 Found 125 hits with Last Name = 'van den heuvel' and Initial = 'm'
Found 125 hits with Last Name = 'van den heuvel' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414981

(CHEMBL567417)Show SMILES CCCC1(CC)Cc2c(O1)cccc2CN1CCC2(CC1)CCN(CC2)C(=O)c1ccc(N)cn1 Show InChI InChI=1S/C29H40N4O2/c1-3-10-29(4-2)19-24-22(6-5-7-26(24)35-29)21-32-15-11-28(12-16-32)13-17-33(18-14-28)27(34)25-9-8-23(30)20-31-25/h5-9,20H,3-4,10-19,21,30H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50414959
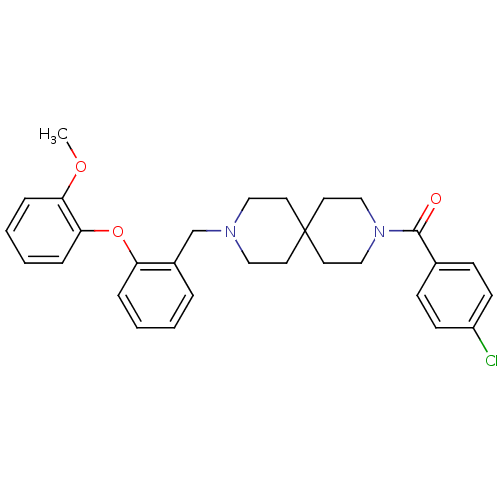
(CHEMBL409224)Show SMILES COc1ccccc1Oc1ccccc1CN1CCC2(CC1)CCN(CC2)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN2O3/c1-35-27-8-4-5-9-28(27)36-26-7-3-2-6-24(26)22-32-18-14-30(15-19-32)16-20-33(21-17-30)29(34)23-10-12-25(31)13-11-23/h2-13H,14-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human recombinant ERG expressed in HEK293 cells by patch clamp method |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414982
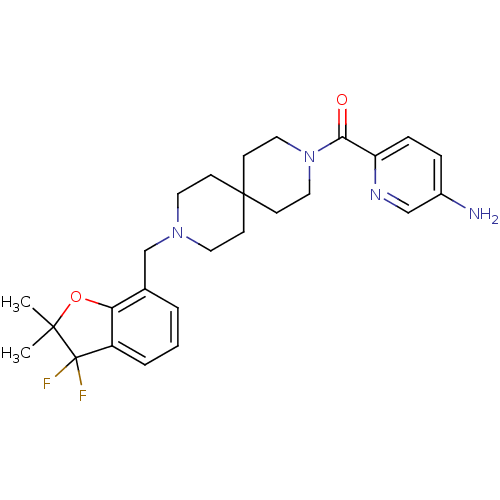
(CHEMBL579072)Show SMILES CC1(C)Oc2c(cccc2CN2CCC3(CC2)CCN(CC3)C(=O)c2ccc(N)cn2)C1(F)F Show InChI InChI=1S/C26H32F2N4O2/c1-24(2)26(27,28)20-5-3-4-18(22(20)34-24)17-31-12-8-25(9-13-31)10-14-32(15-11-25)23(33)21-7-6-19(29)16-30-21/h3-7,16H,8-15,17,29H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414974
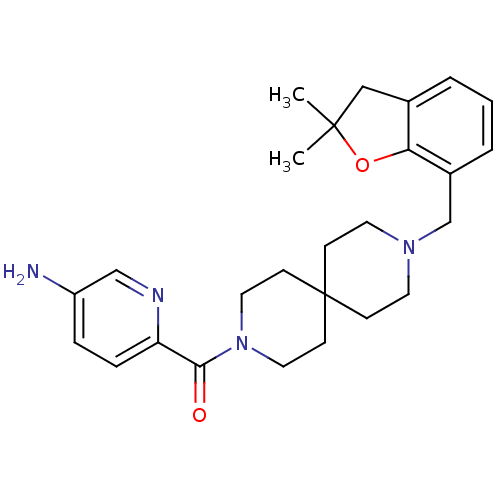
(CHEMBL568523)Show SMILES CC1(C)Cc2cccc(CN3CCC4(CC3)CCN(CC4)C(=O)c3ccc(N)cn3)c2O1 Show InChI InChI=1S/C26H34N4O2/c1-25(2)16-19-4-3-5-20(23(19)32-25)18-29-12-8-26(9-13-29)10-14-30(15-11-26)24(31)22-7-6-21(27)17-28-22/h3-7,17H,8-16,18,27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414980
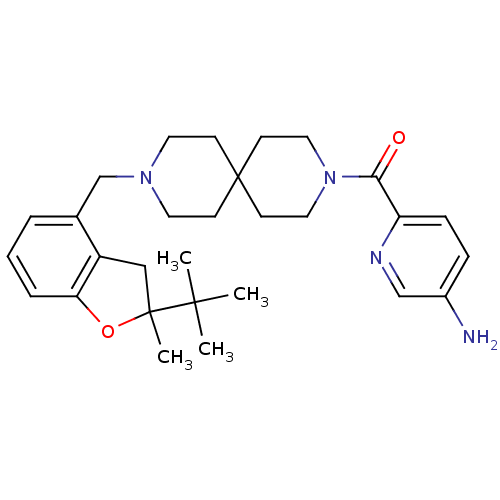
(CHEMBL568294)Show SMILES CC(C)(C)C1(C)Cc2c(O1)cccc2CN1CCC2(CC1)CCN(CC2)C(=O)c1ccc(N)cn1 Show InChI InChI=1S/C29H40N4O2/c1-27(2,3)28(4)18-23-21(6-5-7-25(23)35-28)20-32-14-10-29(11-15-32)12-16-33(17-13-29)26(34)24-9-8-22(30)19-31-24/h5-9,19H,10-18,20,30H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198484

