 Found 1868 hits with Last Name = 'bryan' and Initial = 'mc'
Found 1868 hits with Last Name = 'bryan' and Initial = 'mc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system by mass... |
ACS Med Chem Lett 7: 100-4 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00428
BindingDB Entry DOI: 10.7270/Q25T3NCC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50344100
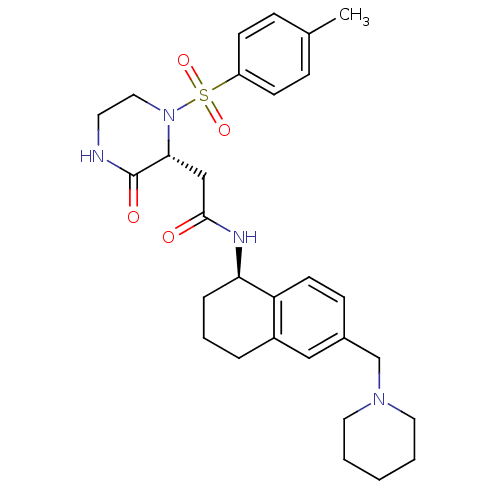
(2-((2R)-1-((4-methylphenyl)sulfonyl)-3-oxo-2-piper...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCNC(=O)[C@H]1CC(=O)N[C@@H]1CCCc2cc(CN3CCCCC3)ccc12 |r| Show InChI InChI=1S/C29H38N4O4S/c1-21-8-11-24(12-9-21)38(36,37)33-17-14-30-29(35)27(33)19-28(34)31-26-7-5-6-23-18-22(10-13-25(23)26)20-32-15-3-2-4-16-32/h8-13,18,26-27H,2-7,14-17,19-20H2,1H3,(H,30,35)(H,31,34)/t26-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human B1 receptor |
J Med Chem 54: 7232-46 (2011)
Article DOI: 10.1021/jm200808v
BindingDB Entry DOI: 10.7270/Q28C9WP1 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504229

(N-[2-[(2R)-2-Fluoro-3-hydroxy- 3-methyl-butyl]-6-i...)Show SMILES CC(C)Oc1cc2C(=O)N(C[C@@H](F)C(C)(C)O)Cc2cc1NC(=O)c1cnn2cccnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636
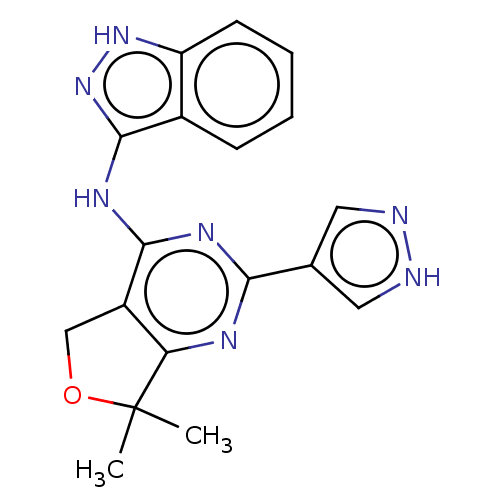
(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504219
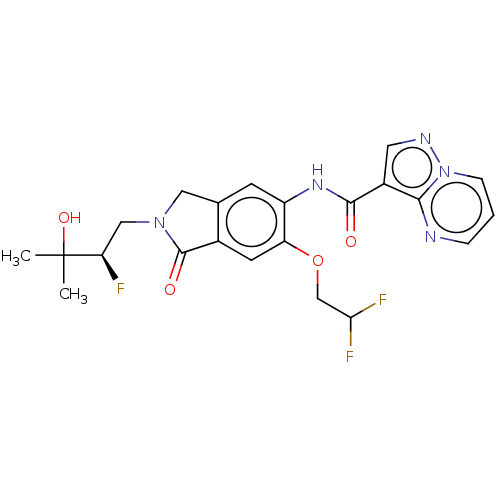
((R)-N-(6-(2,2-Difluoroethoxy)- 2-(2-fluoro-3-hydro...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(OCC(F)F)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504215

