 Found 193 hits with Last Name = 'ford' and Initial = 'mc'
Found 193 hits with Last Name = 'ford' and Initial = 'mc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50156669
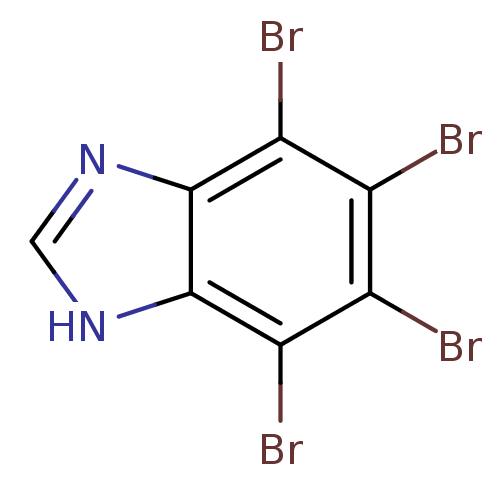
(4,5,6,7-TETRABROMO-BENZIMIDAZOLE | 4,5,6,7-tetrabr...)Show InChI InChI=1S/C7H2Br4N2/c8-2-3(9)5(11)7-6(4(2)10)12-1-13-7/h1H,(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University
Curated by ChEMBL
| Assay Description
Inhibition of human wild type CK2alpha |
J Med Chem 59: 1655-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00997
BindingDB Entry DOI: 10.7270/Q24T6M70 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50156368
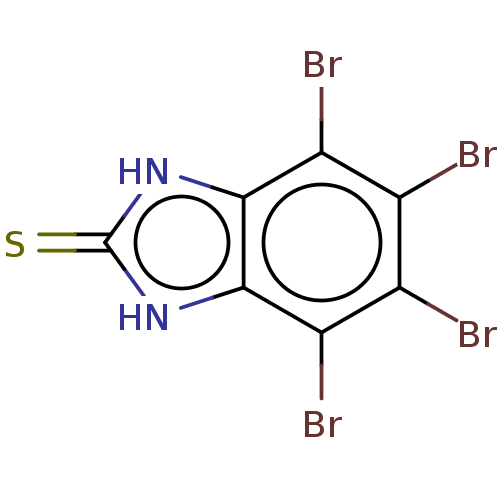
(CHEMBL608394)Show InChI InChI=1S/C7H2Br4N2S/c8-1-2(9)4(11)6-5(3(1)10)12-7(14)13-6/h(H2,12,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University
Curated by ChEMBL
| Assay Description
Inhibition of human wild type CK2alpha |
J Med Chem 59: 1655-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00997
BindingDB Entry DOI: 10.7270/Q24T6M70 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50156367
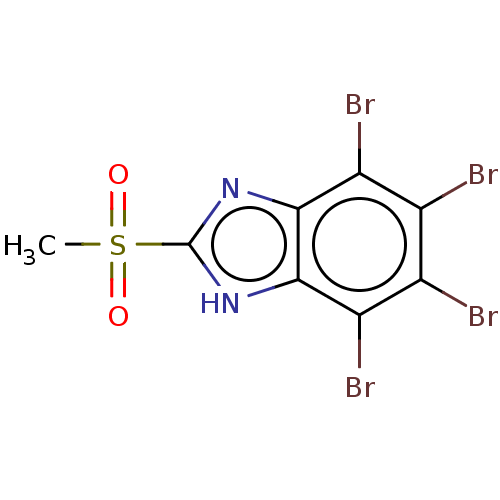
(CHEMBL3793598)Show InChI InChI=1S/C8H4Br4N2O2S/c1-17(15,16)8-13-6-4(11)2(9)3(10)5(12)7(6)14-8/h1H3,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human wild type CK2alpha by Dixon plot analysis |
J Med Chem 59: 1655-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00997
BindingDB Entry DOI: 10.7270/Q24T6M70 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM34045
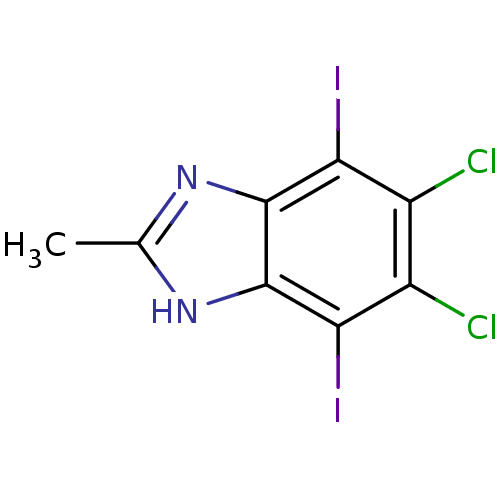
(iodinated benzimidazole, 8a)Show InChI InChI=1S/C8H4Cl2I2N2/c1-2-13-7-5(11)3(9)4(10)6(12)8(7)14-2/h1H3,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human wild type CK2alpha by Dixon plot analysis |
J Med Chem 59: 1655-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00997
BindingDB Entry DOI: 10.7270/Q24T6M70 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM34041
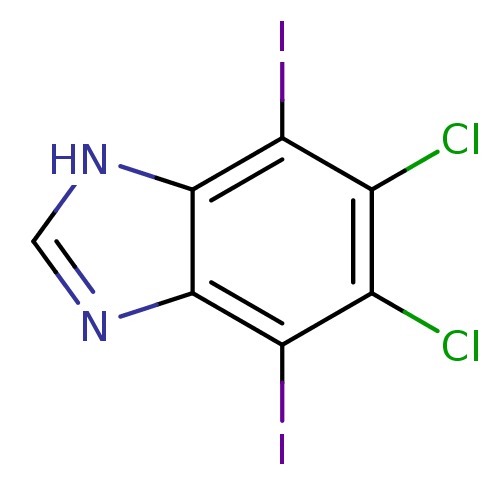
(iodinated benzimidazole, 4a)Show InChI InChI=1S/C7H2Cl2I2N2/c8-2-3(9)5(11)7-6(4(2)10)12-1-13-7/h1H,(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human wild type CK2alpha by Dixon plot analysis |
J Med Chem 59: 1655-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00997
BindingDB Entry DOI: 10.7270/Q24T6M70 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50156369