(5-(5-((2-(benzofuran-4-yl)ethylamino)methyl)pyridi...)Show SMILES ONC(=O)c1ccc(s1)-c1ccc(CNCCc2cccc3occc23)cn1 Show InChI InChI=1S/C21H19N3O3S/c25-21(24-26)20-7-6-19(28-20)17-5-4-14(13-23-17)12-22-10-8-15-2-1-3-18-16(15)9-11-27-18/h1-7,9,11,13,22,26H,8,10,12H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414983

(CHEMBL574655)Show SMILES COc1ccccc1Oc1cccc(CN2CCC3(CC2)CCN(CC3)C(=O)c2ccncn2)c1 Show InChI InChI=1S/C28H32N4O3/c1-34-25-7-2-3-8-26(25)35-23-6-4-5-22(19-23)20-31-15-10-28(11-16-31)12-17-32(18-13-28)27(33)24-9-14-29-21-30-24/h2-9,14,19,21H,10-13,15-18,20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414986

(CHEMBL568522)Show SMILES CC1(C)Oc2c(CN3CCC4(CC3)CCN(CC4)C(=O)c3ccnc(N)c3)cccc2C=C1 |c:35| Show InChI InChI=1S/C27H34N4O2/c1-26(2)8-6-20-4-3-5-22(24(20)33-26)19-30-14-9-27(10-15-30)11-16-31(17-12-27)25(32)21-7-13-29-23(28)18-21/h3-8,13,18H,9-12,14-17,19H2,1-2H3,(H2,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.62 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198461
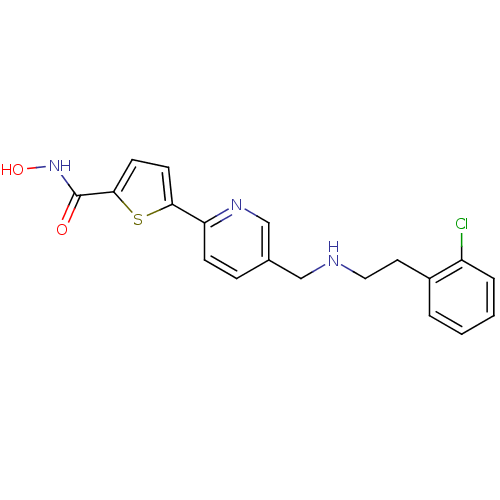
(5-(5-((2-chlorophenethylamino)methyl)pyridin-2-yl)...)Show InChI InChI=1S/C19H18ClN3O2S/c20-15-4-2-1-3-14(15)9-10-21-11-13-5-6-16(22-12-13)17-7-8-18(26-17)19(24)23-25/h1-8,12,21,25H,9-11H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50414958
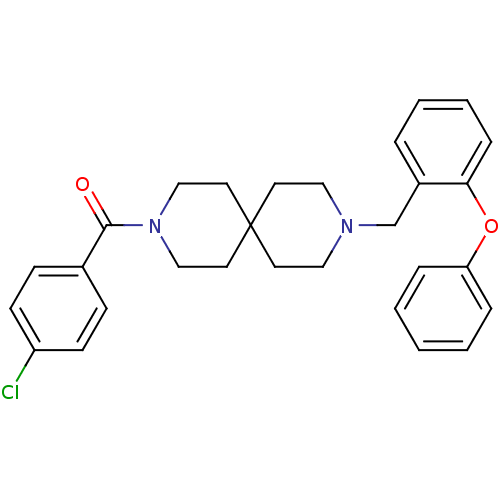
(CHEMBL259243)Show SMILES Clc1ccc(cc1)C(=O)N1CCC2(CCN(Cc3ccccc3Oc3ccccc3)CC2)CC1 Show InChI InChI=1S/C29H31ClN2O2/c30-25-12-10-23(11-13-25)28(33)32-20-16-29(17-21-32)14-18-31(19-15-29)22-24-6-4-5-9-27(24)34-26-7-2-1-3-8-26/h1-13H,14-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human recombinant ERG expressed in HEK293 cells by patch clamp method |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414975