(N-[6-(3-Fluorocyclobutoxy)-2- [(2R)-2-fluoro-3-hyd...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(O[C@@H]3C[C@H](F)C3)cc2C1=O |r,wU:26.27,28.30,wD:4.4,(-7.2,.34,;-7.24,1.88,;-8.73,1.48,;-8.01,3.22,;-5.7,1.88,;-4.93,3.22,;-4.93,.55,;-3.39,.55,;-2.49,1.79,;-1.02,1.32,;.31,2.09,;1.64,1.32,;2.98,2.09,;2.98,3.63,;1.64,4.4,;4.31,4.4,;4.47,5.93,;5.98,6.25,;6.75,4.92,;8.25,4.6,;8.73,3.13,;7.7,1.99,;6.19,2.31,;5.72,3.77,;1.64,-.22,;2.98,-.99,;2.98,-2.53,;1.89,-3.62,;2.98,-4.71,;2.98,-6.25,;4.07,-3.62,;.31,-.99,;-1.02,-.22,;-2.49,-.7,;-2.96,-2.16,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50355063
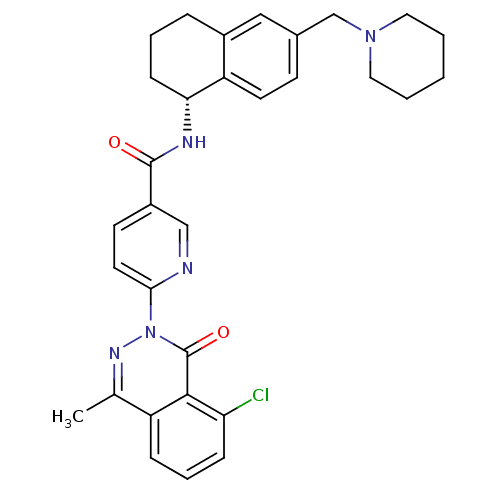
(CHEMBL1834619)Show SMILES Cc1nn(-c2ccc(cn2)C(=O)N[C@@H]2CCCc3cc(CN4CCCCC4)ccc23)c(=O)c2c(Cl)cccc12 |r| Show InChI InChI=1S/C31H32ClN5O2/c1-20-24-8-6-9-26(32)29(24)31(39)37(35-20)28-14-12-23(18-33-28)30(38)34-27-10-5-7-22-17-21(11-13-25(22)27)19-36-15-3-2-4-16-36/h6,8-9,11-14,17-18,27H,2-5,7,10,15-16,19H2,1H3,(H,34,38)/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human B1 receptor |
J Med Chem 54: 7232-46 (2011)
Article DOI: 10.1021/jm200808v
BindingDB Entry DOI: 10.7270/Q28C9WP1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613695
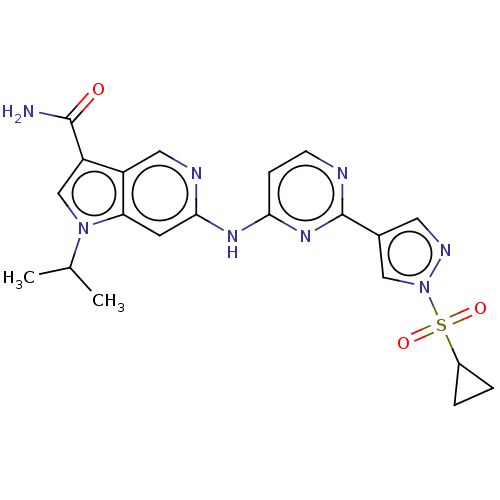
(CHEMBL5274166)Show SMILES CC(C)n1cc(C(N)=O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504233

((R)-N-(6-Ethoxy-2-(2-fluoro-3- hydroxy-3-methylbut...)Show SMILES CCOc1cc2C(=O)N(C[C@@H](F)C(C)(C)O)Cc2cc1NC(=O)c1cnn2cccnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141902

(CHEMBL3758602)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2c(Cl)cccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H16ClN7O/c1-18(2)14-11(8-27-18)16(23-15(22-14)9-6-20-21-7-9)24-17-10-4-3-5-12(19)13(10)25-26-17/h3-7H,8H2,1-2H3,(H,20,21)(H2,22,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504232

((R)-N-(2-(2-Fluoro-3-hydroxy- 3-methylbutyl)-6-iso...)Show SMILES CC(C)Oc1cc2C(=O)N(C[C@@H](F)C(C)(C)O)Cc2cc1NC(=O)c1cnn2cc(C)cnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504234