(CHEMBL227370)Show InChI InChI=1S/C8HBr4F3N2/c9-1-2(10)4(12)6-5(3(1)11)16-7(17-6)8(13,14)15/h(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University
Curated by ChEMBL
| Assay Description
Inhibition of CK2alpha (unknown origin) |
J Med Chem 59: 1655-70 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00997
BindingDB Entry DOI: 10.7270/Q24T6M70 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406127
(CHEMBL5290844)Show SMILES Cc1cc(C)c(CCCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C22H28FO5P/c1-14-9-15(2)19(20(10-14)17-6-7-21(23)16(3)11-17)5-4-8-29(27,28)13-18(24)12-22(25)26/h6-7,9-11,27-29H,4-5,8,12-13H2,1-3H3,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
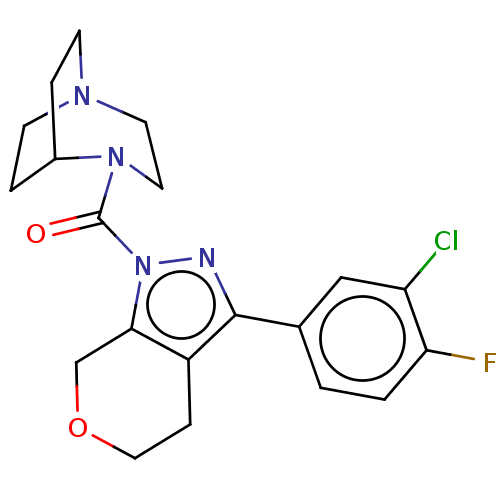
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50422043
(CHEMBL2062144 | CI-988 | PD-137342)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1 |wU:1.1,30.35,wD:1.0,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:21:16:19.18.25,20:19:16:21.23.22,(10.16,-4.53,;9.27,-6.4,;8.87,-4.92,;9.36,-3.46,;8.45,-2.22,;9.36,-.96,;10.83,-1.45,;12.16,-.66,;13.49,-1.43,;13.49,-2.99,;12.16,-3.76,;10.83,-2.99,;7.79,-6,;6.7,-7.08,;7.08,-8.57,;5.2,-6.68,;4.09,-7.77,;2.66,-7.33,;1.36,-7.98,;1.54,-9.46,;.49,-10.86,;1.94,-10.27,;1.75,-8.69,;3.41,-10.67,;4.27,-9.29,;2.92,-9.78,;10.76,-6.8,;11.85,-5.72,;11.15,-8.29,;12.74,-8.08,;14.23,-8.48,;14.61,-9.96,;14.21,-11.44,;12.72,-11.83,;15.28,-12.54,;14.87,-14.03,;15.95,-15.13,;17.44,-14.75,;15.54,-16.61,;14.22,-6.94,;12.88,-6.17,;12.87,-4.64,;14.2,-3.86,;15.54,-4.62,;15.55,-6.17,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21?,22?,24?,25?,29-,32?,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the mouse cerebral cor... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
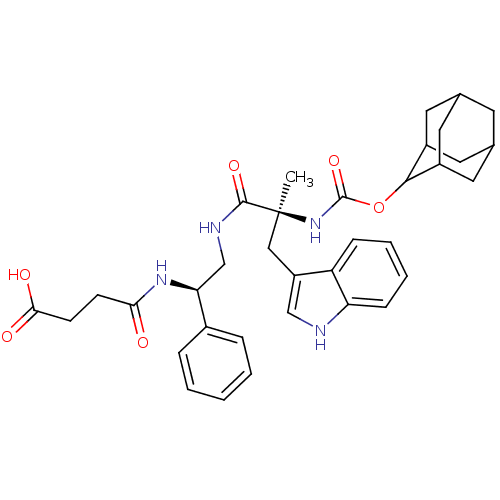
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50449516
(CHEMBL2304152)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCc1nn[nH]n1)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:21:22:31:27.29.26,26:27:22:25.24.31,26:25:22:27.29.28,(14.59,-8.55,;13.05,-8.55,;11.57,-8.13,;10.15,-8.83,;9.76,-7.34,;10.85,-6.25,;8.27,-6.94,;9.17,-5.33,;7.88,-5.46,;8.37,-3.99,;7.46,-2.76,;8.37,-1.51,;9.83,-1.99,;11.15,-1.21,;12.49,-1.98,;12.49,-3.52,;11.15,-4.29,;9.83,-3.52,;6.8,-6.53,;5.71,-7.6,;6.09,-9.09,;4.2,-7.2,;3.13,-8.3,;3.29,-9.81,;1.94,-10.32,;.57,-9.97,;-.48,-11.39,;.96,-10.79,;2.43,-11.2,;.78,-9.22,;1.68,-7.87,;.38,-8.51,;13.44,-10.02,;13.84,-11.51,;12.75,-12.6,;15.32,-11.91,;15.72,-13.4,;17.03,-14.17,;17.17,-15.7,;18.68,-16.03,;19.45,-14.7,;18.45,-13.56,;13.44,-7.06,;12.35,-5.97,;12.74,-4.48,;14.24,-4.06,;15.33,-5.15,;14.93,-6.64,)| Show InChI InChI=1S/C35H42N8O4/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(46)47-32-24-14-21-13-22(16-24)17-25(32)15-21)33(45)37-20-29(23-7-3-2-4-8-23)38-31(44)12-11-30-40-42-43-41-30/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,45)(H,38,44)(H,39,46)(H,40,41,42,43)/t21?,22?,24?,25?,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the mouse cerebral cor... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406126

