 Found 118 hits with Last Name = 'lallena' and Initial = 'mj'
Found 118 hits with Last Name = 'lallena' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50110183
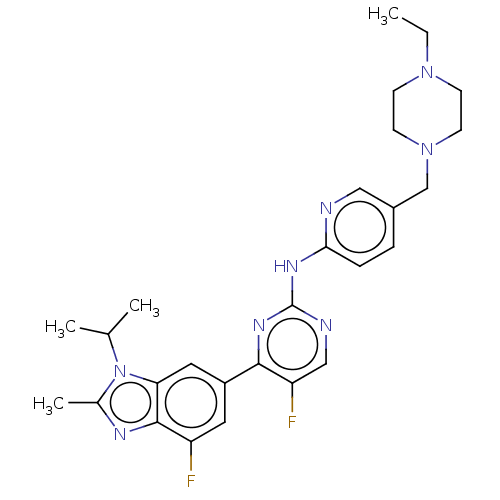
(Abemaciclib | LY-2835219 | US10626107, Example LY2...)Show SMILES CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cc(F)c4nc(C)n(C(C)C)c4c3)nc2)CC1 Show InChI InChI=1S/C27H32F2N8/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive inhibition of CDK6/cyclin D3 (unknown origin) assessed as phosphorylation of CTRF after 50 mins by Michaelis-Menten plot analysis in pres... |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50528812

(CHEMBL4582951)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(nc1)C(=O)N[C@@]1(C)CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C31H35ClN8O2/c1-31(39-29(42)26-13-12-21(17-33-26)36-27(41)11-7-15-40(2)3)14-6-8-20(16-31)37-30-35-19-24(32)28(38-30)23-18-34-25-10-5-4-9-22(23)25/h4-5,7,9-13,17-20,34H,6,8,14-16H2,1-3H3,(H,36,41)(H,39,42)(H,35,37,38)/b11-7+/t20-,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) using 5-FAM YSPTSPSYSPTSPSYSPTSPSKKKK as substrate |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50528814

(CHEMBL4436535)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(nc1)C(=O)N[C@H]1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H33ClN8O2/c1-39(2)14-6-11-27(40)35-21-12-13-26(32-16-21)29(41)36-19-7-5-8-20(15-19)37-30-34-18-24(31)28(38-30)23-17-33-25-10-4-3-9-22(23)25/h3-4,6,9-13,16-20,33H,5,7-8,14-15H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) using 5-FAM YSPTSPSYSPTSPSYSPTSPSKKKK as substrate |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50528811