(US11034698, Example 83 | US11034698, Example 84)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(O[C@@H]3CCOC3)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141610
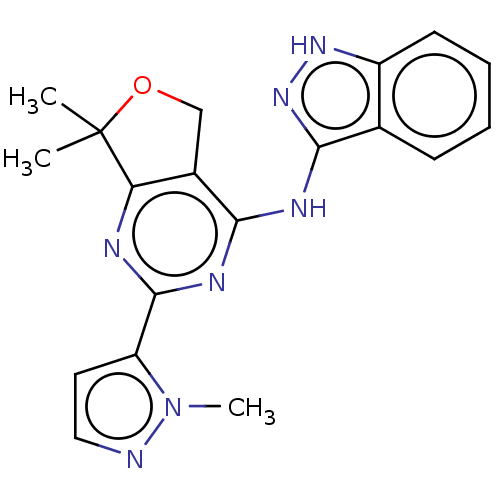
(CHEMBL3759096)Show SMILES Cn1nccc1-c1nc2c(COC2(C)C)c(Nc2n[nH]c3ccccc23)n1 Show InChI InChI=1S/C19H19N7O/c1-19(2)15-12(10-27-19)16(23-18(21-15)14-8-9-20-26(14)3)22-17-11-6-4-5-7-13(11)24-25-17/h4-9H,10H2,1-3H3,(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR deletion (746 to 750 residues) mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141610

(CHEMBL3759096)Show SMILES Cn1nccc1-c1nc2c(COC2(C)C)c(Nc2n[nH]c3ccccc23)n1 Show InChI InChI=1S/C19H19N7O/c1-19(2)15-12(10-27-19)16(23-18(21-15)14-8-9-20-26(14)3)22-17-11-6-4-5-7-13(11)24-25-17/h4-9H,10H2,1-3H3,(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504239
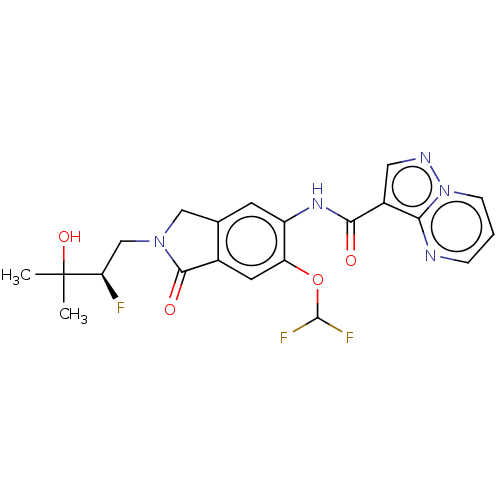
((R)-N-(6-(Difluoromethoxy)-2- (2-fluoro-3-hydroxy-...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(OC(F)F)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50514930

(CHEMBL4464832)Show SMILES CN(C)c1nc2cc(N3CCC(CO)CC3)c(NC(=O)c3cnn4cccnc34)cc2s1 Show InChI InChI=1S/C22H25N7O2S/c1-27(2)22-26-17-10-18(28-8-4-14(13-30)5-9-28)16(11-19(17)32-22)25-21(31)15-12-24-29-7-3-6-23-20(15)29/h3,6-7,10-12,14,30H,4-5,8-9,13H2,1-2H3,(H,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i... |
J Med Chem 62: 6223-6240 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00439
BindingDB Entry DOI: 10.7270/Q2CC1419 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50514929
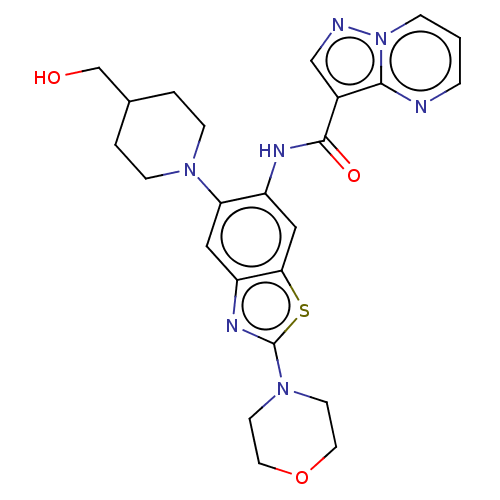
(CHEMBL4474636)Show SMILES OCC1CCN(CC1)c1cc2nc(sc2cc1NC(=O)c1cnn2cccnc12)N1CCOCC1 Show InChI InChI=1S/C24H27N7O3S/c32-15-16-2-6-29(7-3-16)20-12-19-21(35-24(28-19)30-8-10-34-11-9-30)13-18(20)27-23(33)17-14-26-31-5-1-4-25-22(17)31/h1,4-5,12-14,16,32H,2-3,6-11,15H2,(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i... |
J Med Chem 62: 6223-6240 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00439
BindingDB Entry DOI: 10.7270/Q2CC1419 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636

(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R mutant (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504218