(CHEMBL5291288)Show SMILES CC(C)c1c(CCP(O)(O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C23H27FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-10,15,29-31H,11-14H2,1-2H3,(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
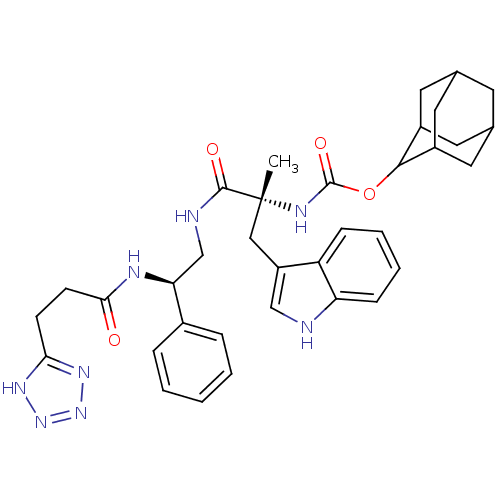
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50449519
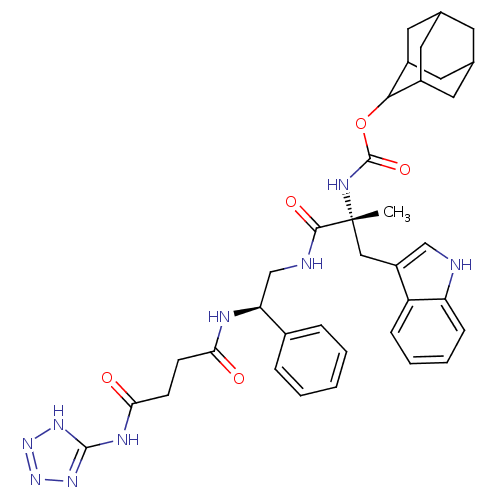
(CHEMBL2304157)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCC(=O)Nc1nn[nH]n1)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:21:22:31:27.29.26,26:27:22:25.24.31,26:25:22:27.29.28,(14.87,-7.82,;13.33,-7.82,;11.85,-7.43,;10.37,-7.82,;9.99,-6.33,;11.07,-5.26,;8.34,-6.7,;9.89,-4.24,;8.1,-4.46,;8.59,-2.99,;7.68,-1.75,;8.59,-.5,;10.04,-.98,;11.37,-.19,;12.72,-.96,;12.72,-2.52,;11.37,-3.29,;10.04,-2.52,;7.01,-5.53,;5.91,-6.61,;6.31,-8.1,;4.42,-6.21,;3.34,-7.3,;3.48,-8.82,;2.15,-9.32,;.77,-8.99,;-.28,-10.39,;1.17,-9.81,;2.64,-10.2,;.98,-8.22,;1.87,-6.87,;.59,-7.51,;13.01,-9.64,;14.12,-10.8,;13.03,-11.88,;15.61,-11.2,;16.01,-12.69,;14.92,-13.77,;13.42,-13.38,;15.31,-15.27,;16.81,-15.66,;17.34,-17.09,;18.88,-17.02,;19.28,-15.52,;18,-14.68,;14.66,-7.05,;16.01,-7.82,;17.32,-7.03,;17.32,-5.49,;15.97,-4.74,;14.64,-5.51,)| Show InChI InChI=1S/C36H43N9O5/c1-36(18-26-19-37-28-10-6-5-9-27(26)28,41-35(49)50-32-24-14-21-13-22(16-24)17-25(32)15-21)33(48)38-20-29(23-7-3-2-4-8-23)39-30(46)11-12-31(47)40-34-42-44-45-43-34/h2-10,19,21-22,24-25,29,32,37H,11-18,20H2,1H3,(H,38,48)(H,39,46)(H,41,49)(H2,40,42,43,44,45,47)/t21?,22?,24?,25?,29-,32?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the mouse cerebral cor... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406123
(CHEMBL5277419)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1CCP(O)(O)CC(=O)CC(O)=O Show InChI InChI=1S/C23H30FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-6,9-11,14,28-30H,7-8,12-13H2,1-4H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50593123
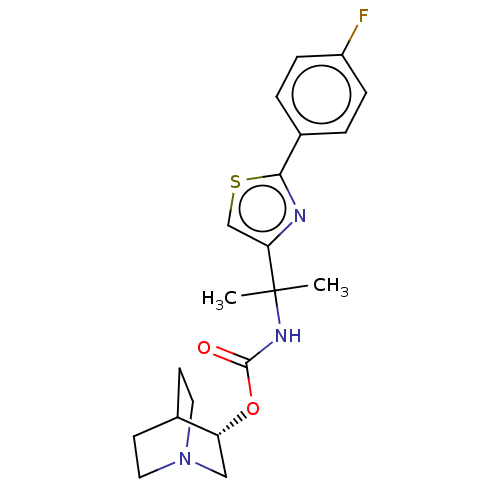
(GENZ-682452 | GZ-402671 | GZ/SAR402671 | GZ402671 ...)Show SMILES CC(C)(NC(=O)O[C@@H]1CN2CCC1CC2)c1csc(n1)-c1ccc(F)cc1 |r,wU:7.6,(-6.59,.2,;-5.85,-1.18,;-5.38,.09,;-4.52,-1.95,;-3.19,-1.18,;-3.19,.36,;-1.85,-1.95,;-.52,-1.18,;.81,-1.95,;2.15,-1.18,;2.15,.36,;.81,1.13,;-.52,.36,;.5,.07,;1.39,-.44,;-7.1,-2.08,;-7.1,-3.62,;-8.57,-4.1,;-9.47,-2.85,;-8.57,-1.6,;-11.01,-2.85,;-11.78,-1.52,;-13.32,-1.52,;-14.09,-2.85,;-15.63,-2.85,;-13.32,-4.18,;-11.78,-4.18,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against bovine cathepsin D |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50449518
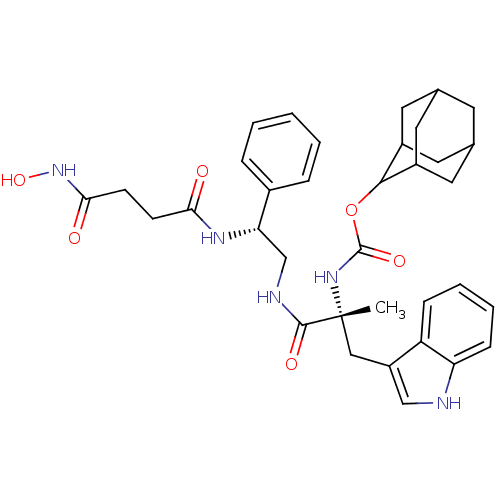
(CHEMBL2304156)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCC(=O)NO)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:21:22:31:27.29.26,26:27:22:25.24.31,26:25:22:27.29.28,(15.78,-7.61,;14.26,-7.61,;12.76,-7.22,;11.43,-7.99,;11.04,-6.49,;12.13,-5.42,;9.24,-6.54,;10.68,-4.57,;9.17,-4.62,;9.64,-3.16,;8.75,-1.91,;9.64,-.66,;11.11,-1.14,;12.44,-.36,;13.77,-1.13,;13.77,-2.68,;12.44,-3.45,;11.11,-2.68,;8.07,-5.69,;6.98,-6.77,;7.37,-8.27,;5.48,-6.38,;4.39,-7.47,;4.56,-8.98,;3.2,-9.48,;1.83,-9.15,;.78,-10.55,;2.22,-9.97,;3.69,-10.37,;2.04,-8.38,;2.94,-7.03,;1.66,-7.68,;14.09,-9.32,;15.03,-10.58,;13.95,-11.67,;16.52,-10.99,;16.92,-12.47,;15.83,-13.56,;14.35,-13.16,;16.22,-15.05,;17.72,-15.45,;15.57,-6.84,;16.92,-7.59,;18.25,-6.82,;18.23,-5.27,;16.88,-4.51,;15.56,-5.3,)| Show InChI InChI=1S/C35H43N5O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)46-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(41)11-12-31(42)40-45/h2-10,19,21-22,24-25,29,32,36,45H,11-18,20H2,1H3,(H,37,43)(H,38,41)(H,39,44)(H,40,42)/t21?,22?,24?,25?,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the mouse cerebral cor... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Mus musculus) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Mus musculus) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406132