(CHEMBL4441405)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N[C@H]1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C31H34ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-4,6,9-15,18-19,22-23,33H,5,7-8,16-17H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+/t22-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) using 5-FAM YSPTSPSYSPTSPSYSPTSPSKKKK as substrate |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50110178
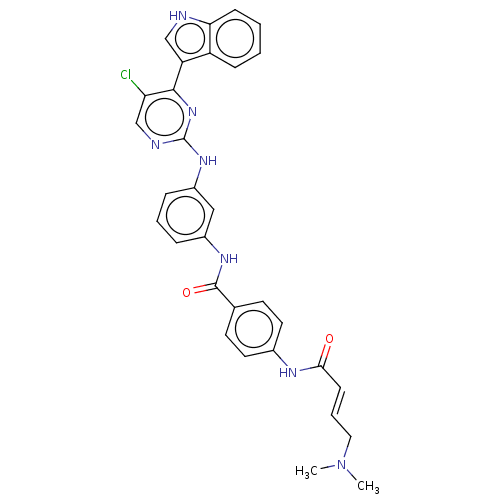
(CHEMBL3603847 | US10787436, Compound I-23)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2ncc(Cl)c(n2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H28ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-15,17-19,33H,16H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+ | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) using 5-FAM YSPTSPSYSPTSPSYSPTSPSKKKK as substrate |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM253928
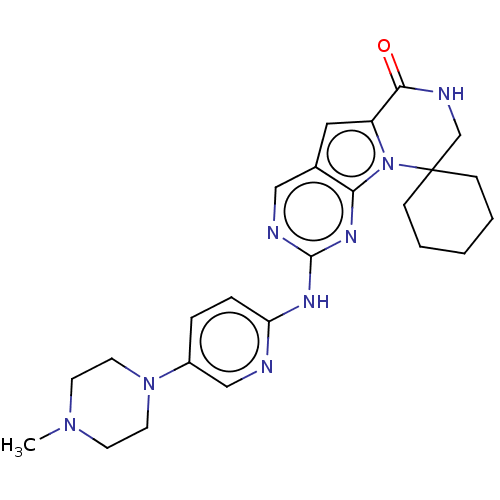
(US10189849, Compound 27 | US10927120, Compound 27 ...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3cc4C(=O)NCC5(CCCCC5)n4c3n2)nc1 Show InChI InChI=1S/C24H30N8O/c1-30-9-11-31(12-10-30)18-5-6-20(25-15-18)28-23-26-14-17-13-19-22(33)27-16-24(7-3-2-4-8-24)32(19)21(17)29-23/h5-6,13-15H,2-4,7-12,16H2,1H3,(H,27,33)(H,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclinD1 |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50110179
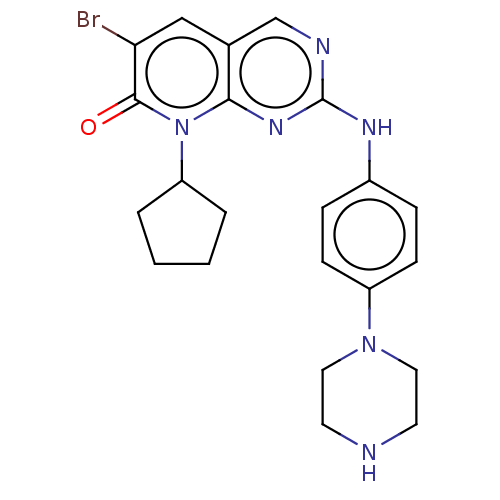
(CHEMBL190281)Show SMILES Brc1cc2cnc(Nc3ccc(cc3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C22H25BrN6O/c23-19-13-15-14-25-22(27-20(15)29(21(19)30)18-3-1-2-4-18)26-16-5-7-17(8-6-16)28-11-9-24-10-12-28/h5-8,13-14,18,24H,1-4,9-12H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 (unknown origin) using GST-fused pRb (792 to 928) as substrate preincubated for 2 mins followed by [gamma-32P]-ATP addition measur... |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50528816
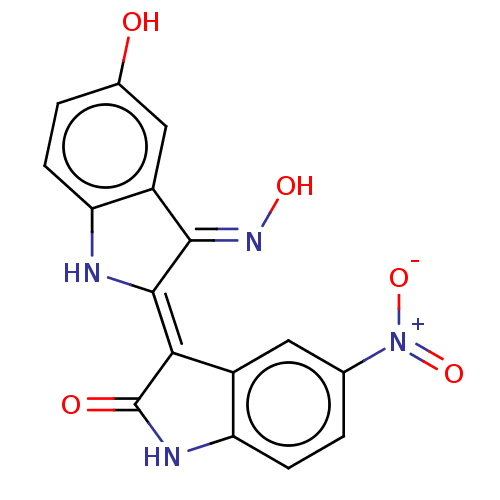
(CHEMBL4457608 | US11370779, Compound 13)Show SMILES O\N=C1\C(\Nc2ccc(O)cc\12)=C1\C(=O)Nc2ccc(cc12)[N+]([O-])=O Show InChI InChI=1S/C16H10N4O5/c21-8-2-4-12-10(6-8)14(19-23)15(17-12)13-9-5-7(20(24)25)1-3-11(9)18-16(13)22/h1-6,17,21,23H,(H,18,22)/b15-13-,19-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM6302
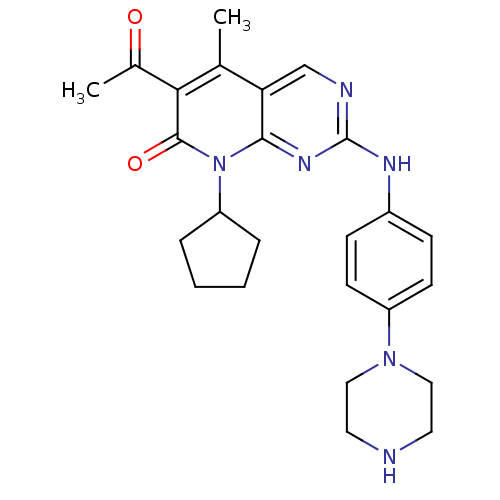
(8-cyclopentyl-6-acetyl-5-methyl-2-{[4-(piperazin-1...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cc3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C25H30N6O2/c1-16-21-15-27-25(28-18-7-9-19(10-8-18)30-13-11-26-12-14-30)29-23(21)31(20-5-3-4-6-20)24(33)22(16)17(2)32/h7-10,15,20,26H,3-6,11-14H2,1-2H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 (unknown origin) using GST-fused pRb (792 to 928) as substrate preincubated for 2 mins followed by [gamma-32P]-ATP addition measur... |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50347388
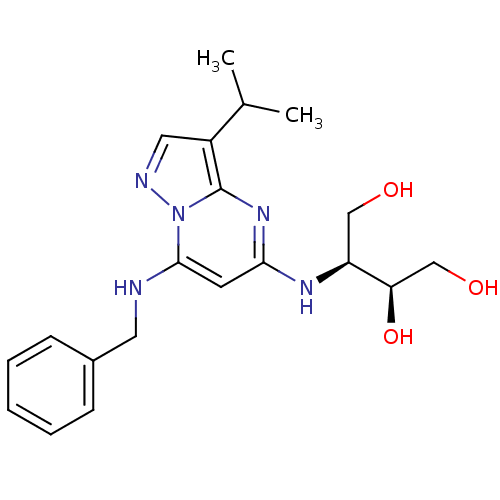
(CHEMBL1234833)Show SMILES CC(C)c1cnn2c(NCc3ccccc3)cc(N[C@@H](CO)[C@H](O)CO)nc12 |r| Show InChI InChI=1S/C20H27N5O3/c1-13(2)15-10-22-25-19(21-9-14-6-4-3-5-7-14)8-18(24-20(15)25)23-16(11-26)17(28)12-27/h3-8,10,13,16-17,21,26-28H,9,11-12H2,1-2H3,(H,23,24)/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CDK2 (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50528809
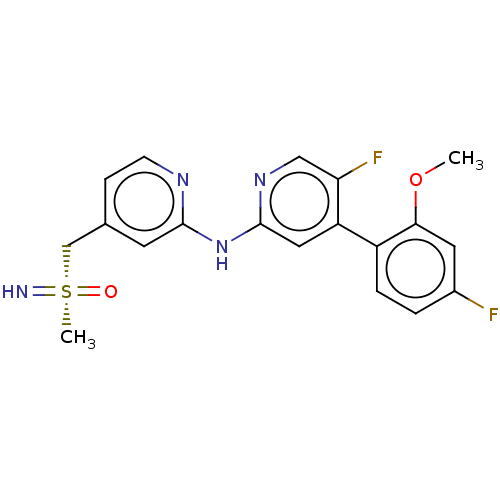
(CHEMBL4589249)Show SMILES COc1cc(F)ccc1-c1cc(Nc2cc(C[S@](C)(=N)=O)ccn2)ncc1F |r| Show InChI InChI=1S/C19H18F2N4O2S/c1-27-17-8-13(20)3-4-14(17)15-9-19(24-10-16(15)21)25-18-7-12(5-6-23-18)11-28(2,22)26/h3-10,22H,11H2,1-2H3,(H,23,24,25)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of recombinant full-length His-tagged human CDK9/cyclin T expressed in baculovirus infected insect cells using biot... |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50110178