(N-[6-(3-Chlorocyclobutoxy)-2- [(2R)-2-fluoro-3-hyd...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(O[C@@H]3C[C@H](Cl)C3)cc2C1=O |r,wU:26.27,28.30,wD:4.4,(-7.2,.34,;-7.24,1.88,;-8.73,1.48,;-8.01,3.22,;-5.7,1.88,;-4.93,3.22,;-4.93,.55,;-3.39,.55,;-2.49,1.79,;-1.02,1.32,;.31,2.09,;1.64,1.32,;2.98,2.09,;2.98,3.63,;1.64,4.4,;4.31,4.4,;4.47,5.93,;5.98,6.25,;6.75,4.92,;8.25,4.6,;8.73,3.13,;7.7,1.99,;6.19,2.31,;5.72,3.77,;1.64,-.22,;2.98,-.99,;2.98,-2.53,;1.89,-3.62,;2.98,-4.71,;2.98,-6.25,;4.07,-3.62,;.31,-.99,;-1.02,-.22,;-2.49,-.7,;-2.96,-2.16,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50498973
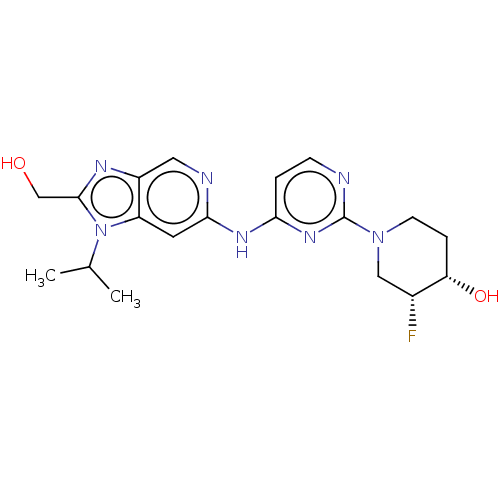
(CHEMBL3734934)Show SMILES CC(C)n1c(CO)nc2cnc(Nc3ccnc(n3)N3CC[C@H](O)[C@H](F)C3)cc12 |r| Show InChI InChI=1S/C19H24FN7O2/c1-11(2)27-14-7-17(22-8-13(14)23-18(27)10-28)24-16-3-5-21-19(25-16)26-6-4-15(29)12(20)9-26/h3,5,7-8,11-12,15,28-29H,4,6,9-10H2,1-2H3,(H,21,22,24,25)/t12-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type EGFR using Fl-EEPLYWSFPAKKK-CONH2 as substrate preincubated for 30 mins followed by addition of substrate measured afte... |
J Med Chem 58: 8877-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01412
BindingDB Entry DOI: 10.7270/Q2833W1G |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504238

((R)-N-(2-(2-Fluoro-3-hydroxy- 3-methylbutyl)-6-(ox...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(OC3COC3)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357645
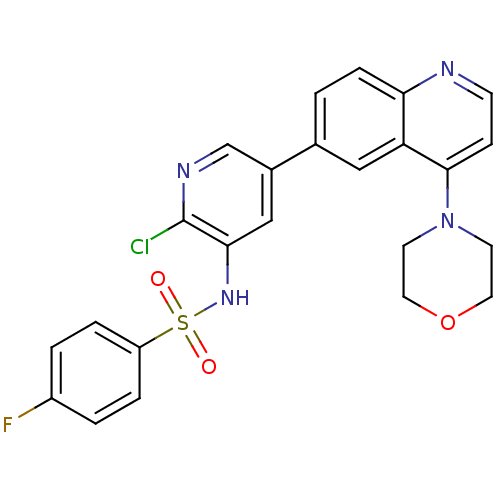
(CHEMBL1738719)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2nccc(N3CCOCC3)c2c1 Show InChI InChI=1S/C24H20ClFN4O3S/c25-24-22(29-34(31,32)19-4-2-18(26)3-5-19)14-17(15-28-24)16-1-6-21-20(13-16)23(7-8-27-21)30-9-11-33-12-10-30/h1-8,13-15,29H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50355062
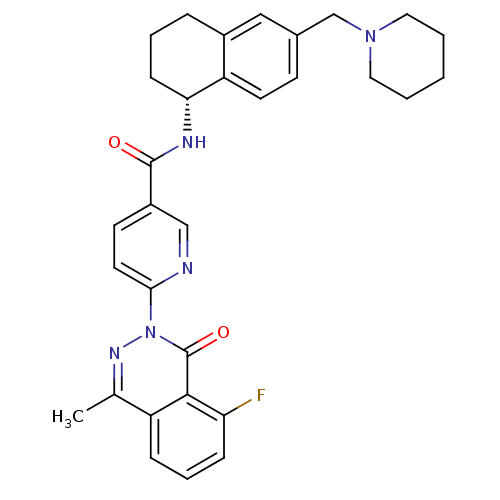
(CHEMBL1834752)Show SMILES Cc1nn(-c2ccc(cn2)C(=O)N[C@@H]2CCCc3cc(CN4CCCCC4)ccc23)c(=O)c2c(F)cccc12 |r| Show InChI InChI=1S/C31H32FN5O2/c1-20-24-8-6-9-26(32)29(24)31(39)37(35-20)28-14-12-23(18-33-28)30(38)34-27-10-5-7-22-17-21(11-13-25(22)27)19-36-15-3-2-4-16-36/h6,8-9,11-14,17-18,27H,2-5,7,10,15-16,19H2,1H3,(H,34,38)/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human B1 receptor |
J Med Chem 54: 7232-46 (2011)
Article DOI: 10.1021/jm200808v
BindingDB Entry DOI: 10.7270/Q28C9WP1 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50355057
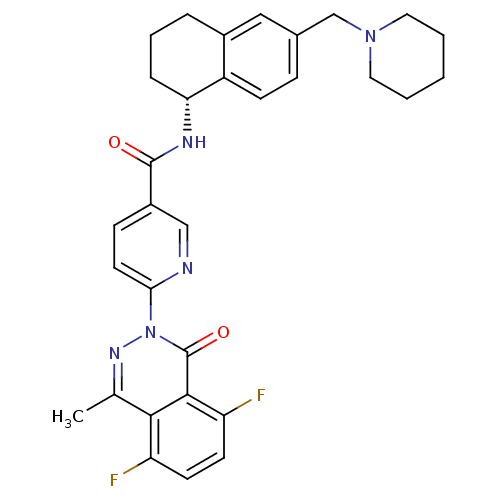
(CHEMBL1834751)Show SMILES Cc1nn(-c2ccc(cn2)C(=O)N[C@@H]2CCCc3cc(CN4CCCCC4)ccc23)c(=O)c2c(F)ccc(F)c12 |r| Show InChI InChI=1S/C31H31F2N5O2/c1-19-28-24(32)11-12-25(33)29(28)31(40)38(36-19)27-13-9-22(17-34-27)30(39)35-26-7-5-6-21-16-20(8-10-23(21)26)18-37-14-3-2-4-15-37/h8-13,16-17,26H,2-7,14-15,18H2,1H3,(H,35,39)/t26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human B1 receptor |
J Med Chem 54: 7232-46 (2011)
Article DOI: 10.1021/jm200808v
BindingDB Entry DOI: 10.7270/Q28C9WP1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141636