(CHEMBL584087)Show SMILES CC1(C)Oc2cccc(CN3CCC4(CC3)CCN(CC4)C(=O)c3ccnc(N)c3)c2O1 Show InChI InChI=1S/C25H32N4O3/c1-24(2)31-20-5-3-4-19(22(20)32-24)17-28-12-7-25(8-13-28)9-14-29(15-10-25)23(30)18-6-11-27-21(26)16-18/h3-6,11,16H,7-10,12-15,17H2,1-2H3,(H2,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.61 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198462
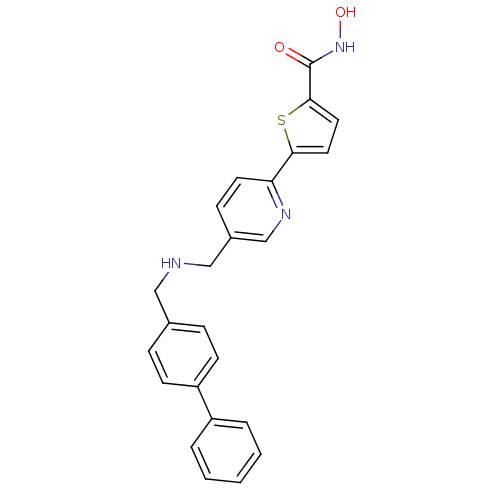
(5-(5-((biphenyl-4-ylmethylamino)methyl)pyridin-2-y...)Show SMILES ONC(=O)c1ccc(s1)-c1ccc(CNCc2ccc(cc2)-c2ccccc2)cn1 Show InChI InChI=1S/C24H21N3O2S/c28-24(27-29)23-13-12-22(30-23)21-11-8-18(16-26-21)15-25-14-17-6-9-20(10-7-17)19-4-2-1-3-5-19/h1-13,16,25,29H,14-15H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198480
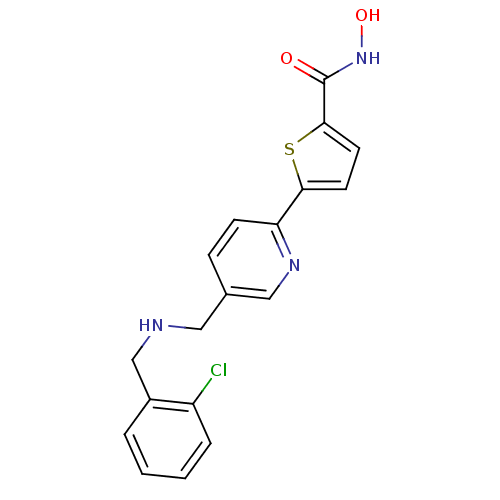
(5-(5-((2-chlorobenzylamino)methyl)pyridin-2-yl)-N-...)Show InChI InChI=1S/C18H16ClN3O2S/c19-14-4-2-1-3-13(14)11-20-9-12-5-6-15(21-10-12)16-7-8-17(25-16)18(23)22-24/h1-8,10,20,24H,9,11H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198469

(CHEMBL441699 | N-hydroxy-5-(5-((2-methylphenethyla...)Show InChI InChI=1S/C20H21N3O2S/c1-14-4-2-3-5-16(14)10-11-21-12-15-6-7-17(22-13-15)18-8-9-19(26-18)20(24)23-25/h2-9,13,21,25H,10-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198473
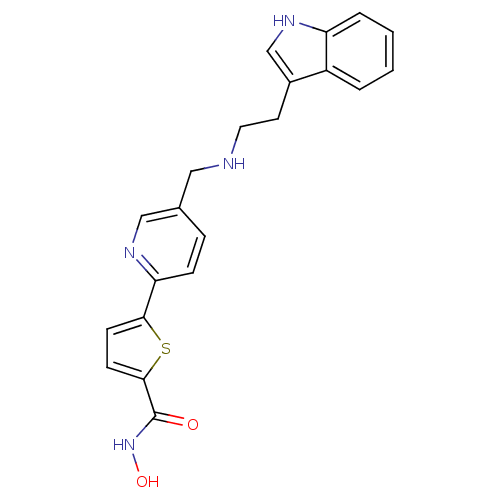
(5-(5-((2-(1H-indol-3-yl)ethylamino)methyl)pyridin-...)Show SMILES ONC(=O)c1ccc(s1)-c1ccc(CNCCc2c[nH]c3ccccc23)cn1 Show InChI InChI=1S/C21H20N4O2S/c26-21(25-27)20-8-7-19(28-20)18-6-5-14(12-23-18)11-22-10-9-15-13-24-17-4-2-1-3-16(15)17/h1-8,12-13,22,24,27H,9-11H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198475

(5-(5-((benzo[d][1,3]dioxol-5-ylmethylamino)methyl)...)Show SMILES ONC(=O)c1ccc(s1)-c1ccc(CNCc2ccc3OCOc3c2)cn1 Show InChI InChI=1S/C19H17N3O4S/c23-19(22-24)18-6-5-17(27-18)14-3-1-13(10-21-14)9-20-8-12-2-4-15-16(7-12)26-11-25-15/h1-7,10,20,24H,8-9,11H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414978

(CHEMBL566543)Show SMILES CC1(C)Cc2c(O1)cccc2CN1CCC2(CC1)CCN(CC2)C(=O)c1ccc(N)cn1 Show InChI InChI=1S/C26H34N4O2/c1-25(2)16-21-19(4-3-5-23(21)32-25)18-29-12-8-26(9-13-29)10-14-30(15-11-26)24(31)22-7-6-20(27)17-28-22/h3-7,17H,8-16,18,27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198478