(CHEMBL3603847 | US10787436, Compound I-23)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2ncc(Cl)c(n2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H28ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-15,17-19,33H,16H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive inhibition of human CDK7 in presence of ATP |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50110180
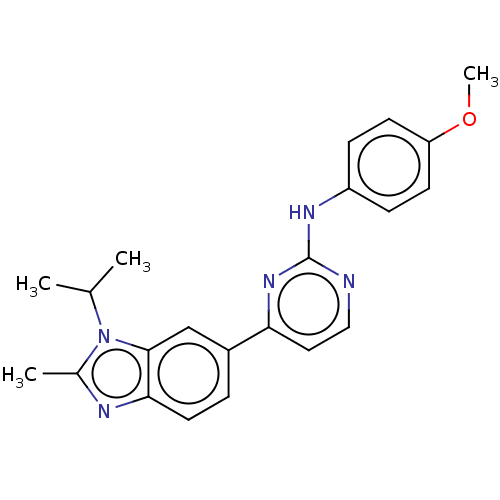
(CHEMBL3604462)Show SMILES COc1ccc(Nc2nccc(n2)-c2ccc3nc(C)n(C(C)C)c3c2)cc1 Show InChI InChI=1S/C22H23N5O/c1-14(2)27-15(3)24-20-10-5-16(13-21(20)27)19-11-12-23-22(26-19)25-17-6-8-18(28-4)9-7-17/h5-14H,1-4H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human CDK1/cyclin B1 (unknown origin) |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM253928

(US10189849, Compound 27 | US10927120, Compound 27 ...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3cc4C(=O)NCC5(CCCCC5)n4c3n2)nc1 Show InChI InChI=1S/C24H30N8O/c1-30-9-11-31(12-10-30)18-5-6-20(25-15-18)28-23-26-14-17-13-19-22(33)27-16-24(7-3-2-4-8-24)32(19)21(17)29-23/h5-6,13-15H,2-4,7-12,16H2,1H3,(H,27,33)(H,25,26,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of human CDK6/cyclin D3 |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50528817

(CHEMBL4462530 | US10717746, Example 14 | US2023041...)Show SMILES CC(=O)N[C@@H]1CCC[C@@H](C1)C(=O)Nc1cc(-c2cnn3CC(C)(C)Cc23)c(Cl)cn1 |r| Show InChI InChI=1S/C22H28ClN5O2/c1-13(29)26-15-6-4-5-14(7-15)21(30)27-20-8-16(18(23)11-24-20)17-10-25-28-12-22(2,3)9-19(17)28/h8,10-11,14-15H,4-7,9,12H2,1-3H3,(H,26,29)(H,24,27,30)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK9 (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50528809