(CHEMBL3758502)Show SMILES CC1(C)OCc2c1nc(nc2Nc1n[nH]c2ccccc12)-c1cn[nH]c1 Show InChI InChI=1S/C18H17N7O/c1-18(2)14-12(9-26-18)16(22-15(21-14)10-7-19-20-8-10)23-17-11-5-3-4-6-13(11)24-25-17/h3-8H,9H2,1-2H3,(H,19,20)(H2,21,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) |
Bioorg Med Chem Lett 26: 534-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.078
BindingDB Entry DOI: 10.7270/Q2HH6MZQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357674
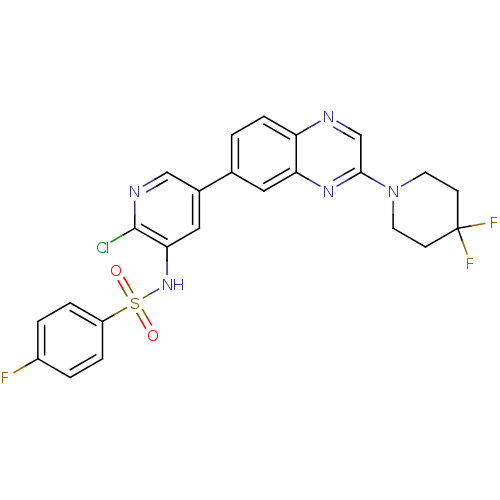
(CHEMBL1914739)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(nc2c1)N1CCC(F)(F)CC1 Show InChI InChI=1S/C24H19ClF3N5O2S/c25-23-21(32-36(34,35)18-4-2-17(26)3-5-18)12-16(13-30-23)15-1-6-19-20(11-15)31-22(14-29-19)33-9-7-24(27,28)8-10-33/h1-6,11-14,32H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613688