(CHEMBL5273314)Show SMILES CC(C)n1c(C=CP(O)(=O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50449521
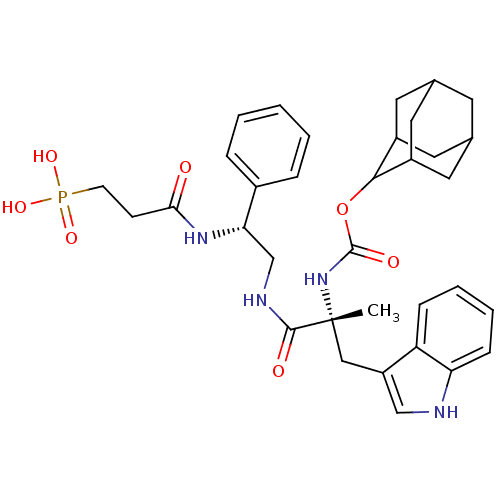
(CHEMBL2304155)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCP(O)(O)=O)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:26:27:22:25.24.31,26:25:22:27.29.28,21:22:31:27.29.26,(15.55,-8.55,;14.01,-8.55,;12.53,-8.15,;11.2,-8.92,;10.81,-7.43,;11.9,-6.35,;9.32,-7.42,;10.1,-5.77,;8.94,-5.55,;9.41,-4.09,;8.52,-2.85,;9.41,-1.59,;10.87,-2.08,;12.2,-1.29,;13.54,-2.06,;13.54,-3.62,;12.2,-4.39,;10.87,-3.62,;7.84,-6.63,;6.74,-7.71,;7.14,-9.2,;5.25,-7.31,;4.16,-8.4,;4.32,-9.92,;2.97,-10.41,;1.59,-10.09,;.54,-11.49,;1.99,-10.9,;3.46,-11.3,;1.8,-9.32,;2.71,-7.96,;1.42,-8.61,;14.4,-10.03,;14.79,-11.53,;13.7,-12.61,;16.29,-11.92,;16.68,-13.42,;15.59,-14.5,;14.1,-14.1,;17.13,-14.5,;15.98,-15.99,;15.34,-7.77,;16.68,-8.54,;18,-7.75,;17.99,-6.21,;16.64,-5.46,;15.31,-6.24,)| Show InChI InChI=1S/C34H43N4O7P/c1-34(18-26-19-35-28-10-6-5-9-27(26)28,38-33(41)45-31-24-14-21-13-22(16-24)17-25(31)15-21)32(40)36-20-29(23-7-3-2-4-8-23)37-30(39)11-12-46(42,43)44/h2-10,19,21-22,24-25,29,31,35H,11-18,20H2,1H3,(H,36,40)(H,37,39)(H,38,41)(H2,42,43,44)/t21?,22?,24?,25?,29-,31?,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the mouse cerebral cor... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406136