(5-(5-(2-(benzylamino)ethyl)pyridin-2-yl)-N-hydroxy...)Show InChI InChI=1S/C19H19N3O2S/c23-19(22-24)18-9-8-17(25-18)16-7-6-15(13-21-16)10-11-20-12-14-4-2-1-3-5-14/h1-9,13,20,24H,10-12H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198470
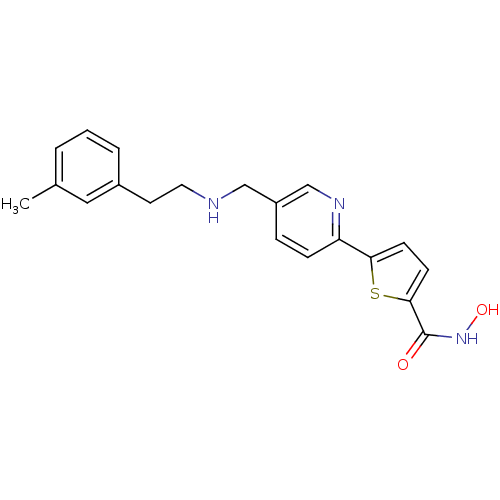
(CHEMBL436964 | N-hydroxy-5-(5-((3-methylphenethyla...)Show InChI InChI=1S/C20H21N3O2S/c1-14-3-2-4-15(11-14)9-10-21-12-16-5-6-17(22-13-16)18-7-8-19(26-18)20(24)23-25/h2-8,11,13,21,25H,9-10,12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198454

(5-(5-((4-chlorobenzylamino)methyl)pyridin-2-yl)-N-...)Show InChI InChI=1S/C18H16ClN3O2S/c19-14-4-1-12(2-5-14)9-20-10-13-3-6-15(21-11-13)16-7-8-17(25-16)18(23)22-24/h1-8,11,20,24H,9-10H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198463

(5-(5-((4-fluorophenethylamino)methyl)pyridin-2-yl)...)Show InChI InChI=1S/C19H18FN3O2S/c20-15-4-1-13(2-5-15)9-10-21-11-14-3-6-16(22-12-14)17-7-8-18(26-17)19(24)23-25/h1-8,12,21,25H,9-11H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198472
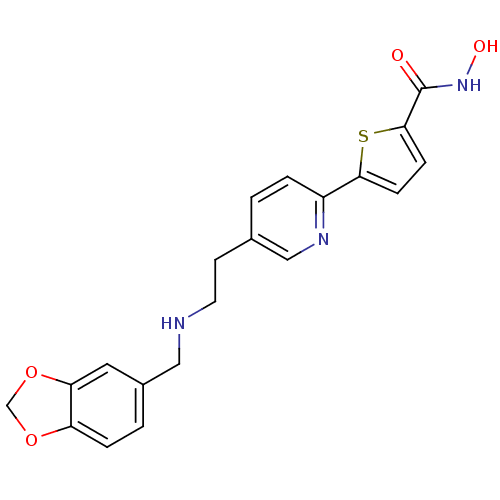
(5-(5-(2-(benzo[d][1,3]dioxol-5-ylmethylamino)ethyl...)Show SMILES ONC(=O)c1ccc(s1)-c1ccc(CCNCc2ccc3OCOc3c2)cn1 Show InChI InChI=1S/C20H19N3O4S/c24-20(23-25)19-6-5-18(28-19)15-3-1-13(11-22-15)7-8-21-10-14-2-4-16-17(9-14)27-12-26-16/h1-6,9,11,21,25H,7-8,10,12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198453
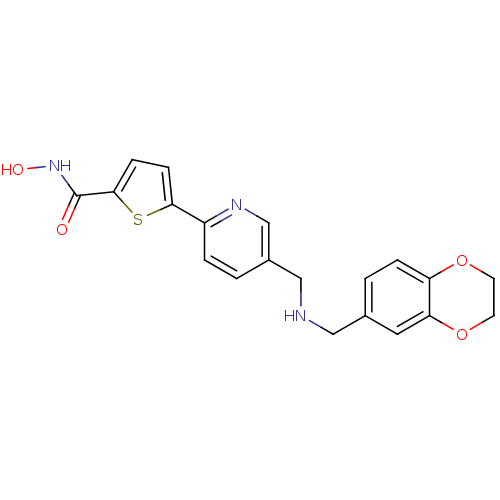
(5-(5-(((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)methyl...)Show SMILES ONC(=O)c1ccc(s1)-c1ccc(CNCc2ccc3OCCOc3c2)cn1 Show InChI InChI=1S/C20H19N3O4S/c24-20(23-25)19-6-5-18(28-19)15-3-1-14(12-22-15)11-21-10-13-2-4-16-17(9-13)27-8-7-26-16/h1-6,9,12,21,25H,7-8,10-11H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198465
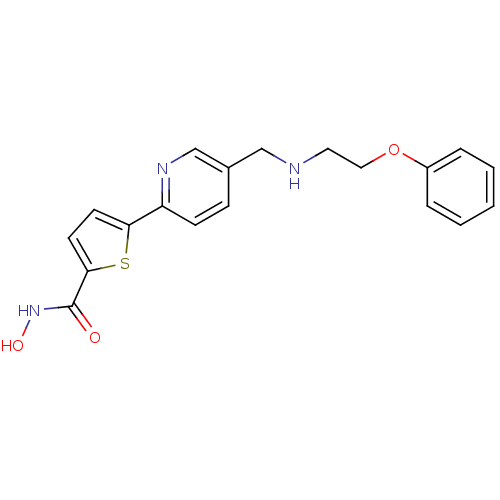
(CHEMBL387247 | N-hydroxy-5-(5-((2-phenoxyethylamin...)Show InChI InChI=1S/C19H19N3O3S/c23-19(22-24)18-9-8-17(26-18)16-7-6-14(13-21-16)12-20-10-11-25-15-4-2-1-3-5-15/h1-9,13,20,24H,10-12H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414964