(CHEMBL5282716)Show SMILES CC(C)n1nc(N2CC(C2)C(C)(C)O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613687
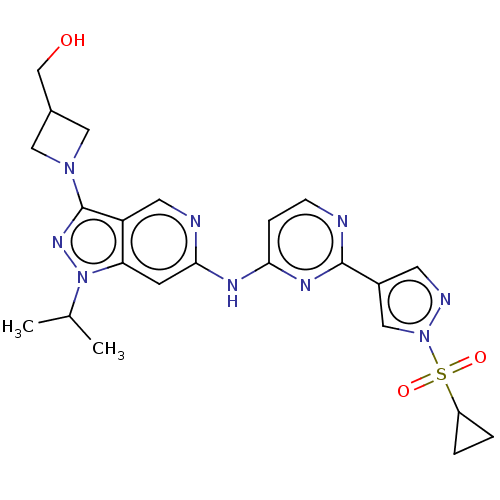
(CHEMBL5285503)Show SMILES CC(C)n1nc(N2CC(CO)C2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM494431
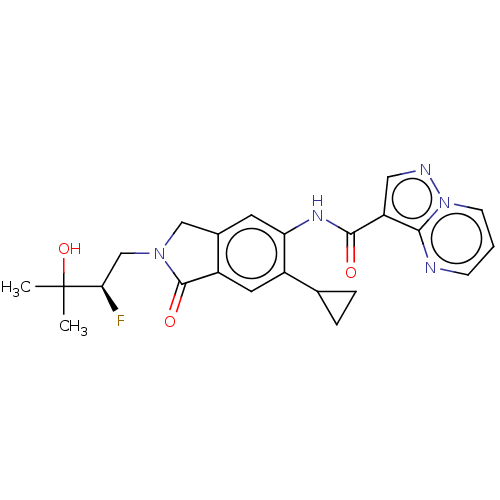
((R)-N-(6-cyclopropyl-2- (2-fluoro-3-hydroxy-3- met...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2C1=O)C1CC1 |r| Show InChI InChI=1S/C23H24FN5O3/c1-23(2,32)19(24)12-28-11-14-8-18(15(13-4-5-13)9-16(14)22(28)31)27-21(30)17-10-26-29-7-3-6-25-20(17)29/h3,6-10,13,19,32H,4-5,11-12H2,1-2H3,(H,27,30)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ... |
US Patent US10988478 (2021)
BindingDB Entry DOI: 10.7270/Q2K077DT |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504241

((R)-N-(6-(tert-Butoxy)-2-(2- fluoro-3-hydroxy-3- m...)Show SMILES CC(C)(C)Oc1cc2C(=O)N(C[C@@H](F)C(C)(C)O)Cc2cc1NC(=O)c1cnn2cccnc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM494507

((R)-N-(6- (dimethylamino)-2-(2- fluoro-3-hydroxy-3...)Show SMILES CN(C)c1cc2C(=O)N(C[C@@H](F)C(C)(C)O)Cc2cc1NC(=O)c1cnn2cccnc12 |r| Show InChI InChI=1S/C22H25FN6O3/c1-22(2,32)18(23)12-28-11-13-8-16(17(27(3)4)9-14(13)21(28)31)26-20(30)15-10-25-29-7-5-6-24-19(15)29/h5-10,18,32H,11-12H2,1-4H3,(H,26,30)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ... |
US Patent US10988478 (2021)
BindingDB Entry DOI: 10.7270/Q2K077DT |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504267
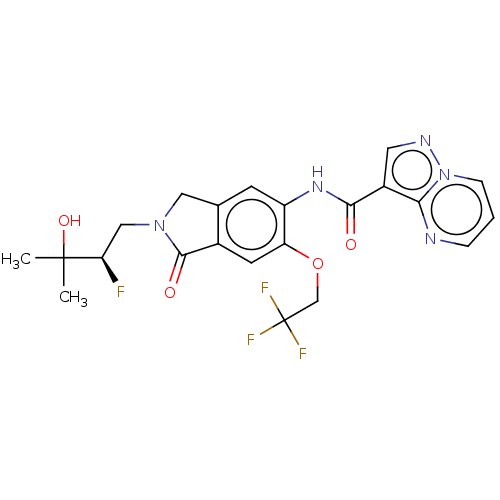
((R)-N-(2-(2-Fluoro-3-hydroxy- 3-methylbutyl)-1-oxo...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(OCC(F)(F)F)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504228

((R)-N-(6-Cyclopropoxy-2-(2- fluoro-3-hydroxy-3- me...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(OC3CC3)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504220

