

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415001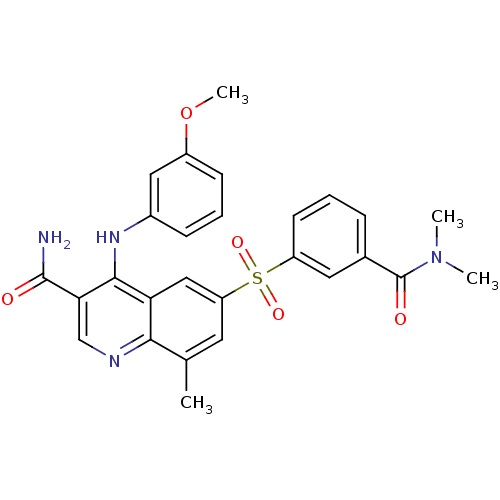 (CHEMBL570015 | GSK-256066 | GSK-256066 (3)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.00794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414999 (CHEMBL569791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415009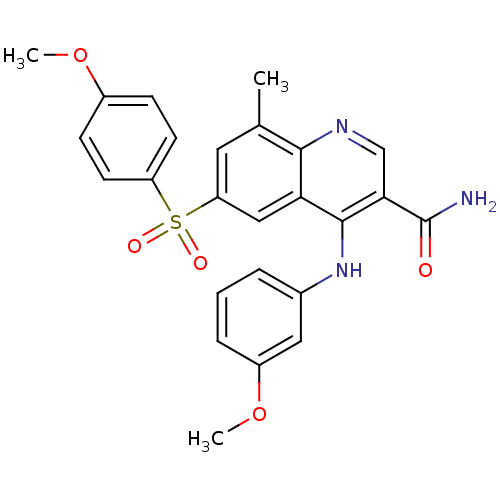 (CHEMBL571381) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415008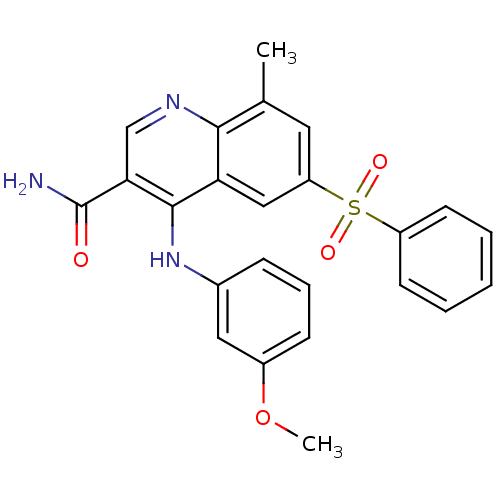 (CHEMBL584327) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414998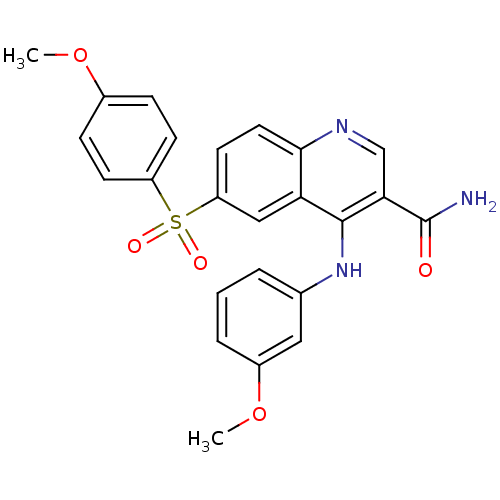 (CHEMBL569556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14774 (3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415007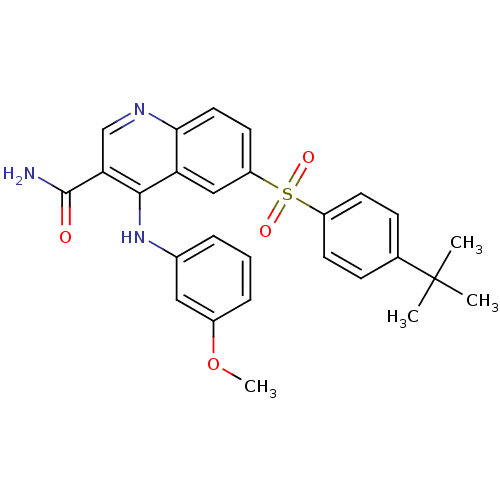 (CHEMBL571171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414992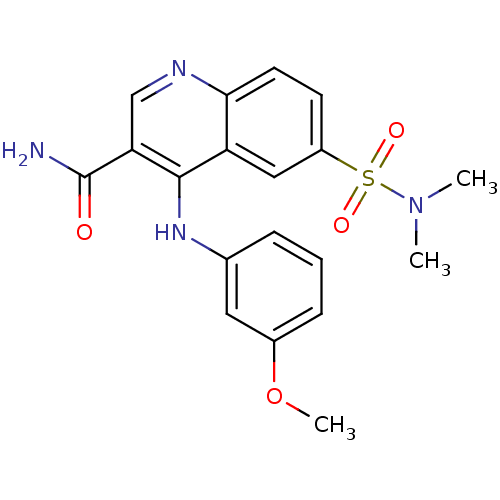 (CHEMBL576479) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50413291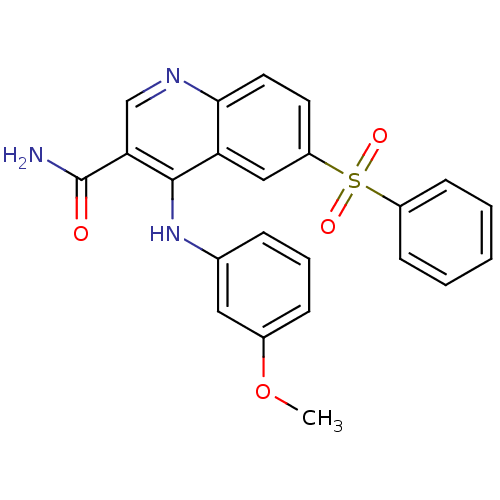 (CHEMBL515240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415000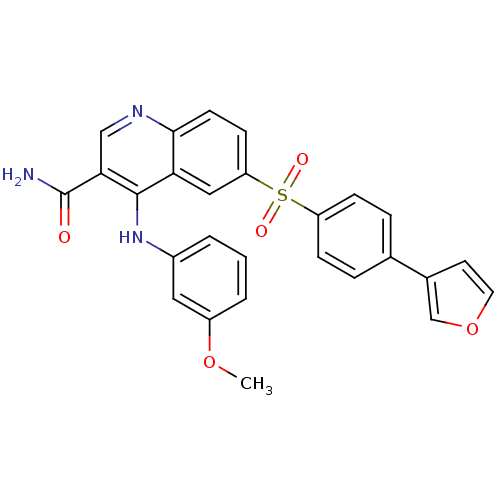 (CHEMBL570029) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415004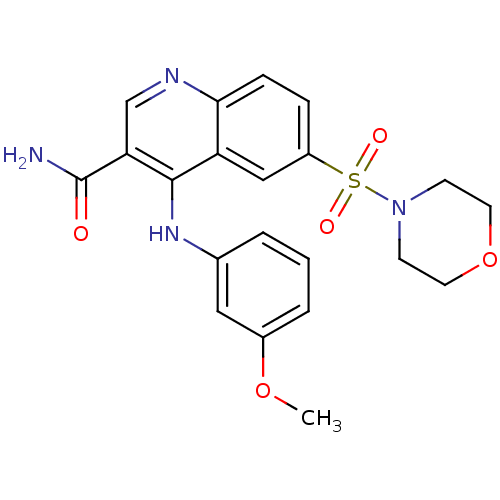 (CHEMBL571593) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415006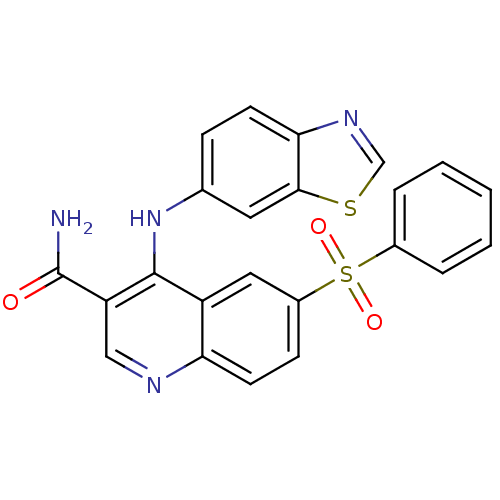 (CHEMBL585528) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414994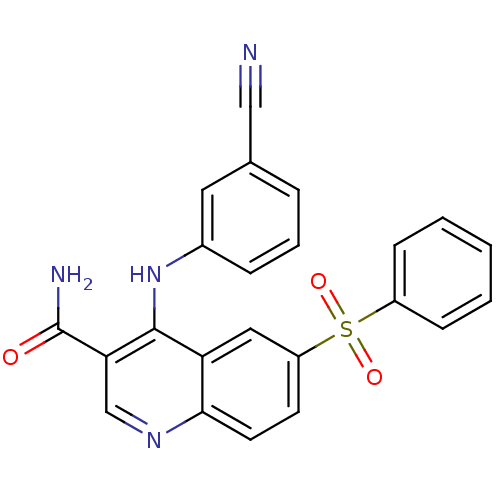 (CHEMBL571386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414989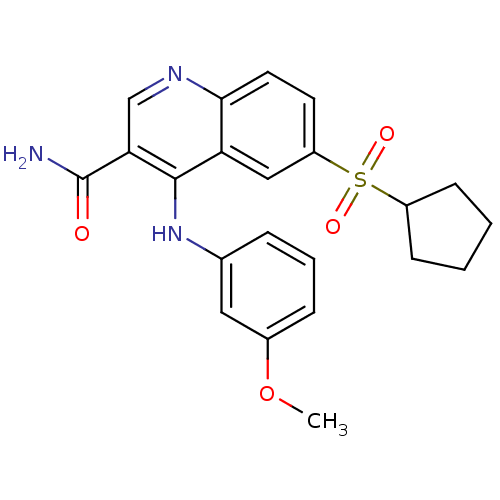 (CHEMBL585937) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50413298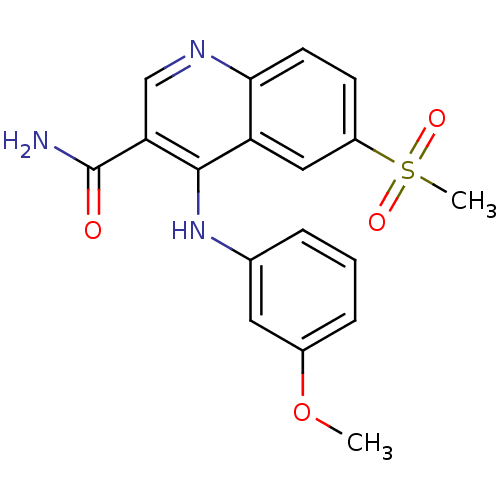 (CHEMBL462150) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498874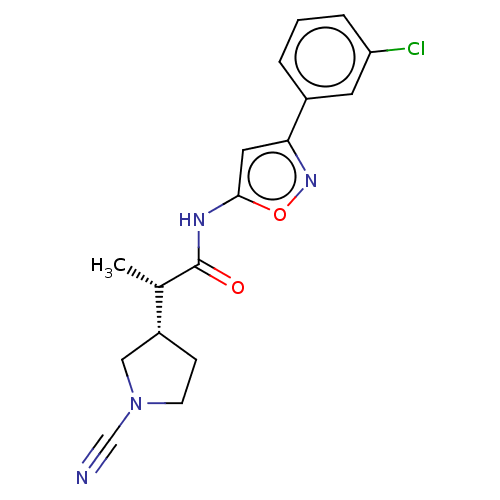 (US11014912, Example 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498875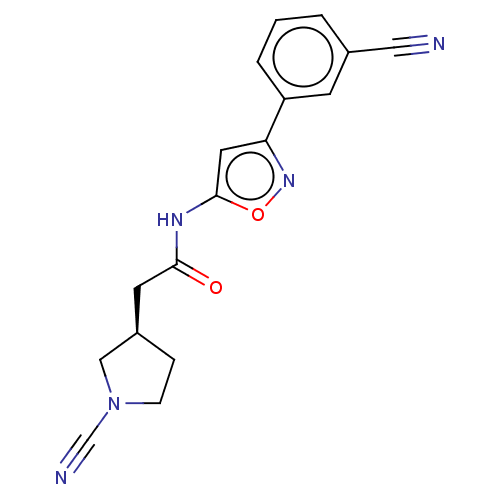 (US11014912, Example 51) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498860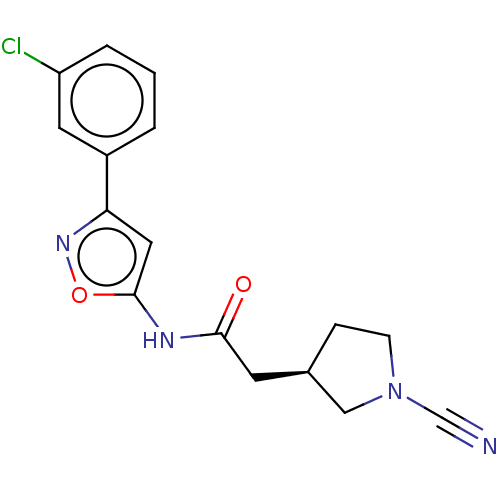 ((R)—N-(3-(3-Chlorophenyl)isoxazol-5-yl)-2-(1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498879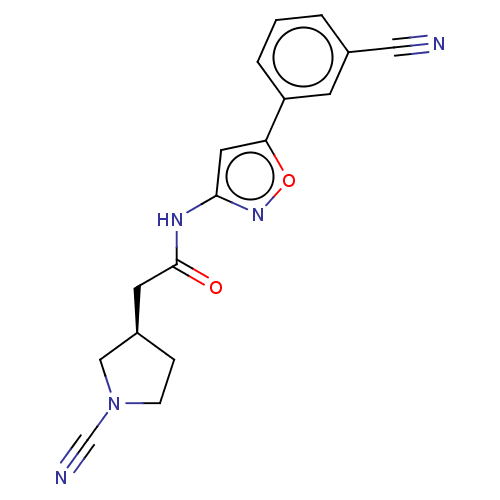 (US11014912, Example 55) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498878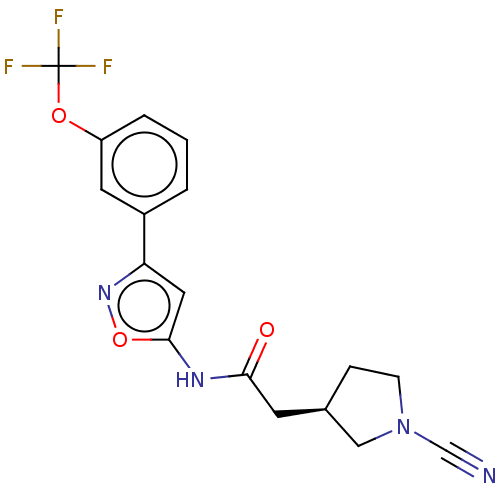 (US11014912, Example 54) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415005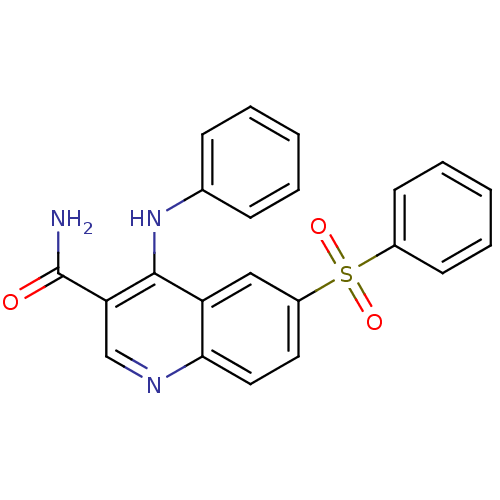 (CHEMBL569352) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414993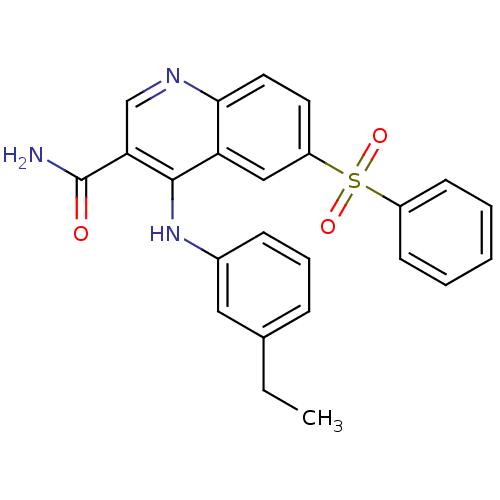 (CHEMBL571594) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414997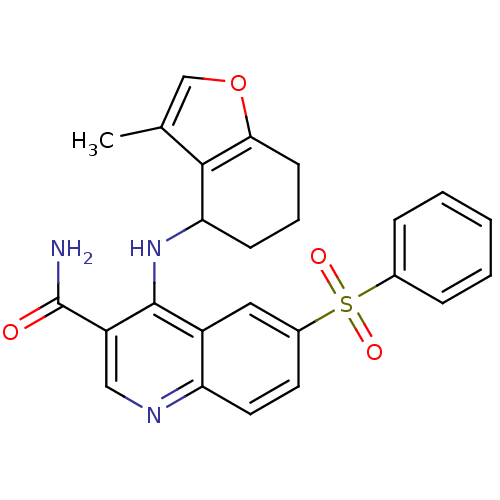 (CHEMBL569555) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414991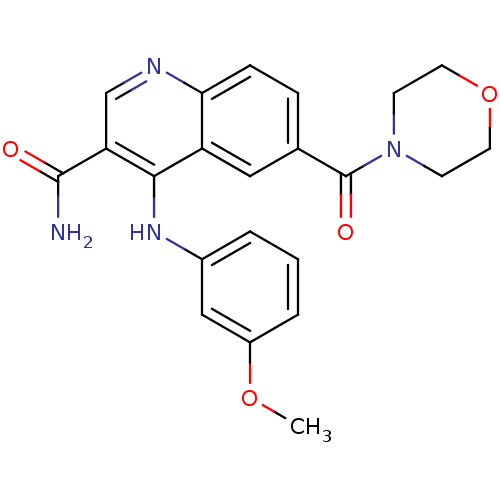 (CHEMBL569790) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50415003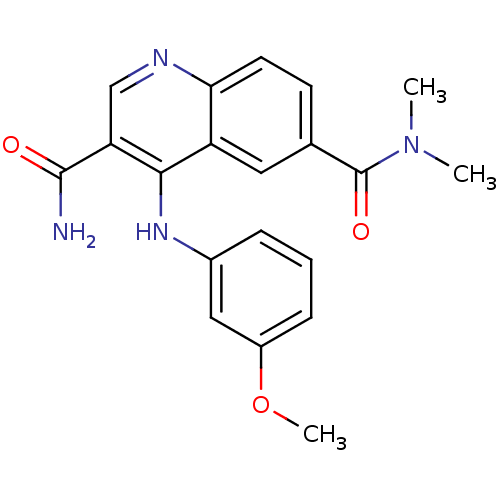 (CHEMBL570956) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human C-terminal BRD4 bromodomain expressed in Escherichia coli BL21(DE3) after 30 mins by luminescence proximity homogeneous assay | J Med Chem 55: 587-96 (2012) Article DOI: 10.1021/jm201283q BindingDB Entry DOI: 10.7270/Q22R3SSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414996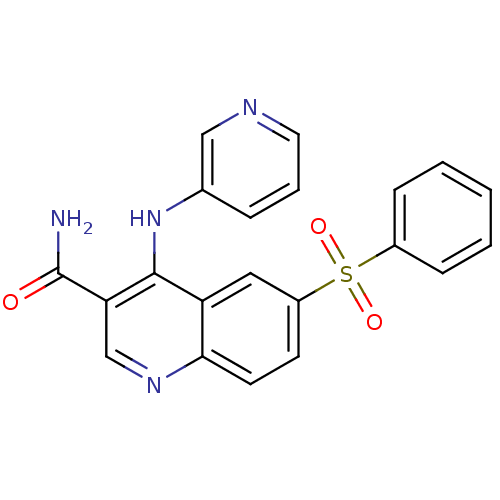 (CHEMBL571170) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50414995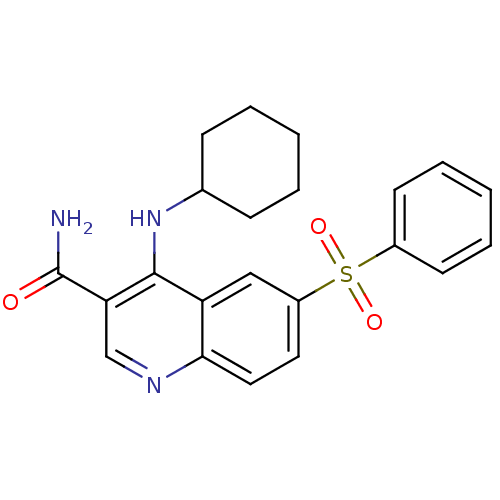 (CHEMBL585537) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50042058 ((-)-rolipram | (4R)-4-[3-(cyclopentyloxy)-4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4B by scintillation proximity assay | Bioorg Med Chem Lett 19: 5261-5 (2009) Article DOI: 10.1016/j.bmcl.2009.04.012 BindingDB Entry DOI: 10.7270/Q2K075HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498877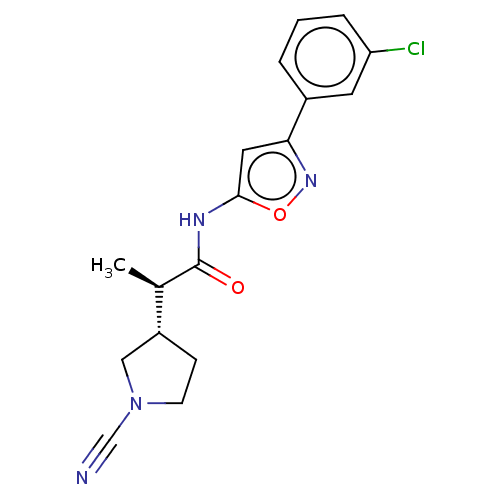 (US11014912, Example 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498867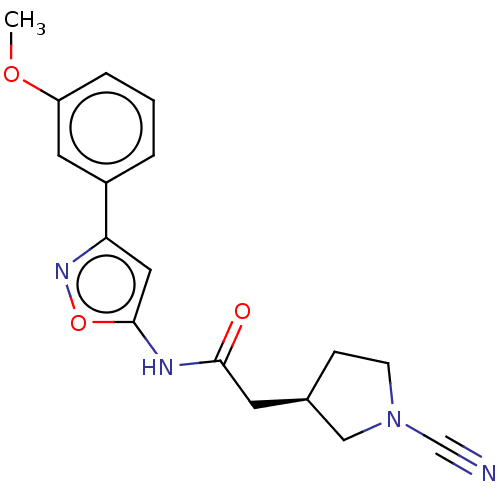 (US11014912, Example 43) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498866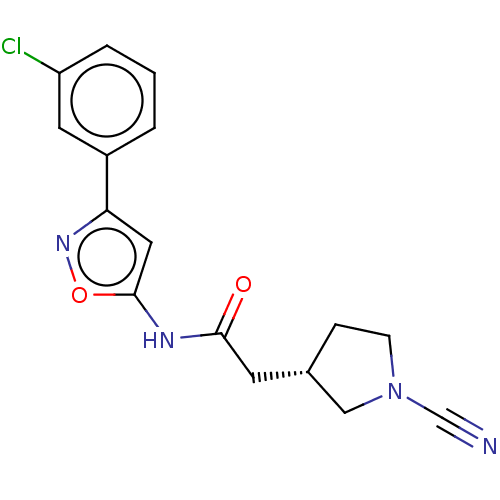 (US11014912, Example 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498864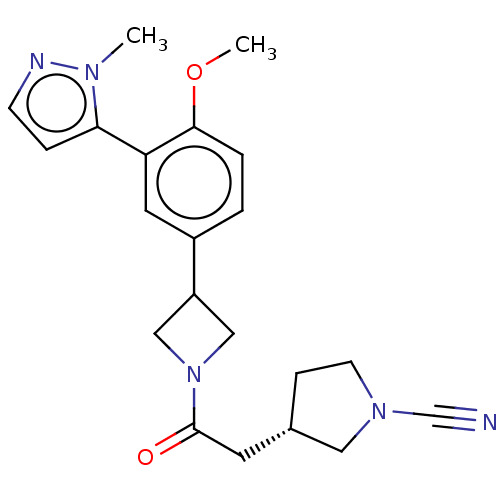 ((S)-3-(2-(3-(4-Methoxy-3-(1-methyl-1H-pyrazol-5-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498863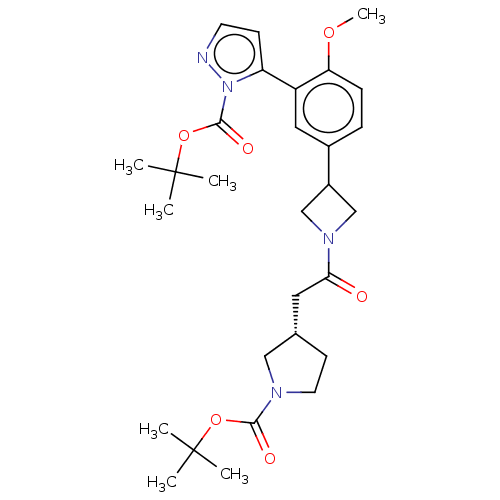 ((S)-3-(2-(3-(4-Methoxy-3-(1H-pyrazol-5-yl)phenyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498862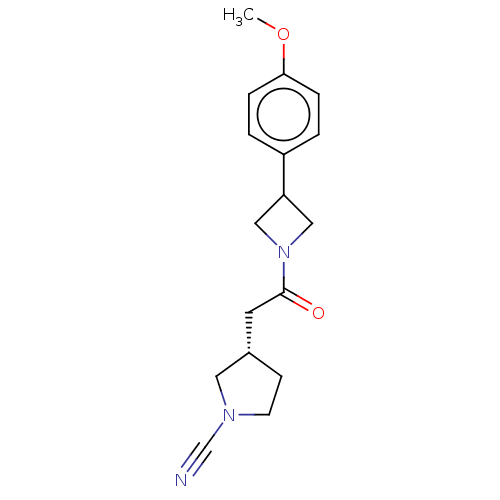 (US11014912, Example 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498846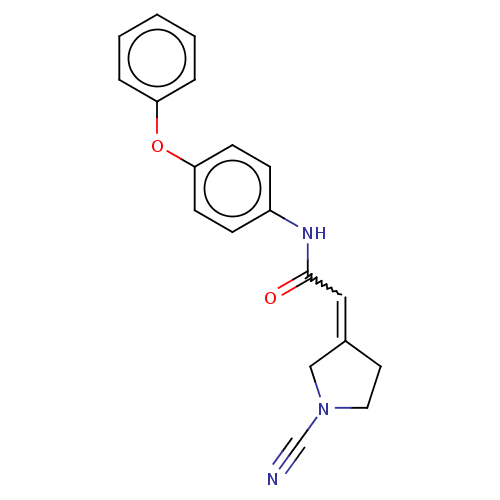 (2-(1-Cyanopyrrolidin-3- ylidene)-N-(4- phenoxyphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498889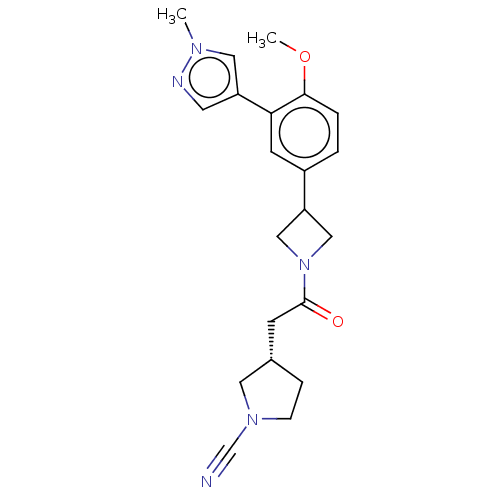 (US11014912, Example 67) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498883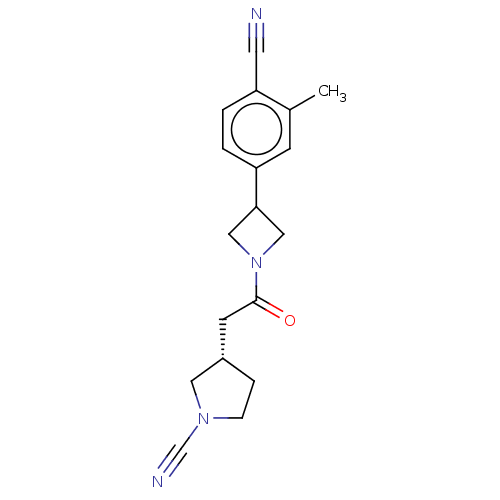 (US11014912, Example 59) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM498890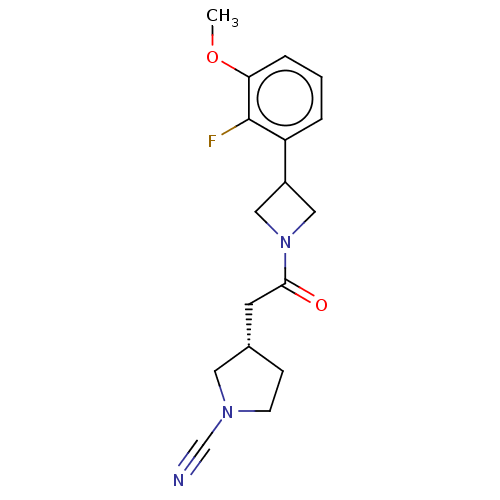 (US11014912, Example 68) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US11014912 (2021) BindingDB Entry DOI: 10.7270/Q2ZW1Q27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of human N-terminal BRD4 bromodomain expressed in Escherichia coli BL21(DE3) after 30 mins by luminescence proximity homogeneous assay | J Med Chem 55: 587-96 (2012) Article DOI: 10.1021/jm201283q BindingDB Entry DOI: 10.7270/Q22R3SSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM556946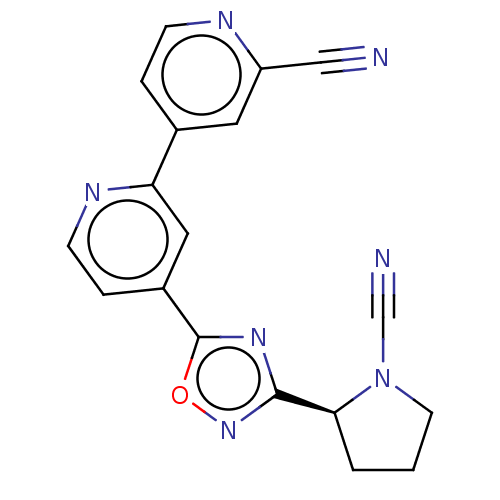 ((S)-4-(3-(1-Cyanopyrrolidin-2-yl)-1,2,4-oxadiazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | Citation and Details BindingDB Entry DOI: 10.7270/Q28K7D9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM556948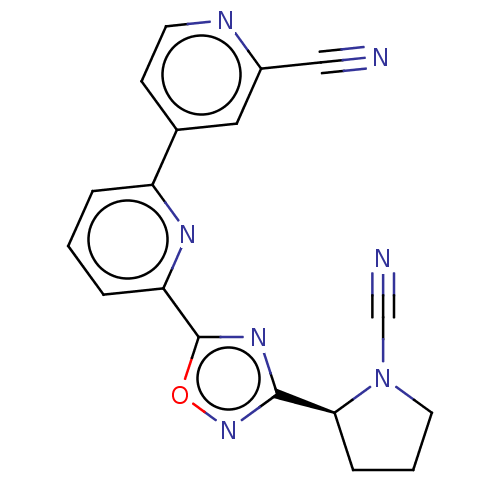 ((S)-6-(3-(1-Cyanopyrrolidin-2-yl)-1,2,4-oxadiazol-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | Citation and Details BindingDB Entry DOI: 10.7270/Q28K7D9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OTU domain-containing protein 7B (Human) | BDBM436072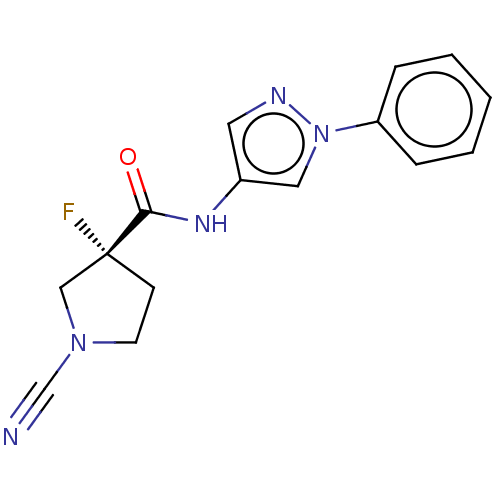 ((R)-1-cyano-3-fluoro-N-(1-phenyl-1H-pyrazol-4-yl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US10590075 (2020) BindingDB Entry DOI: 10.7270/Q2280BNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OTU domain-containing protein 7B (Human) | BDBM443251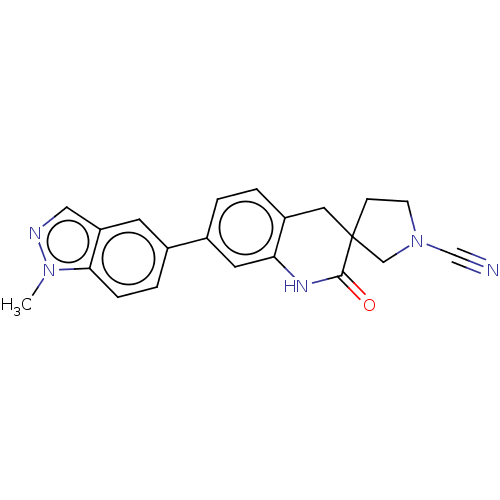 (7'-(1-Methyl-1H-indazol-5-yl)- 2'-oxo-1',4'-dihydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US10654853 (2020) BindingDB Entry DOI: 10.7270/Q21V5J02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OTU