(CHEMBL5278676)Show SMILES CCc1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:5.5| Show InChI InChI=1S/C22H21FNO5P/c1-2-18-19-5-3-4-6-20(19)24(16-9-7-15(23)8-10-16)21(18)11-12-30(28,29)14-17(25)13-22(26)27/h3-12H,2,13-14H2,1H3,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against aspartic proteinases pepsin from porcine |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406133

(CHEMBL5288633)Show SMILES COP(=O)(C[C@@H](O)CC(O)=O)NCc1c(C)cc(C)cc1-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H27FNO5P/c1-13-7-14(2)19(18(8-13)16-5-6-20(22)15(3)9-16)11-23-29(27,28-4)12-17(24)10-21(25)26/h5-9,17,24H,10-12H2,1-4H3,(H,23,27)(H,25,26)/t17-,29?/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406137

(CHEMBL5286066)Show InChI InChI=1S/C21H27N3O/c1-18-7-5-6-10-20(18)21(25)22-11-12-23-13-15-24(16-14-23)17-19-8-3-2-4-9-19/h2-10H,11-17H2,1H3,(H,22,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406138
(CHEMBL5286071)Show SMILES [O-][N+](=O)c1ccccc1NC(=O)CCN1CCN2Cc3[nH]c4ccccc4c3CC2C1 Show InChI InChI=1S/C23H25N5O3/c29-23(25-20-7-3-4-8-22(20)28(30)31)9-10-26-11-12-27-15-21-18(13-16(27)14-26)17-5-1-2-6-19(17)24-21/h1-8,16,24H,9-15H2,(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406131
(CHEMBL5278405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1C=CP(O)(=O)CC(=O)CC(O)=O |w:19.21| Show InChI InChI=1S/C23H26FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-11,14H,12-13H2,1-4H3,(H,26,27)(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406135

(CHEMBL5278640)Show SMILES CC(C)c1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50593123

(GENZ-682452 | GZ-402671 | GZ/SAR402671 | GZ402671 ...)Show SMILES CC(C)(NC(=O)O[C@@H]1CN2CCC1CC2)c1csc(n1)-c1ccc(F)cc1 |r,wU:7.6,(-6.59,.2,;-5.85,-1.18,;-5.38,.09,;-4.52,-1.95,;-3.19,-1.18,;-3.19,.36,;-1.85,-1.95,;-.52,-1.18,;.81,-1.95,;2.15,-1.18,;2.15,.36,;.81,1.13,;-.52,.36,;.5,.07,;1.39,-.44,;-7.1,-2.08,;-7.1,-3.62,;-8.57,-4.1,;-9.47,-2.85,;-8.57,-1.6,;-11.01,-2.85,;-11.78,-1.52,;-13.32,-1.52,;-14.09,-2.85,;-15.63,-2.85,;-13.32,-4.18,;-11.78,-4.18,)| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50449517
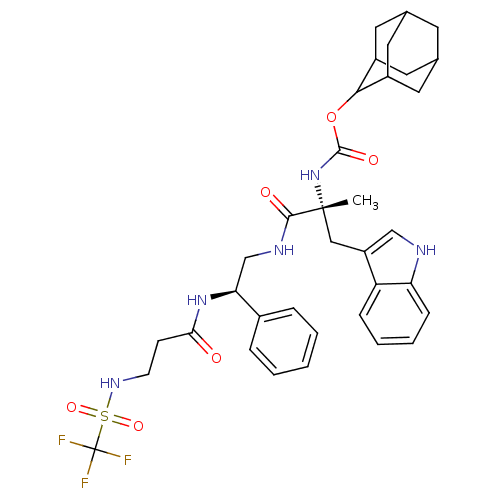
(CHEMBL2304151)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCNS(=O)(=O)C(F)(F)F)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:26:27:22:25.24.31,26:25:22:27.29.28,21:22:31:27.29.26,(14.08,-8.89,;12.55,-8.89,;11.02,-8.61,;9.54,-9.01,;9.15,-7.52,;10.24,-6.44,;7.66,-7.12,;8.5,-5.14,;7.26,-5.63,;7.75,-4.18,;6.84,-2.92,;7.75,-1.69,;9.22,-2.15,;10.55,-1.38,;11.88,-2.15,;11.88,-3.69,;10.55,-4.46,;9.22,-3.69,;6.18,-6.7,;5.09,-7.79,;5.47,-9.29,;3.59,-7.4,;2.5,-8.48,;2.66,-9.99,;1.31,-10.51,;-.07,-10.17,;-1.12,-11.58,;.33,-10.99,;1.8,-11.39,;.14,-9.41,;1.05,-8.06,;-.26,-8.69,;12.93,-10.37,;13.33,-11.86,;12.25,-12.95,;14.82,-12.26,;15.21,-13.75,;16.71,-14.15,;17.8,-13.07,;17.41,-11.57,;19.28,-13.46,;18.57,-14.4,;18,-15.9,;20.16,-14.66,;19.27,-15.61,;13.87,-8.1,;13.86,-6.58,;15.17,-5.79,;16.53,-6.54,;16.54,-8.08,;15.21,-8.87,)| Show InChI InChI=1S/C35H42F3N5O6S/c1-34(18-26-19-39-28-10-6-5-9-27(26)28,43-33(46)49-31-24-14-21-13-22(16-24)17-25(31)15-21)32(45)40-20-29(23-7-3-2-4-8-23)42-30(44)11-12-41-50(47,48)35(36,37)38/h2-10,19,21-22,24-25,29,31,39,41H,11-18,20H2,1H3,(H,40,45)(H,42,44)(H,43,46)/t21?,22?,24?,25?,29-,31?,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the mouse cerebral cor... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406119