(US11034698, Example 66 | US11034698, Example 67)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(O[C@H]3CC[C@@H](O)C3)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM480659
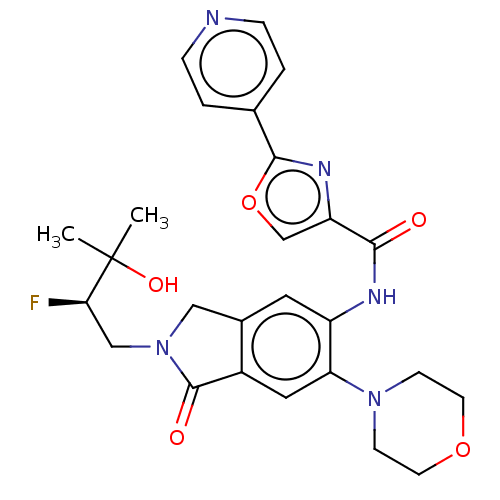
(N-[6-morpholino-1-oxo-2-[rac- (2R)-2-fluoro-3-hydr...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3coc(n3)-c3ccncc3)c(cc2C1=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H28FN5O5/c1-26(2,35)22(27)14-32-13-17-11-19(21(12-18(17)25(32)34)31-7-9-36-10-8-31)29-23(33)20-15-37-24(30-20)16-3-5-28-6-4-16/h3-6,11-12,15,22,35H,7-10,13-14H2,1-2H3,(H,29,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ... |
US Patent US10899772 (2021)
BindingDB Entry DOI: 10.7270/Q2CC13SR |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504224

((R)-N-(2-(2-Fluoro-3-hydroxy- 3-methylbutyl)-1-oxo...)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(OC3CCNCC3)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357652
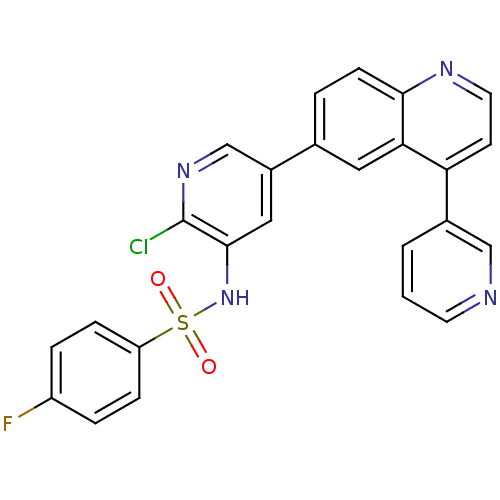
(CHEMBL1914726)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2nccc(-c3cccnc3)c2c1 Show InChI InChI=1S/C25H16ClFN4O2S/c26-25-24(31-34(32,33)20-6-4-19(27)5-7-20)13-18(15-30-25)16-3-8-23-22(12-16)21(9-11-29-23)17-2-1-10-28-14-17/h1-15,31H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357665

(CHEMBL1914728)Show SMILES CN(C)c1ccc(cc1)-c1ccnc2ccc(cc12)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C28H22ClFN4O2S/c1-34(2)22-8-3-18(4-9-22)24-13-14-31-26-12-5-19(15-25(24)26)20-16-27(28(29)32-17-20)33-37(35,36)23-10-6-21(30)7-11-23/h3-17,33H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma
(Homo sapiens (Human)) | BDBM504234