domain-containing protein 7B (Human) | BDBM443372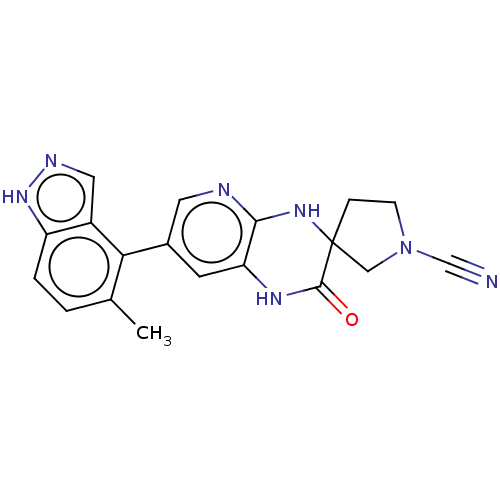 (7-(5-Methyl-1H- indazol-4-yl)-2- oxo-1,4-dihydro- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US10654853 (2020) BindingDB Entry DOI: 10.7270/Q21V5J02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OTU domain-containing protein 7B (Human) | BDBM443364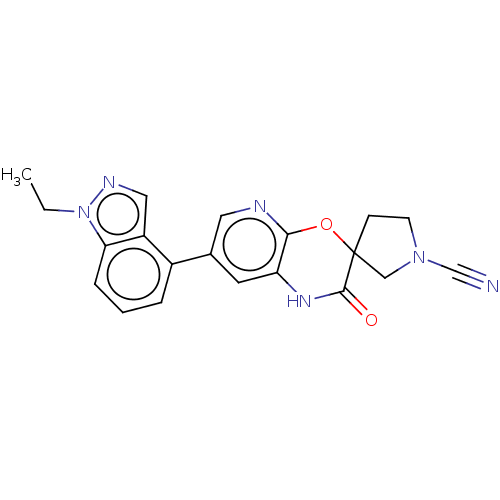 (7-(1-(2-Hydroxyethyl)- 1H-indazol-4-yl)-2- oxo-1,2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US10654853 (2020) BindingDB Entry DOI: 10.7270/Q21V5J02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OTU domain-containing protein 7B (Human) | BDBM443363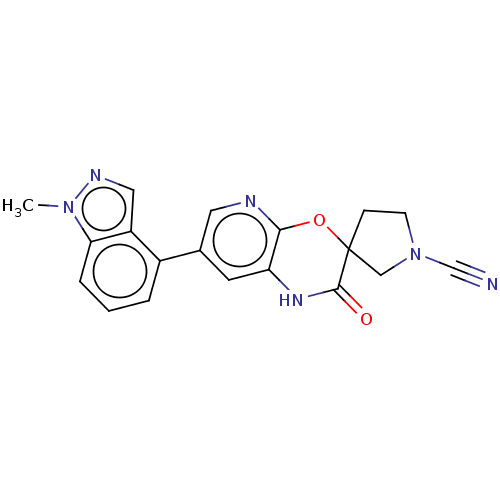 (7-(1-Methyl-1H-indazol- 4-yl)-2-oxo-1,2- dihydrosp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US10654853 (2020) BindingDB Entry DOI: 10.7270/Q21V5J02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OTU domain-containing protein 7B (Human) | BDBM443362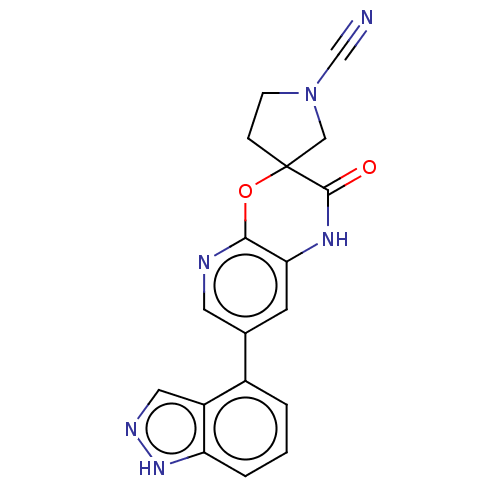 (7-(1H-Indazol-4-yl)-2- oxo-1,2-dihydrospiro [pyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 