(CHEMBL5273405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1COP(O)(=O)C[C@@H](O)CC(O)=O Show InChI InChI=1S/C22H28FO6P/c1-13(2)18-7-14(3)8-19(16-5-6-21(23)15(4)9-16)20(18)11-29-30(27,28)12-17(24)10-22(25)26/h5-9,13,17,24H,10-12H2,1-4H3,(H,25,26)(H,27,28)/t17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50449520
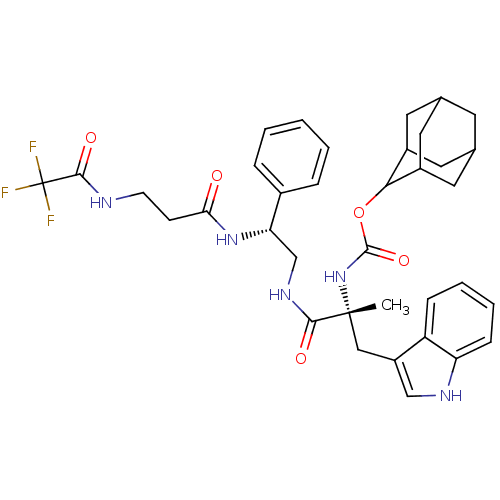
(CHEMBL2304154)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@H](NC(=O)CCNC(=O)C(F)(F)F)c1ccccc1 |TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:21:16:19.18.25,20:19:16:21.23.22,15:16:25:21.23.20| Show InChI InChI=1S/C36H42F3N5O5/c1-35(18-26-19-41-28-10-6-5-9-27(26)28,44-34(48)49-31-24-14-21-13-22(16-24)17-25(31)15-21)32(46)42-20-29(23-7-3-2-4-8-23)43-30(45)11-12-40-33(47)36(37,38)39/h2-10,19,21-22,24-25,29,31,41H,11-18,20H2,1H3,(H,40,47)(H,42,46)(H,43,45)(H,44,48)/t21?,22?,24?,25?,29-,31?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the mouse cerebral cor... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406128

(CHEMBL5270537)Show SMILES OC(=O)CC(=O)CP(O)(=O)CCC1=C(c2ccccc2C11CCCC1)c1ccc(F)cc1 |t:12| Show InChI InChI=1S/C25H26FO5P/c26-18-9-7-17(8-10-18)24-20-5-1-2-6-21(20)25(12-3-4-13-25)22(24)11-14-32(30,31)16-19(27)15-23(28)29/h1-2,5-10H,3-4,11-16H2,(H,28,29)(H,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406131

(CHEMBL5278405)Show SMILES CC(C)c1cc(C)cc(-c2ccc(F)c(C)c2)c1C=CP(O)(=O)CC(=O)CC(O)=O |w:19.21| Show InChI InChI=1S/C23H26FO5P/c1-14(2)20-9-15(3)10-21(17-5-6-22(24)16(4)11-17)19(20)7-8-30(28,29)13-18(25)12-23(26)27/h5-11,14H,12-13H2,1-4H3,(H,26,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 338 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
ETA receptor antagonist activity was measured by inhibition of ET-1 induced vasoconstriction in isolated porcine coronary artery |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406135

(CHEMBL5278640)Show SMILES CC(C)c1c(C=CP(O)(=O)CC(=O)CC(O)=O)n(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)23-19-5-3-4-6-20(19)25(17-9-7-16(24)8-10-17)21(23)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 585 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50449517

(CHEMBL2304151)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCNS(=O)(=O)C(F)(F)F)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:26:27:22:25.24.31,26:25:22:27.29.28,21:22:31:27.29.26,(14.08,-8.89,;12.55,-8.89,;11.02,-8.61,;9.54,-9.01,;9.15,-7.52,;10.24,-6.44,;7.66,-7.12,;8.5,-5.14,;7.26,-5.63,;7.75,-4.18,;6.84,-2.92,;7.75,-1.69,;9.22,-2.15,;10.55,-1.38,;11.88,-2.15,;11.88,-3.69,;10.55,-4.46,;9.22,-3.69,;6.18,-6.7,;5.09,-7.79,;5.47,-9.29,;3.59,-7.4,;2.5,-8.48,;2.66,-9.99,;1.31,-10.51,;-.07,-10.17,;-1.12,-11.58,;.33,-10.99,;1.8,-11.39,;.14,-9.41,;1.05,-8.06,;-.26,-8.69,;12.93,-10.37,;13.33,-11.86,;12.25,-12.95,;14.82,-12.26,;15.21,-13.75,;16.71,-14.15,;17.8,-13.07,;17.41,-11.57,;19.28,-13.46,;18.57,-14.4,;18,-15.9,;20.16,-14.66,;19.27,-15.61,;13.87,-8.1,;13.86,-6.58,;15.17,-5.79,;16.53,-6.54,;16.54,-8.08,;15.21,-8.87,)| Show InChI InChI=1S/C35H42F3N5O6S/c1-34(18-26-19-39-28-10-6-5-9-27(26)28,43-33(46)49-31-24-14-21-13-22(16-24)17-25(31)15-21)32(45)40-20-29(23-7-3-2-4-8-23)42-30(44)11-12-41-50(47,48)35(36,37)38/h2-10,19,21-22,24-25,29,31,39,41H,11-18,20H2,1H3,(H,40,45)(H,42,44)(H,43,46)/t21?,22?,24?,25?,29-,31?,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the rat pancreas (CCK-... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406118
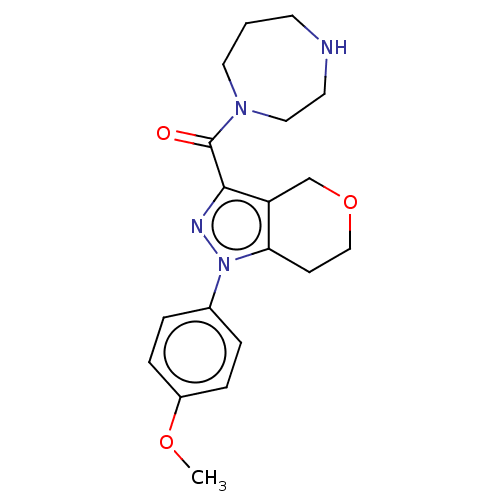
(CHEMBL5281181)Show SMILES CC(C)n1c(CCP(O)(O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 Show InChI InChI=1S/C23H27FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-10,15,29-31H,11-14H2,1-2H3,(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro Oxytocin receptor antagonistic activity against rat uterine strips |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50449520

