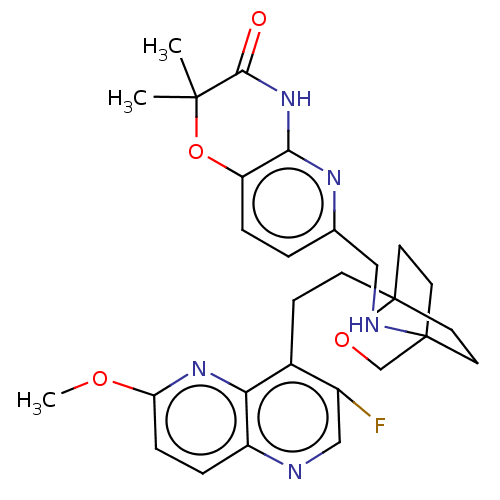
(CHEMBL3604800)Show SMILES COc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OC(C)(C)C(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.92,16.47,;7.84,17.1,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-26(2)25(35)34-24-21(38-26)6-4-17(32-24)14-31-27-10-12-28(13-11-27,37-16-27)9-8-18-19(29)15-30-20-5-7-22(36-3)33-23(18)20/h4-7,15,31H,8-14,16H2,1-3H3,(H,32,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 3636-43 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.057
BindingDB Entry DOI: 10.7270/Q2VH5QNN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data