μM for a final concentration of 100 μM) in 50% DMSO in a 96-well po... | US Patent US10654853 (2020) BindingDB Entry DOI: 10.7270/Q21V5J02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM441759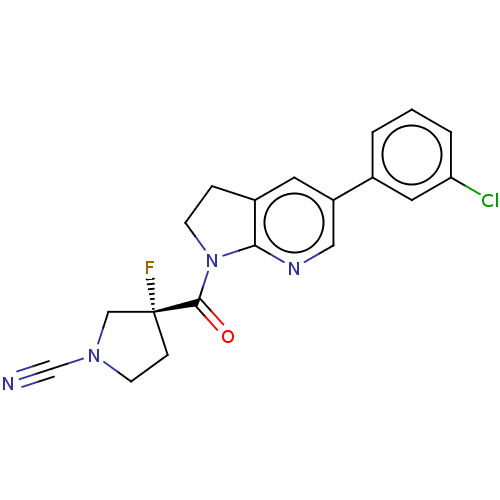 ((R)-3-(5-(3-Chlorophenyl)-2,3-dihydro-1H-pyrrolo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 M for a final concentration of 100 μM) in 50% DMSO in a 96-well polyprop... | US Patent US10640498 (2020) BindingDB Entry DOI: 10.7270/Q2J10663 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 30 (Homo sapiens (Human)) | BDBM441743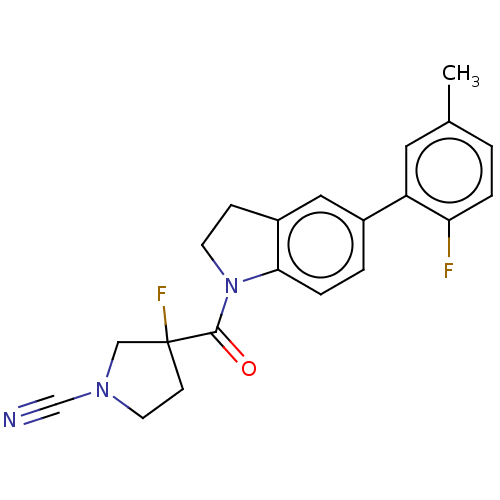 (3-Fluoro-3-(5-(2-fluoro-5- methylphenyl)indoline-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
MISSION THERAPEUTICS LIMITED US Patent | Assay Description Dilution plates were prepared at 21 times the final concentration (2100 M for a final concentration of 100 μM) in 50% DMSO in a 96-well polyprop... | US Patent US10640498 (2020) BindingDB Entry DOI: 10.7270/Q2J10663 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1001 total ) | Next | Last >> |