(CHEMBL4589249)Show SMILES COc1cc(F)ccc1-c1cc(Nc2cc(C[S@](C)(=N)=O)ccn2)ncc1F |r| Show InChI InChI=1S/C19H18F2N4O2S/c1-27-17-8-13(20)3-4-14(17)15-9-19(24-10-16(15)21)25-18-7-12(5-6-23-18)11-28(2,22)26/h3-10,22H,11H2,1-2H3,(H,23,24,25)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of recombinant full-length His-tagged human CDK9/cyclin T expressed in baculovirus infected insect cells using biot... |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM6301

(6-bromo-8-cyclopentyl-5-methyl-2-{[4-(piperazin-1-...)Show SMILES Cc1c(Br)c(=O)n(C2CCCC2)c2nc(Nc3ccc(cc3)N3CCNCC3)ncc12 Show InChI InChI=1S/C23H27BrN6O/c1-15-19-14-26-23(27-16-6-8-17(9-7-16)29-12-10-25-11-13-29)28-21(19)30(22(31)20(15)24)18-4-2-3-5-18/h6-9,14,18,25H,2-5,10-13H2,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 (unknown origin) using GST-fused pRb (792 to 928) as substrate preincubated for 2 mins followed by [gamma-32P]-ATP addition measur... |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM106950

(US8592581, 1)Show SMILES CC[C@H](Nc1nc(NCc2cnc(C)cc2C)c2ncn(C(C)C)c2n1)[C@@H](C)O |r| Show InChI InChI=1S/C21H31N7O/c1-7-17(15(6)29)25-21-26-19(18-20(27-21)28(11-24-18)12(2)3)23-10-16-9-22-14(5)8-13(16)4/h8-9,11-12,15,17,29H,7,10H2,1-6H3,(H2,23,25,26,27)/t15-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CDK2/cyclin E (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-Y/Cyclin-dependent kinase 16
(Homo sapiens (Human)) | BDBM50528810

(CHEMBL4580787)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)S(=O)(=O)N1CCC(CC1)NC(=O)c1n[nH]cc1NC(=O)c1c(Cl)cc(Cl)cc1Cl Show InChI InChI=1S/C28H30Cl3N7O5S/c1-37(2)10-4-7-24(39)33-19-5-3-6-20(15-19)44(42,43)38-11-8-18(9-12-38)34-28(41)26-23(16-32-36-26)35-27(40)25-21(30)13-17(29)14-22(25)31/h3-7,13-16,18H,8-12H2,1-2H3,(H,32,36)(H,33,39)(H,34,41)(H,35,40)/b7-4+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK16/cyclin Y (unknown origin) in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM148264

(7-cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)p...)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n1C1CCCC1 Show InChI InChI=1S/C23H30N8O/c1-29(2)22(32)19-13-16-14-26-23(28-21(16)31(19)17-5-3-4-6-17)27-20-8-7-18(15-25-20)30-11-9-24-10-12-30/h7-8,13-15,17,24H,3-6,9-12H2,1-2H3,(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 (unknown origin) |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50528818

(CHEMBL4439321)Show SMILES COc1cc(F)ccc1-c1ncnc(Nc2cccc(C[S@](C)(=N)=O)c2)n1 |r| Show InChI InChI=1S/C18H18FN5O2S/c1-26-16-9-13(19)6-7-15(16)17-21-11-22-18(24-17)23-14-5-3-4-12(8-14)10-27(2,20)25/h3-9,11,20H,10H2,1-2H3,(H,21,22,23,24)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of recombinant full-length His-tagged human CDK9/cyclin T expressed in baculovirus infected insect cells using biot... |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50528812