(CHEMBL2304154)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@H](NC(=O)CCNC(=O)C(F)(F)F)c1ccccc1 |TLB:22:17:25:21.23.20,22:21:16.17.18:25,THB:20:21:16:19.18.25,20:19:16:21.23.22,15:16:25:21.23.20| Show InChI InChI=1S/C36H42F3N5O5/c1-35(18-26-19-41-28-10-6-5-9-27(26)28,44-34(48)49-31-24-14-21-13-22(16-24)17-25(31)15-21)32(46)42-20-29(23-7-3-2-4-8-23)43-30(45)11-12-40-33(47)36(37,38)39/h2-10,19,21-22,24-25,29,31,41H,11-18,20H2,1H3,(H,40,47)(H,42,46)(H,43,45)(H,44,48)/t21?,22?,24?,25?,29-,31?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the rat pancreas (CCK-... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50406128

(CHEMBL5270537)Show SMILES OC(=O)CC(=O)CP(O)(=O)CCC1=C(c2ccccc2C11CCCC1)c1ccc(F)cc1 |t:12| Show InChI InChI=1S/C25H26FO5P/c26-18-9-7-17(8-10-18)24-20-5-1-2-6-21(20)25(12-3-4-13-25)22(24)11-14-32(30,31)16-19(27)15-23(28)29/h1-2,5-10H,3-4,11-16H2,(H,28,29)(H,30,31) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory potency against human kidney renin. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50449516

(CHEMBL2304152)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCc1nn[nH]n1)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:21:22:31:27.29.26,26:27:22:25.24.31,26:25:22:27.29.28,(14.59,-8.55,;13.05,-8.55,;11.57,-8.13,;10.15,-8.83,;9.76,-7.34,;10.85,-6.25,;8.27,-6.94,;9.17,-5.33,;7.88,-5.46,;8.37,-3.99,;7.46,-2.76,;8.37,-1.51,;9.83,-1.99,;11.15,-1.21,;12.49,-1.98,;12.49,-3.52,;11.15,-4.29,;9.83,-3.52,;6.8,-6.53,;5.71,-7.6,;6.09,-9.09,;4.2,-7.2,;3.13,-8.3,;3.29,-9.81,;1.94,-10.32,;.57,-9.97,;-.48,-11.39,;.96,-10.79,;2.43,-11.2,;.78,-9.22,;1.68,-7.87,;.38,-8.51,;13.44,-10.02,;13.84,-11.51,;12.75,-12.6,;15.32,-11.91,;15.72,-13.4,;17.03,-14.17,;17.17,-15.7,;18.68,-16.03,;19.45,-14.7,;18.45,-13.56,;13.44,-7.06,;12.35,-5.97,;12.74,-4.48,;14.24,-4.06,;15.33,-5.15,;14.93,-6.64,)| Show InChI InChI=1S/C35H42N8O4/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(46)47-32-24-14-21-13-22(16-24)17-25(32)15-21)33(45)37-20-29(23-7-3-2-4-8-23)38-31(44)12-11-30-40-42-43-41-30/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,45)(H,38,44)(H,39,46)(H,40,41,42,43)/t21?,22?,24?,25?,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the rat pancreas (CCK-... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50449519

(CHEMBL2304157)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCC(=O)Nc1nn[nH]n1)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:21:22:31:27.29.26,26:27:22:25.24.31,26:25:22:27.29.28,(14.87,-7.82,;13.33,-7.82,;11.85,-7.43,;10.37,-7.82,;9.99,-6.33,;11.07,-5.26,;8.34,-6.7,;9.89,-4.24,;8.1,-4.46,;8.59,-2.99,;7.68,-1.75,;8.59,-.5,;10.04,-.98,;11.37,-.19,;12.72,-.96,;12.72,-2.52,;11.37,-3.29,;10.04,-2.52,;7.01,-5.53,;5.91,-6.61,;6.31,-8.1,;4.42,-6.21,;3.34,-7.3,;3.48,-8.82,;2.15,-9.32,;.77,-8.99,;-.28,-10.39,;1.17,-9.81,;2.64,-10.2,;.98,-8.22,;1.87,-6.87,;.59,-7.51,;13.01,-9.64,;14.12,-10.8,;13.03,-11.88,;15.61,-11.2,;16.01,-12.69,;14.92,-13.77,;13.42,-13.38,;15.31,-15.27,;16.81,-15.66,;17.34,-17.09,;18.88,-17.02,;19.28,-15.52,;18,-14.68,;14.66,-7.05,;16.01,-7.82,;17.32,-7.03,;17.32,-5.49,;15.97,-4.74,;14.64,-5.51,)| Show InChI InChI=1S/C36H43N9O5/c1-36(18-26-19-37-28-10-6-5-9-27(26)28,41-35(49)50-32-24-14-21-13-22(16-24)17-25(32)15-21)33(48)38-20-29(23-7-3-2-4-8-23)39-30(46)11-12-31(47)40-34-42-44-45-43-34/h2-10,19,21-22,24-25,29,32,37H,11-18,20H2,1H3,(H,38,48)(H,39,46)(H,41,49)(H2,40,42,43,44,45,47)/t21?,22?,24?,25?,29-,32?,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the rat pancreas (CCK-... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406132