(US11034698, Example 83 | US11034698, Example 84)Show SMILES CC(C)(O)[C@H](F)CN1Cc2cc(NC(=O)c3cnn4cccnc34)c(O[C@@H]3CCOC3)cc2C1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The IRAK4 reaction conditions were optimized using an IRAK1-derived peptide (sequence H-KKARFSRFAGSSPSQSSMVAR) to provide a linear reaction rate over... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q24B34FX |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613684
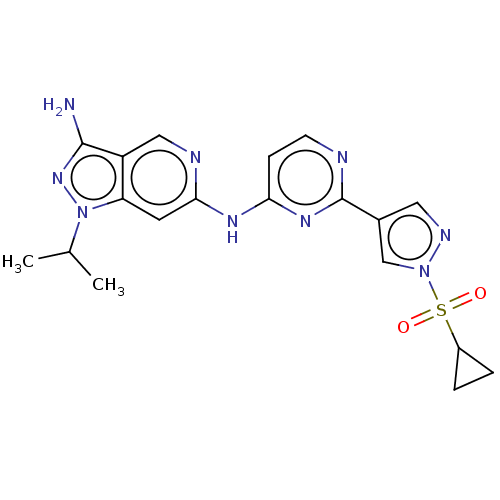
(CHEMBL5280555)Show SMILES CC(C)n1nc(N)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357650
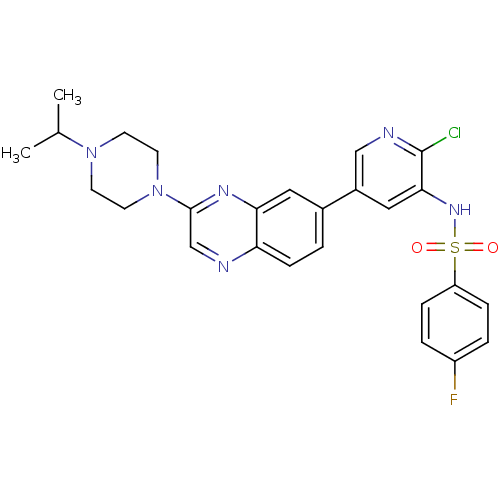
(CHEMBL1914742)Show SMILES CC(C)N1CCN(CC1)c1cnc2ccc(cc2n1)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H26ClFN6O2S/c1-17(2)33-9-11-34(12-10-33)25-16-29-22-8-3-18(13-23(22)31-25)19-14-24(26(27)30-15-19)32-37(35,36)21-6-4-20(28)5-7-21/h3-8,13-17,32H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357653
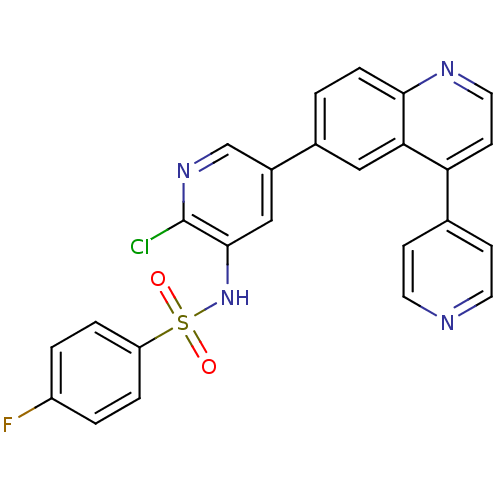
(CHEMBL1914727)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2nccc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H16ClFN4O2S/c26-25-24(31-34(32,33)20-4-2-19(27)3-5-20)14-18(15-30-25)17-1-6-23-22(13-17)21(9-12-29-23)16-7-10-28-11-8-16/h1-15,31H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357668
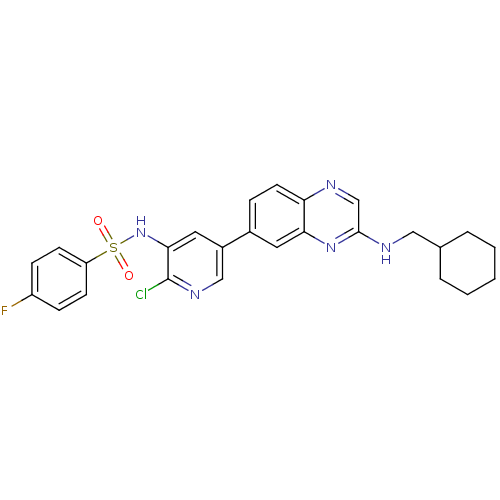
(CHEMBL1914731)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(NCC3CCCCC3)nc2c1 Show InChI InChI=1S/C26H25ClFN5O2S/c27-26-24(33-36(34,35)21-9-7-20(28)8-10-21)13-19(15-31-26)18-6-11-22-23(12-18)32-25(16-29-22)30-14-17-4-2-1-3-5-17/h6-13,15-17,33H,1-5,14H2,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50357670
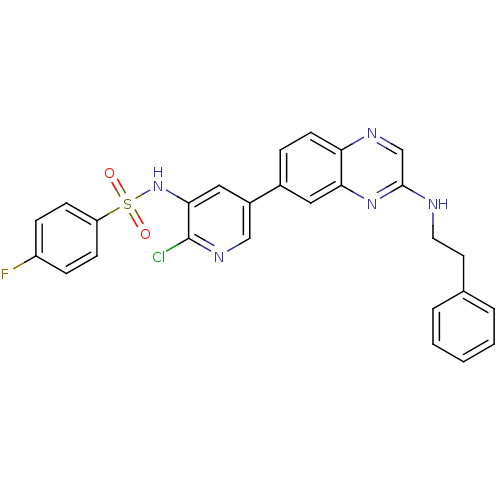
(CHEMBL1914734)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1ccc2ncc(NCCc3ccccc3)nc2c1 Show InChI InChI=1S/C27H21ClFN5O2S/c28-27-25(34-37(35,36)22-9-7-21(29)8-10-22)15-20(16-32-27)19-6-11-23-24(14-19)33-26(17-31-23)30-13-12-18-4-2-1-3-5-18/h1-11,14-17,34H,12-13H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus polyHis-tagged human recombinant PI3Kalpha expressed in baculovirus infected insect Sf9 cells using phosphoinositol-4,5-bisp... |
J Med Chem 54: 4735-51 (2011)
Article DOI: 10.1021/jm200386s
BindingDB Entry DOI: 10.7270/Q2FB53C5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data