(CHEMBL4582951)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(nc1)C(=O)N[C@@]1(C)CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C31H35ClN8O2/c1-31(39-29(42)26-13-12-21(17-33-26)36-27(41)11-7-15-40(2)3)14-6-8-20(16-31)37-30-35-19-24(32)28(38-30)23-18-34-25-10-5-4-9-22(23)25/h4-5,7,9-13,17-20,34H,6,8,14-16H2,1-3H3,(H,36,41)(H,39,42)(H,35,37,38)/b11-7+/t20-,31+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK12/cyclinK (unknown origin) in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50528815
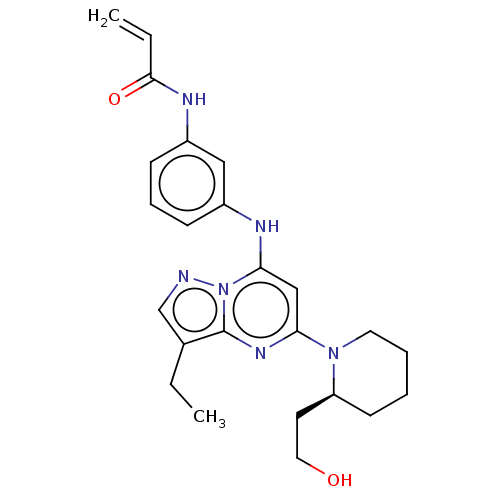
(CHEMBL4468670)Show SMILES CCc1cnn2c(Nc3cccc(NC(=O)C=C)c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C24H30N6O2/c1-3-17-16-25-30-22(26-18-8-7-9-19(14-18)27-23(32)4-2)15-21(28-24(17)30)29-12-6-5-10-20(29)11-13-31/h4,7-9,14-16,20,26,31H,2-3,5-6,10-13H2,1H3,(H,27,32)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK9/cyclin T (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50347389
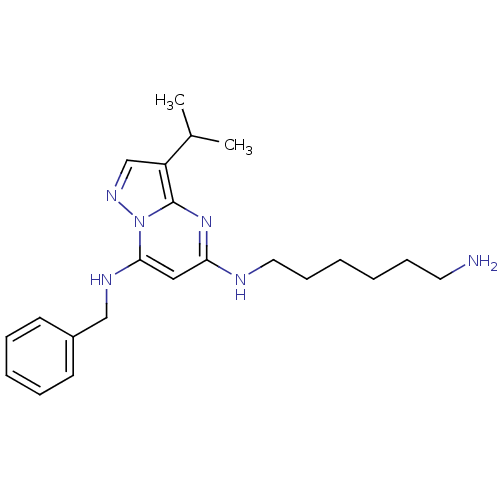
(CHEMBL1801932)Show InChI InChI=1S/C22H32N6/c1-17(2)19-16-26-28-21(25-15-18-10-6-5-7-11-18)14-20(27-22(19)28)24-13-9-4-3-8-12-23/h5-7,10-11,14,16-17,25H,3-4,8-9,12-13,15,23H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CDK7 (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50528814

(CHEMBL4436535)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(nc1)C(=O)N[C@H]1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H33ClN8O2/c1-39(2)14-6-11-27(40)35-21-12-13-26(32-16-21)29(41)36-19-7-5-8-20(15-19)37-30-34-18-24(31)28(38-30)23-17-33-25-10-4-3-9-22(23)25/h3-4,6,9-13,16-20,33H,5,7-8,14-15H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) using 5-FAM YSPTSPSYSPTSPSYSPTSPSKKKK as substrate in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM106950

(US8592581, 1)Show SMILES CC[C@H](Nc1nc(NCc2cnc(C)cc2C)c2ncn(C(C)C)c2n1)[C@@H](C)O |r| Show InChI InChI=1S/C21H31N7O/c1-7-17(15(6)29)25-21-26-19(18-20(27-21)28(11-24-18)12(2)3)23-10-16-9-22-14(5)8-13(16)4/h8-9,11-12,15,17,29H,7,10H2,1-6H3,(H2,23,25,26,27)/t15-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CDK5 (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM106950

(US8592581, 1)Show SMILES CC[C@H](Nc1nc(NCc2cnc(C)cc2C)c2ncn(C(C)C)c2n1)[C@@H](C)O |r| Show InChI InChI=1S/C21H31N7O/c1-7-17(15(6)29)25-21-26-19(18-20(27-21)28(11-24-18)12(2)3)23-10-16-9-22-14(5)8-13(16)4/h8-9,11-12,15,17,29H,7,10H2,1-6H3,(H2,23,25,26,27)/t15-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CDK9/CyclinT (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50347388