(CHEMBL271128)Show SMILES COc1ccccc1Oc1ccccc1CN1CCC2(CC1)CCN(CC2)C(=O)c1ccncc1 Show InChI InChI=1S/C29H33N3O3/c1-34-26-8-4-5-9-27(26)35-25-7-3-2-6-24(25)22-31-18-12-29(13-19-31)14-20-32(21-15-29)28(33)23-10-16-30-17-11-23/h2-11,16-17H,12-15,18-22H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198466

(5-(5-((2-fluorophenethylamino)methyl)pyridin-2-yl)...)Show InChI InChI=1S/C19H18FN3O2S/c20-15-4-2-1-3-14(15)9-10-21-11-13-5-6-16(22-12-13)17-7-8-18(26-17)19(24)23-25/h1-8,12,21,25H,9-11H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198455
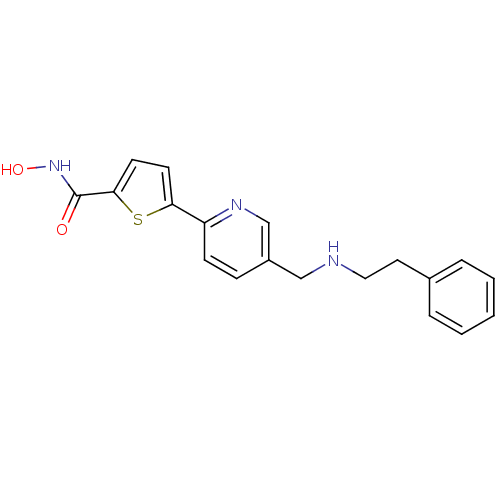
(CHEMBL217531 | N-hydroxy-5-(5-((phenethylamino)met...)Show InChI InChI=1S/C19H19N3O2S/c23-19(22-24)18-9-8-17(25-18)16-7-6-15(13-21-16)12-20-11-10-14-4-2-1-3-5-14/h1-9,13,20,24H,10-12H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198464
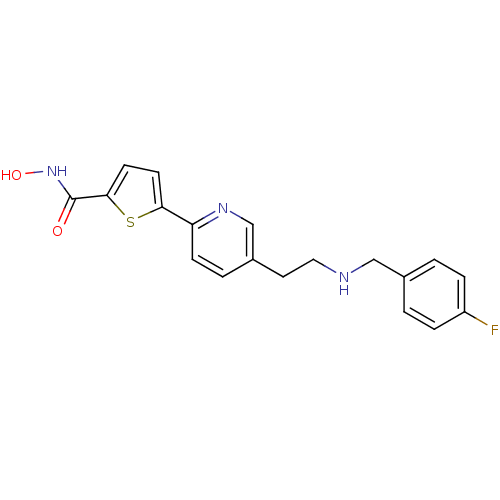
(5-(5-(2-(4-fluorobenzylamino)ethyl)pyridin-2-yl)-N...)Show InChI InChI=1S/C19H18FN3O2S/c20-15-4-1-13(2-5-15)11-21-10-9-14-3-6-16(22-12-14)17-7-8-18(26-17)19(24)23-25/h1-8,12,21,25H,9-11H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198468
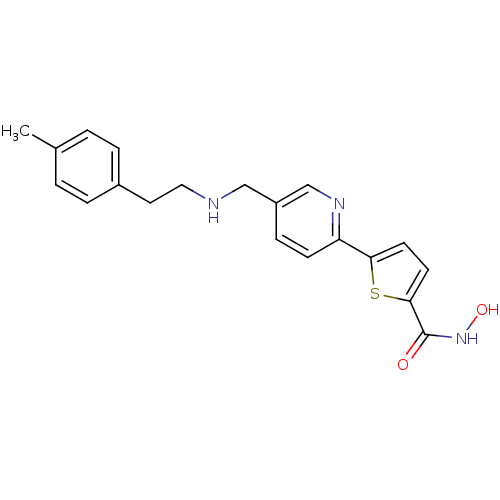
(CHEMBL216896 | N-hydroxy-5-(5-((4-methylphenethyla...)Show InChI InChI=1S/C20H21N3O2S/c1-14-2-4-15(5-3-14)10-11-21-12-16-6-7-17(22-13-16)18-8-9-19(26-18)20(24)23-25/h2-9,13,21,25H,10-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414966