(CHEMBL5273314)Show SMILES CC(C)n1c(C=CP(O)(=O)CC(=O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |w:6.6| Show InChI InChI=1S/C23H23FNO5P/c1-15(2)25-20-6-4-3-5-19(20)23(16-7-9-17(24)10-8-16)21(25)11-12-31(29,30)14-18(26)13-22(27)28/h3-12,15H,13-14H2,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50449518

(CHEMBL2304156)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCC(=O)NO)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:21:22:31:27.29.26,26:27:22:25.24.31,26:25:22:27.29.28,(15.78,-7.61,;14.26,-7.61,;12.76,-7.22,;11.43,-7.99,;11.04,-6.49,;12.13,-5.42,;9.24,-6.54,;10.68,-4.57,;9.17,-4.62,;9.64,-3.16,;8.75,-1.91,;9.64,-.66,;11.11,-1.14,;12.44,-.36,;13.77,-1.13,;13.77,-2.68,;12.44,-3.45,;11.11,-2.68,;8.07,-5.69,;6.98,-6.77,;7.37,-8.27,;5.48,-6.38,;4.39,-7.47,;4.56,-8.98,;3.2,-9.48,;1.83,-9.15,;.78,-10.55,;2.22,-9.97,;3.69,-10.37,;2.04,-8.38,;2.94,-7.03,;1.66,-7.68,;14.09,-9.32,;15.03,-10.58,;13.95,-11.67,;16.52,-10.99,;16.92,-12.47,;15.83,-13.56,;14.35,-13.16,;16.22,-15.05,;17.72,-15.45,;15.57,-6.84,;16.92,-7.59,;18.25,-6.82,;18.23,-5.27,;16.88,-4.51,;15.56,-5.3,)| Show InChI InChI=1S/C35H43N5O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)46-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(41)11-12-31(42)40-45/h2-10,19,21-22,24-25,29,32,36,45H,11-18,20H2,1H3,(H,37,43)(H,38,41)(H,39,44)(H,40,42)/t21?,22?,24?,25?,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the rat pancreas (CCK-... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406137

(CHEMBL5286066)Show InChI InChI=1S/C21H27N3O/c1-18-7-5-6-10-20(18)21(25)22-11-12-23-13-15-24(16-14-23)17-19-8-3-2-4-9-19/h2-10H,11-17H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50406134

(CHEMBL5275403)Show SMILES Cc1cc(C)c(C=CP(O)(=O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 |w:7.7| Show InChI InChI=1S/C21H22FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-10H,11-12H2,1-3H3,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50406130

(CHEMBL5272946)Show SMILES Cc1cc(C)c(CCP(O)(O)CC(=O)CC(O)=O)c(c1)-c1ccc(F)c(C)c1 Show InChI InChI=1S/C21H26FO5P/c1-13-8-14(2)18(6-7-28(26,27)12-17(23)11-21(24)25)19(9-13)16-4-5-20(22)15(3)10-16/h4-5,8-10,26-28H,6-7,11-12H2,1-3H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50406138

(CHEMBL5286071)Show SMILES [O-][N+](=O)c1ccccc1NC(=O)CCN1CCN2Cc3[nH]c4ccccc4c3CC2C1 Show InChI InChI=1S/C23H25N5O3/c29-23(25-20-7-3-4-8-22(20)28(30)31)9-10-26-11-12-27-15-21-18(13-16(27)14-26)17-5-1-2-6-19(17)24-21/h1-8,16,24H,9-15H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Choline Acetyltransferase |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50449521

(CHEMBL2304155)Show SMILES [H][C@@](CNC(=O)[C@@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CCP(O)(O)=O)c1ccccc1 |wU:1.36,6.6,wD:6.7,1.0,TLB:28:23:31:27.29.26,28:27:22.23.24:31,THB:26:27:22:25.24.31,26:25:22:27.29.28,21:22:31:27.29.26,(15.55,-8.55,;14.01,-8.55,;12.53,-8.15,;11.2,-8.92,;10.81,-7.43,;11.9,-6.35,;9.32,-7.42,;10.1,-5.77,;8.94,-5.55,;9.41,-4.09,;8.52,-2.85,;9.41,-1.59,;10.87,-2.08,;12.2,-1.29,;13.54,-2.06,;13.54,-3.62,;12.2,-4.39,;10.87,-3.62,;7.84,-6.63,;6.74,-7.71,;7.14,-9.2,;5.25,-7.31,;4.16,-8.4,;4.32,-9.92,;2.97,-10.41,;1.59,-10.09,;.54,-11.49,;1.99,-10.9,;3.46,-11.3,;1.8,-9.32,;2.71,-7.96,;1.42,-8.61,;14.4,-10.03,;14.79,-11.53,;13.7,-12.61,;16.29,-11.92,;16.68,-13.42,;15.59,-14.5,;14.1,-14.1,;17.13,-14.5,;15.98,-15.99,;15.34,-7.77,;16.68,-8.54,;18,-7.75,;17.99,-6.21,;16.64,-5.46,;15.31,-6.24,)| Show InChI InChI=1S/C34H43N4O7P/c1-34(18-26-19-35-28-10-6-5-9-27(26)28,38-33(41)45-31-24-14-21-13-22(16-24)17-25(31)15-21)32(40)36-20-29(23-7-3-2-4-8-23)37-30(39)11-12-46(42,43)44/h2-10,19,21-22,24-25,29,31,35H,11-18,20H2,1H3,(H,36,40)(H,37,39)(H,38,41)(H2,42,43,44)/t21?,22?,24?,25?,29-,31?,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The concentration (nM) producing half-maximal inhibition of specific binding of [1251] Bolton Hunter CCK-8 to CCK receptors in the rat pancreas (CCK-... |
Bioorg Med Chem Lett 2: 45-8 (1992)
BindingDB Entry DOI: 10.7270/Q2K0746W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data