(CHEMBL1234833)Show SMILES CC(C)c1cnn2c(NCc3ccccc3)cc(N[C@@H](CO)[C@H](O)CO)nc12 |r| Show InChI InChI=1S/C20H27N5O3/c1-13(2)15-10-22-25-19(21-9-14-6-4-3-5-7-14)8-18(24-20(15)25)23-16(11-26)17(28)12-27/h3-8,10,13,16-17,21,26-28H,9,11-12H2,1-2H3,(H,23,24)/t16-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CDK5 (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50113342
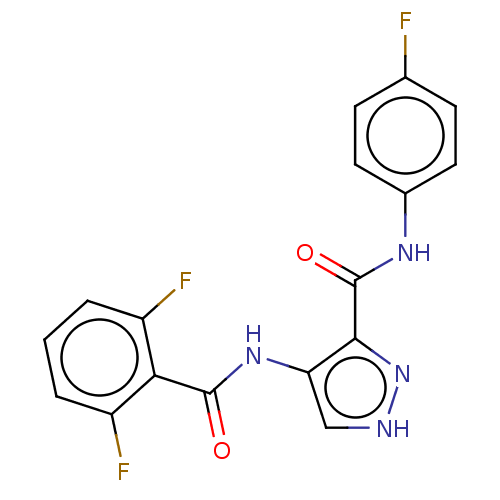
(CHEMBL518383)Show SMILES Fc1ccc(NC(=O)c2n[nH]cc2NC(=O)c2c(F)cccc2F)cc1 Show InChI InChI=1S/C17H11F3N4O2/c18-9-4-6-10(7-5-9)22-17(26)15-13(8-21-24-15)23-16(25)14-11(19)2-1-3-12(14)20/h1-8H,(H,21,24)(H,22,26)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin A (unknown origin) by radiometric filter binding assay |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50110187
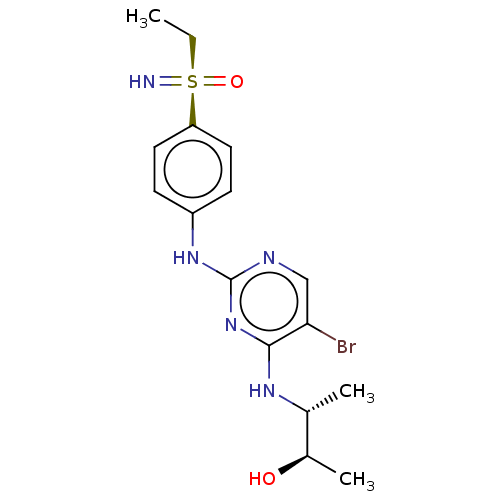
(CHEMBL3604469)Show SMILES CC[S@@](=N)(=O)c1ccc(Nc2ncc(Br)c(N[C@H](C)[C@@H](C)O)n2)cc1 |r| Show InChI InChI=1S/C16H22BrN5O2S/c1-4-25(18,24)13-7-5-12(6-8-13)21-16-19-9-14(17)15(22-16)20-10(2)11(3)23/h5-11,18,23H,4H2,1-3H3,(H2,19,20,21,22)/t10-,11-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50110184
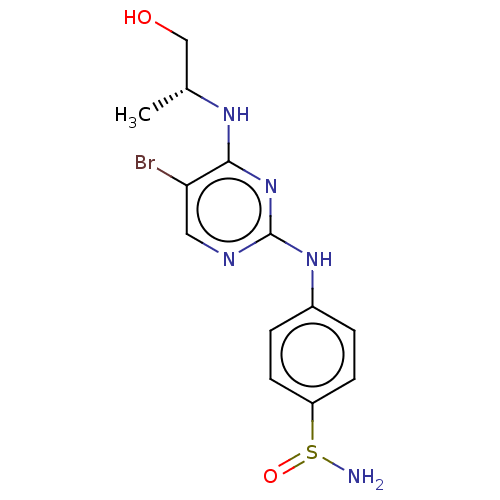
(CHEMBL3604466)Show SMILES C[C@H](CO)Nc1nc(Nc2ccc(cc2)S(N)=O)ncc1Br |r| Show InChI InChI=1S/C13H16BrN5O2S/c1-8(7-20)17-12-11(14)6-16-13(19-12)18-9-2-4-10(5-3-9)22(15)21/h2-6,8,20H,7,15H2,1H3,(H2,16,17,18,19)/t8-,22?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM148264

(7-cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)p...)Show SMILES CN(C)C(=O)c1cc2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n1C1CCCC1 Show InChI InChI=1S/C23H30N8O/c1-29(2)22(32)19-13-16-14-26-23(28-21(16)31(19)17-5-3-4-6-17)27-20-8-7-18(15-25-20)30-11-9-24-10-12-30/h7-8,13-15,17,24H,3-6,9-12H2,1-2H3,(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 (unknown origin) |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50526797
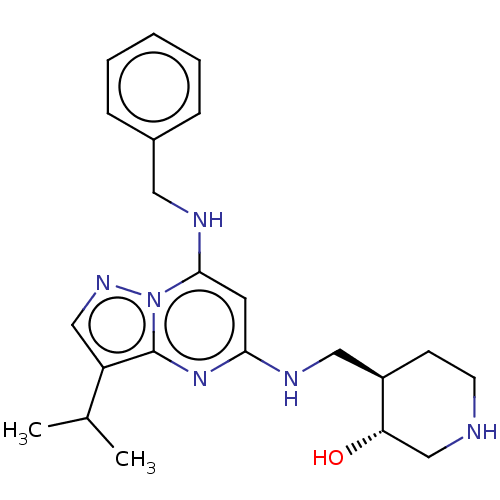
(Ct-7001 | Ct7001 | ICEC-0942 | ICEC0942 | Samuraci...)Show SMILES CC(C)c1cnn2c(NCc3ccccc3)cc(NC[C@H]3CCNC[C@@H]3O)nc12 Show InChI InChI=1S/C22H30N6O/c1-15(2)18-13-26-28-21(25-11-16-6-4-3-5-7-16)10-20(27-22(18)28)24-12-17-8-9-23-14-19(17)29/h3-7,10,13,15,17,19,23,25,29H,8-9,11-12,14H2,1-2H3,(H,24,27)/t17-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CDK7 (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50110179