(CHEMBL573216)Show SMILES COc1ccccc1Oc1ccccc1CN1CCC2(CC1)CCN(CC2)C(=O)c1ccncn1 Show InChI InChI=1S/C28H32N4O3/c1-34-25-8-4-5-9-26(25)35-24-7-3-2-6-22(24)20-31-16-11-28(12-17-31)13-18-32(19-14-28)27(33)23-10-15-29-21-30-23/h2-10,15,21H,11-14,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198483
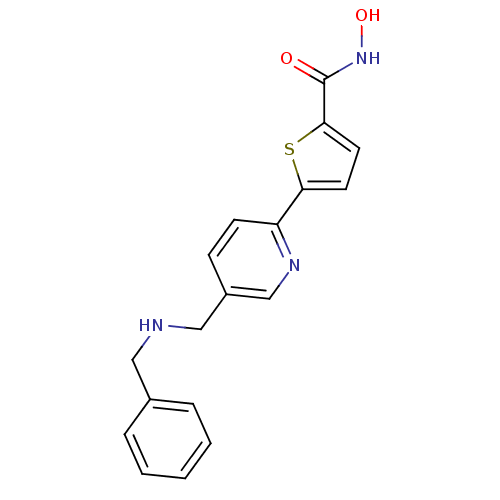
(5-(5-((benzylamino)methyl)pyridin-2-yl)-N-hydroxyt...)Show InChI InChI=1S/C18H17N3O2S/c22-18(21-23)17-9-8-16(24-17)15-7-6-14(12-20-15)11-19-10-13-4-2-1-3-5-13/h1-9,12,19,23H,10-11H2,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198474
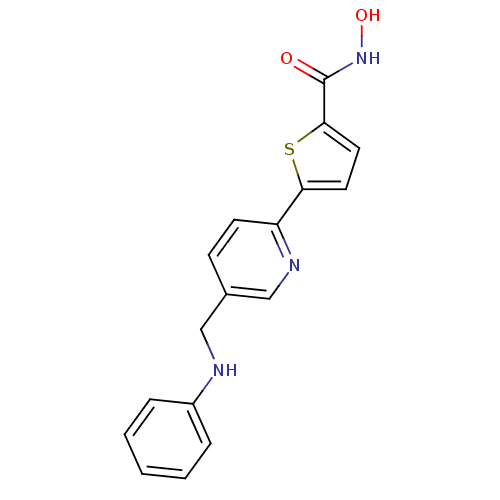
(CHEMBL263570 | N-hydroxy-5-(5-((phenylamino)methyl...)Show InChI InChI=1S/C17H15N3O2S/c21-17(20-22)16-9-8-15(23-16)14-7-6-12(11-19-14)10-18-13-4-2-1-3-5-13/h1-9,11,18,22H,10H2,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198458

(CHEMBL385934 | N-hydroxy-5-(5-((2-methoxyphenethyl...)Show InChI InChI=1S/C20H21N3O3S/c1-26-17-5-3-2-4-15(17)10-11-21-12-14-6-7-16(22-13-14)18-8-9-19(27-18)20(24)23-25/h2-9,13,21,25H,10-12H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414967
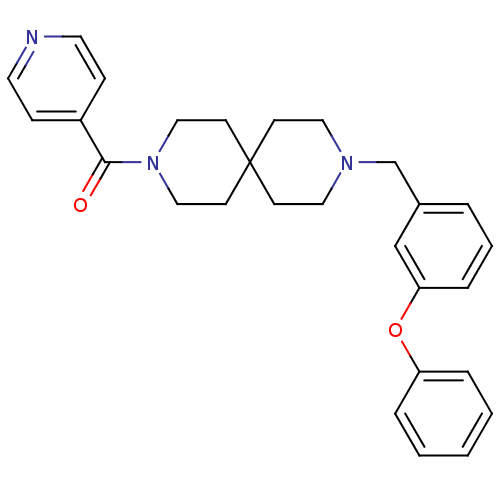
(CHEMBL271801)Show SMILES O=C(N1CCC2(CCN(Cc3cccc(Oc4ccccc4)c3)CC2)CC1)c1ccncc1 Show InChI InChI=1S/C28H31N3O2/c32-27(24-9-15-29-16-10-24)31-19-13-28(14-20-31)11-17-30(18-12-28)22-23-5-4-8-26(21-23)33-25-6-2-1-3-7-25/h1-10,15-16,21H,11-14,17-20,22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198456
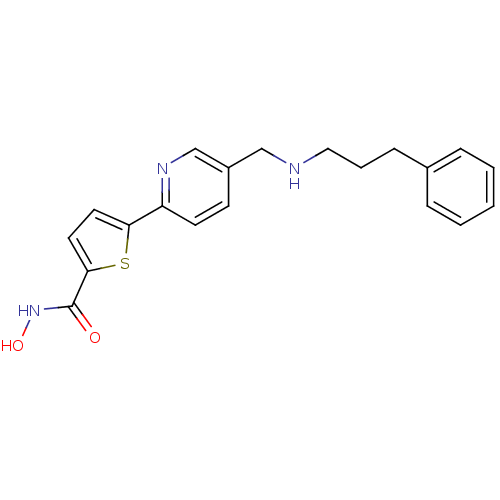
(CHEMBL217529 | N-hydroxy-5-(5-((3-phenylpropylamin...)Show InChI InChI=1S/C20H21N3O2S/c24-20(23-25)19-11-10-18(26-19)17-9-8-16(14-22-17)13-21-12-4-7-15-5-2-1-3-6-15/h1-3,5-6,8-11,14,21,25H,4,7,12-13H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414957