(CHEMBL190281)Show SMILES Brc1cc2cnc(Nc3ccc(cc3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C22H25BrN6O/c23-19-13-15-14-25-22(27-20(15)29(21(19)30)18-3-1-2-4-18)26-16-5-7-17(8-6-16)28-11-9-24-10-12-28/h5-8,13-14,18,24H,1-4,9-12H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) using GST-fused pRb (792 to 928) as substrate preincubated for 2 mins followed by [gamma-32P]-ATP addition measur... |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin A (unknown origin) by radiometric filter binding assay |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50526795
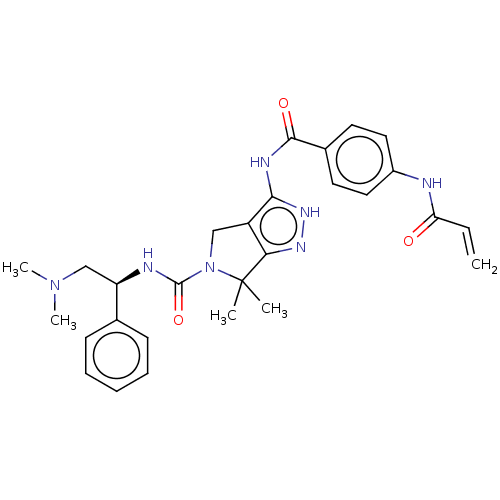
(CHEMBL4450322)Show SMILES CN(C)C[C@@H](NC(=O)N1Cc2c(NC(=O)c3ccc(NC(=O)C=C)cc3)[nH]nc2C1(C)C)c1ccccc1 |r| Show InChI InChI=1S/C28H33N7O3/c1-6-23(36)29-20-14-12-19(13-15-20)26(37)31-25-21-16-35(28(2,3)24(21)32-33-25)27(38)30-22(17-34(4)5)18-10-8-7-9-11-18/h6-15,22H,1,16-17H2,2-5H3,(H,29,36)(H,30,38)(H2,31,32,33,37)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) in presence of 1 mM ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50110185

(CHEMBL3604467)Show SMILES C[C@@H](O)[C@@H](C)Nc1nc(Nc2ccc(cc2)S(N)=O)ncc1Br |r| Show InChI InChI=1S/C14H18BrN5O2S/c1-8(9(2)21)18-13-12(15)7-17-14(20-13)19-10-3-5-11(6-4-10)23(16)22/h3-9,21H,16H2,1-2H3,(H2,17,18,19,20)/t8-,9-,23?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50528813
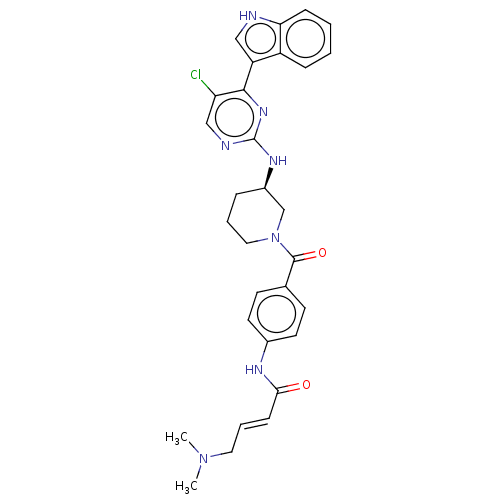
(CHEMBL4163879)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H32ClN7O2/c1-37(2)15-6-10-27(39)34-21-13-11-20(12-14-21)29(40)38-16-5-7-22(19-38)35-30-33-18-25(31)28(36-30)24-17-32-26-9-4-3-8-23(24)26/h3-4,6,8-14,17-18,22,32H,5,7,15-16,19H2,1-2H3,(H,34,39)(H,33,35,36)/b10-6+/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK13/cyclinK (unknown origin) in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50528812

(CHEMBL4582951)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(nc1)C(=O)N[C@@]1(C)CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C31H35ClN8O2/c1-31(39-29(42)26-13-12-21(17-33-26)36-27(41)11-7-15-40(2)3)14-6-8-20(16-31)37-30-35-19-24(32)28(38-30)23-18-34-25-10-5-4-9-22(23)25/h4-5,7,9-13,17-20,34H,6,8,14-16H2,1-3H3,(H,36,41)(H,39,42)(H,35,37,38)/b11-7+/t20-,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) using 5-FAM YSPTSPSYSPTSPSYSPTSPSKKKK as substrate in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-Y
(Homo sapiens) | BDBM50528810

(CHEMBL4580787)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)S(=O)(=O)N1CCC(CC1)NC(=O)c1n[nH]cc1NC(=O)c1c(Cl)cc(Cl)cc1Cl Show InChI InChI=1S/C28H30Cl3N7O5S/c1-37(2)10-4-7-24(39)33-19-5-3-6-20(15-19)44(42,43)38-11-8-18(9-12-38)34-28(41)26-23(16-32-36-26)35-27(40)25-21(30)13-17(29)14-22(25)31/h3-7,13-16,18H,8-12H2,1-2H3,(H,32,36)(H,33,39)(H,34,41)(H,35,40)/b7-4+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK14/cyclin Y (unknown origin) in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50347388

(CHEMBL1234833)Show SMILES CC(C)c1cnn2c(NCc3ccccc3)cc(N[C@@H](CO)[C@H](O)CO)nc12 |r| Show InChI InChI=1S/C20H27N5O3/c1-13(2)15-10-22-25-19(21-9-14-6-4-3-5-7-14)8-18(24-20(15)25)23-16(11-26)17(28)12-27/h3-8,10,13,16-17,21,26-28H,9,11-12H2,1-2H3,(H,23,24)/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CDK8 (unknown origin) |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50528811

(CHEMBL4441405)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N[C@H]1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C31H34ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-4,6,9-15,18-19,22-23,33H,5,7-8,16-17H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+/t22-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) using 5-FAM YSPTSPSYSPTSPSYSPTSPSKKKK as substrate in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50110186
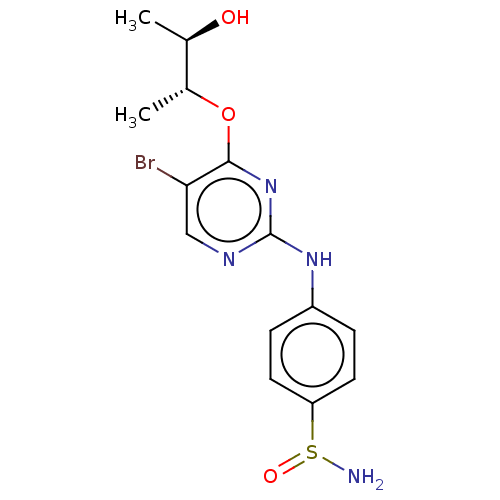
(CHEMBL3604468)Show SMILES C[C@@H](O)[C@@H](C)Oc1nc(Nc2ccc(cc2)S(N)=O)ncc1Br |r| Show InChI InChI=1S/C14H17BrN4O3S/c1-8(20)9(2)22-13-12(15)7-17-14(19-13)18-10-3-5-11(6-4-10)23(16)21/h3-9,20H,16H2,1-2H3,(H,17,18,19)/t8-,9-,23?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50113349

(CHEMBL457388)Show InChI InChI=1S/C16H17F2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin A (unknown origin) by radiometric filter binding assay |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50528813

(CHEMBL4163879)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H32ClN7O2/c1-37(2)15-6-10-27(39)34-21-13-11-20(12-14-21)29(40)38-16-5-7-22(19-38)35-30-33-18-25(31)28(36-30)24-17-32-26-9-4-3-8-23(24)26/h3-4,6,8-14,17-18,22,32H,5,7,15-16,19H2,1-2H3,(H,34,39)(H,33,35,36)/b10-6+/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK12/cyclinK (unknown origin) in presence of Km ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) by radiometric filter binding assay |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) by ELISA |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/cyclin B (unknown origin) by radiometric filter binding assay |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D3
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibition of CDK6/cyclin D3 (unknown origin) by ELISA |
Bioorg Med Chem Lett 25: 3420-35 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.100
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | |
CDK-activating kinase assembly factor MAT1/Cyclin-H/Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50528814

(CHEMBL4436535)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(nc1)C(=O)N[C@H]1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H33ClN8O2/c1-39(2)14-6-11-27(40)35-21-12-13-26(32-16-21)29(41)36-19-7-5-8-20(15-19)37-30-34-18-24(31)28(38-30)23-17-33-25-10-4-3-9-22(23)25/h3-4,6,9-13,16-20,33H,5,7-8,14-15H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive irreversible inhibition of CDK7/cyclinH/MAT1 (unknown origin) using 5-FAM YSPTSPSYSPTSPSYSPTSPSKKKK as substrate in presence of 2 mM ATP |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126637
BindingDB Entry DOI: 10.7270/Q23F4T3T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data