(CHEMBL429436)Show SMILES Clc1ccc(cc1)C(=O)N1CCC2(CCN(Cc3cccc(Oc4ccccc4)c3)CC2)CC1 Show InChI InChI=1S/C29H31ClN2O2/c30-25-11-9-24(10-12-25)28(33)32-19-15-29(16-20-32)13-17-31(18-14-29)22-23-5-4-8-27(21-23)34-26-6-2-1-3-7-26/h1-12,21H,13-20,22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414976
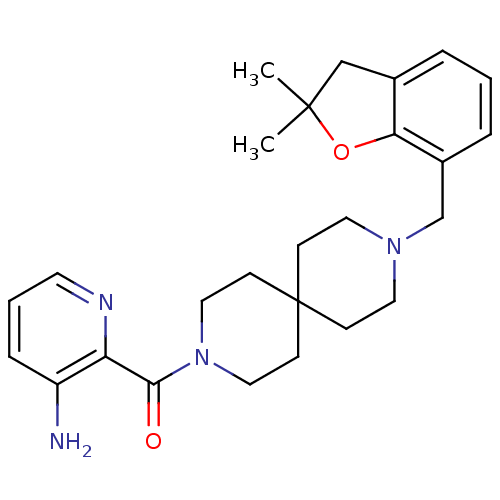
(CHEMBL566324)Show SMILES CC1(C)Cc2cccc(CN3CCC4(CC3)CCN(CC4)C(=O)c3ncccc3N)c2O1 Show InChI InChI=1S/C26H34N4O2/c1-25(2)17-19-5-3-6-20(23(19)32-25)18-29-13-8-26(9-14-29)10-15-30(16-11-26)24(31)22-21(27)7-4-12-28-22/h3-7,12H,8-11,13-18,27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414977

(CHEMBL567627)Show SMILES CC1(C)Cc2c(O1)cccc2CN1CCC2(CC1)CCN(CC2)C(=O)c1ccnc(N)c1 Show InChI InChI=1S/C26H34N4O2/c1-25(2)17-21-20(4-3-5-22(21)32-25)18-29-12-7-26(8-13-29)9-14-30(15-10-26)24(31)19-6-11-28-23(27)16-19/h3-6,11,16H,7-10,12-15,17-18H2,1-2H3,(H2,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414968

(CHEMBL410885)Show SMILES O=C(N1CCC2(CCN(Cc3cccc(Oc4ccccc4)c3)CC2)CC1)c1ccncn1 Show InChI InChI=1S/C27H30N4O2/c32-26(25-9-14-28-21-29-25)31-17-12-27(13-18-31)10-15-30(16-11-27)20-22-5-4-8-24(19-22)33-23-6-2-1-3-7-23/h1-9,14,19,21H,10-13,15-18,20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198218
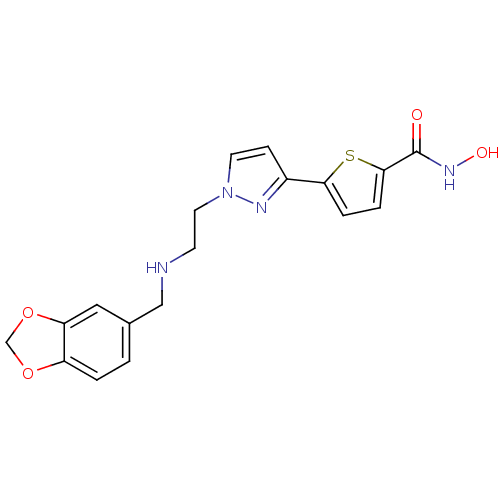
(5-(1-(2-(benzo[d][1,3]dioxol-5-ylmethylamino)ethyl...)Show SMILES ONC(=O)c1ccc(s1)-c1ccn(CCNCc2ccc3OCOc3c2)n1 Show InChI InChI=1S/C18H18N4O4S/c23-18(21-24)17-4-3-16(27-17)13-5-7-22(20-13)8-6-19-10-12-1-2-14-15(9-12)26-11-25-14/h1-5,7,9,19,24H,6,8,10-11H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198482

(5-(5-((3,4-dihydroisoquinolin-2(1H)-yl)methyl)pyri...)Show InChI InChI=1S/C20H19N3O2S/c24-20(22-25)19-8-7-18(26-19)17-6-5-14(11-21-17)12-23-10-9-15-3-1-2-4-16(15)13-23/h1-8,11,25H,9-10,12-13H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50414964

(CHEMBL271128)Show SMILES COc1ccccc1Oc1ccccc1CN1CCC2(CC1)CCN(CC2)C(=O)c1ccncc1 Show InChI InChI=1S/C29H33N3O3/c1-34-26-8-4-5-9-27(26)35-25-7-3-2-6-24(25)22-31-18-12-29(13-19-31)14-20-32(21-15-29)28(33)23-10-16-30-17-11-23/h2-11,16-17H,12-15,18-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human recombinant ERG expressed in HEK293 cells by patch clamp method |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414979
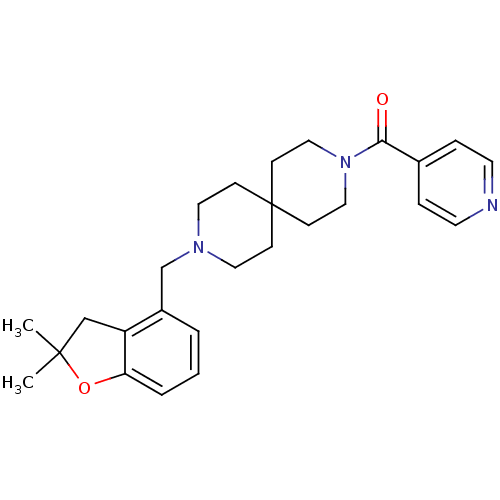
(CHEMBL583621)Show SMILES CC1(C)Cc2c(O1)cccc2CN1CCC2(CC1)CCN(CC2)C(=O)c1ccncc1 Show InChI InChI=1S/C26H33N3O2/c1-25(2)18-22-21(4-3-5-23(22)31-25)19-28-14-8-26(9-15-28)10-16-29(17-11-26)24(30)20-6-12-27-13-7-20/h3-7,12-13H,8-11,14-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198471

(CHEMBL279353 | N-hydroxy-5-(5-(2-(phenethylamino)e...)Show InChI InChI=1S/C20H21N3O2S/c24-20(23-25)19-9-8-18(26-19)17-7-6-16(14-22-17)11-13-21-12-10-15-4-2-1-3-5-15/h1-9,14,21,25H,10-13H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414969

(CHEMBL272022)Show SMILES COc1ccccc1Oc1cccc(CN2CCC3(CC2)CCN(CC3)C(=O)c2cc[n+]([O-])cc2)c1 Show InChI InChI=1S/C29H33N3O4/c1-35-26-7-2-3-8-27(26)36-25-6-4-5-23(21-25)22-30-17-11-29(12-18-30)13-19-31(20-14-29)28(33)24-9-15-32(34)16-10-24/h2-10,15-16,21H,11-14,17-20,22H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414959

(CHEMBL409224)Show SMILES COc1ccccc1Oc1ccccc1CN1CCC2(CC1)CCN(CC2)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN2O3/c1-35-27-8-4-5-9-28(27)36-26-7-3-2-6-24(26)22-32-18-14-30(15-19-32)16-20-33(21-17-30)29(34)23-10-12-25(31)13-11-23/h2-13H,14-22H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34.7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50198459
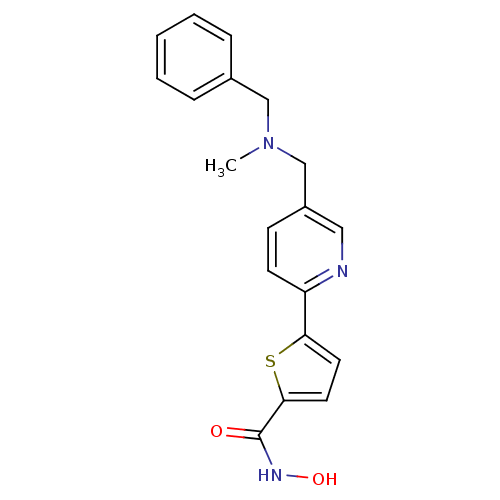
(5-(5-((benzyl(methyl)amino)methyl)pyridin-2-yl)-N-...)Show InChI InChI=1S/C19H19N3O2S/c1-22(12-14-5-3-2-4-6-14)13-15-7-8-16(20-11-15)17-9-10-18(25-17)19(23)21-24/h2-11,24H,12-13H2,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC activity measured by HDAC Fluorescent Activity Assay (mean of two experiments) |
Bioorg Med Chem Lett 17: 363-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.045
BindingDB Entry DOI: 10.7270/Q2VT1RR0 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50414964

(CHEMBL271128)Show SMILES COc1ccccc1Oc1ccccc1CN1CCC2(CC1)CCN(CC2)C(=O)c1ccncc1 Show InChI InChI=1S/C29H33N3O3/c1-34-26-8-4-5-9-27(26)35-25-7-3-2-6-24(25)22-31-18-12-29(13-19-31)14-20-32(21-15-29)28(33)23-10-16-30-17-11-23/h2-11,16-17H,12-15,18-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45.7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ERG expressed in CHOK1 cells by whole-cell plate-based electrophysiology |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 8
(Homo sapiens (Human)) | BDBM50414970

(CHEMBL583843)Show SMILES COc1ccccc1Oc1ccccc1CN1CCC2(CC1)CCN(CC2)C(=O)c1ccc[nH]c1=O Show InChI InChI=1S/C29H33N3O4/c1-35-25-10-4-5-11-26(25)36-24-9-3-2-7-22(24)21-31-17-12-29(13-18-31)14-19-32(20-15-29)28(34)23-8-6-16-30-27(23)33/h2-11,16H,12-15,17-21H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55.0 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR8 |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50414966

(CHEMBL573216)Show SMILES COc1ccccc1Oc1ccccc1CN1CCC2(CC1)CCN(CC2)C(=O)c1ccncn1 Show InChI InChI=1S/C28H32N4O3/c1-34-25-8-4-5-9-26(25)35-24-7-3-2-6-22(24)20-31-16-11-28(12-17-31)13-18-32(19-14-28)27(33)23-10-15-29-21-30-23/h2-10,15,21H,11-14,16-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type human ERG expressed in CHOK1 cells by whole-cell plate-based electrophysiology |
J Med Chem 52: 7706-23 (2009)
Article DOI: 10.1021/jm900713y
BindingDB Entry DOI: 10.7270/Q2TH8NZP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data