

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366322 (CHEMBL2367489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against estrone sulfatase in placental preparation | Bioorg Med Chem Lett 3: 313-318 (1993) Article DOI: 10.1016/S0960-894X(01)80900-8 BindingDB Entry DOI: 10.7270/Q2K074R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366322 (CHEMBL2367489) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against estrone sulfatase in breast tumor preparations | Bioorg Med Chem Lett 3: 313-318 (1993) Article DOI: 10.1016/S0960-894X(01)80900-8 BindingDB Entry DOI: 10.7270/Q2K074R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Rattus norvegicus) | BDBM50147505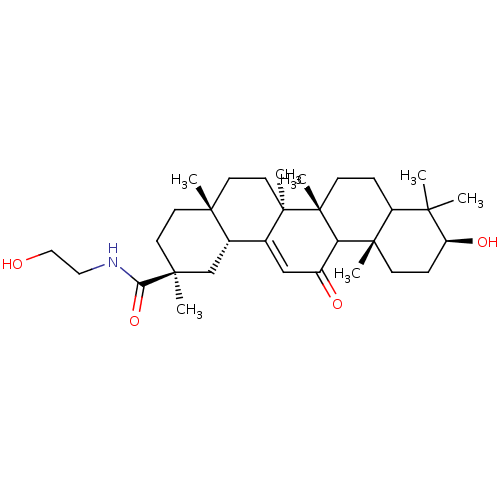 ((2S,4aS,6aS,6bR,10S,12aS,14bR)-10-Hydroxy-2,4a,6a,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Percent inhibition against 11-beta-hydroxysteroid dehydrogenase 2 of wistar rat kidney at 10 microM was determined using [3H]-cortisol | Bioorg Med Chem Lett 14: 3263-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.107 BindingDB Entry DOI: 10.7270/Q2H41QWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307918 (2-(5-((1H-1,2,4-triazol-1-yl)methyl)-3'-chloro-4'-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307901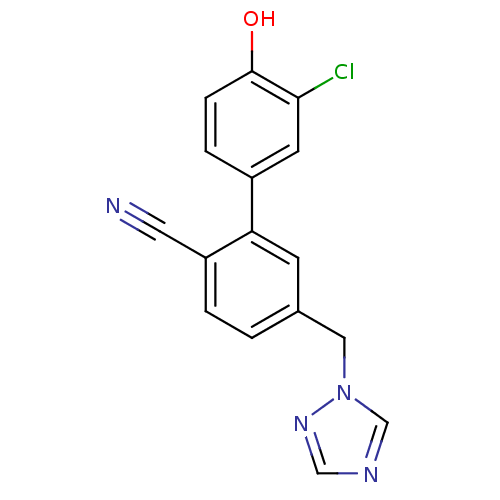 (5-((1H-1,2,4-triazol-1-yl)methyl)-3'-chloro-4'-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24341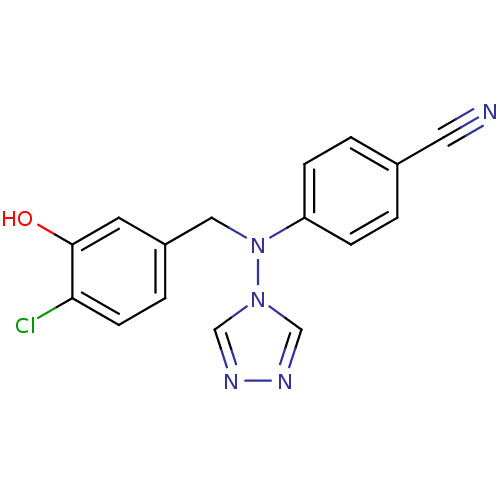 (4-{[(4-chloro-3-hydroxyphenyl)methyl](4H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307900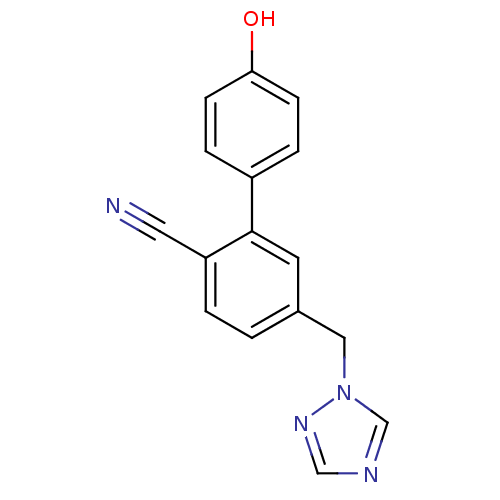 (5-((1H-1,2,4-triazol-1-yl)methyl)-4'-hydroxybiphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307899 (5-((1H-1,2,4-triazol-1-yl)methyl)biphenyl-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307919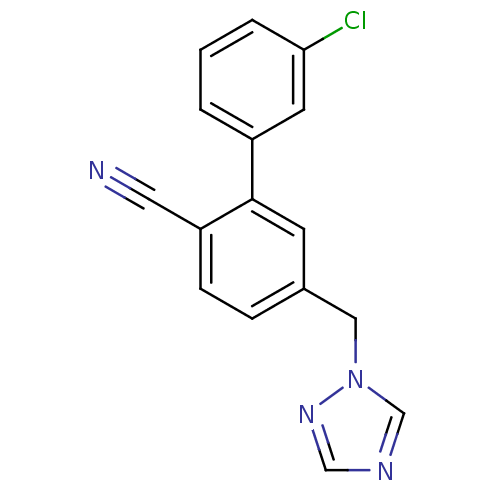 (5-((1H-1,2,4-triazol-1-yl)methyl)-3'-chlorobipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307902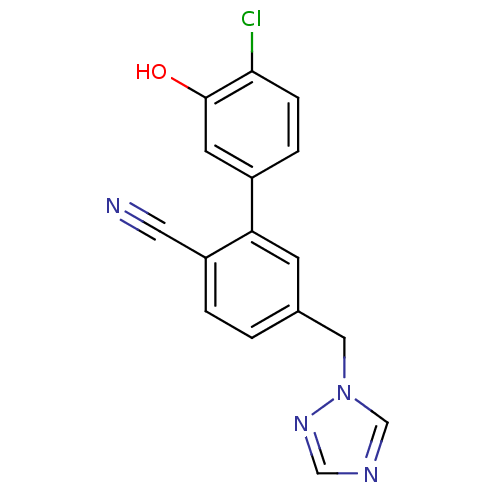 (5-((1H-1,2,4-triazol-1-yl)methyl)-4'-chloro-3'-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307881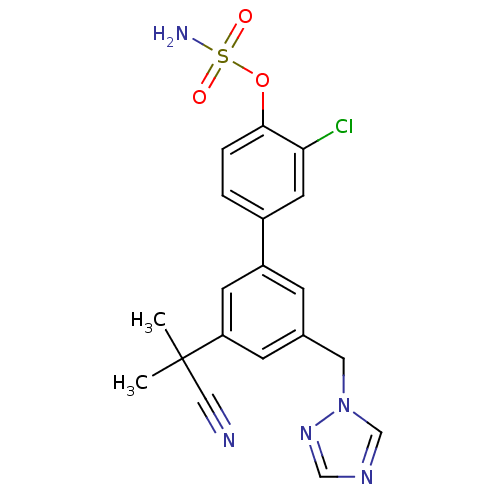 (3'-((1H-1,2,4-triazol-1-yl)methyl)-3-chloro-5'-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24328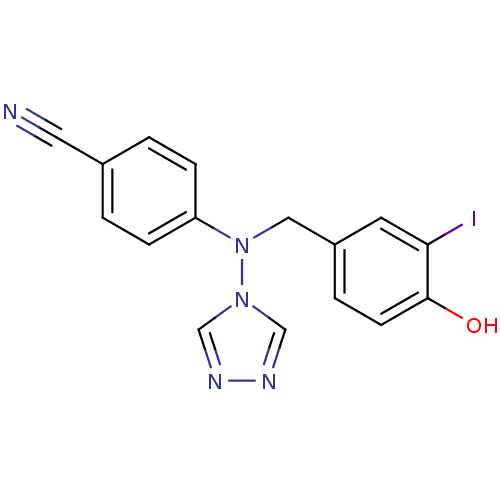 (4-{[(4-hydroxy-3-iodophenyl)methyl](4H-1,2,4-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307882 (5'-((1H-1,2,4-triazol-1-yl)methyl)-4-chloro-2'-cya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24344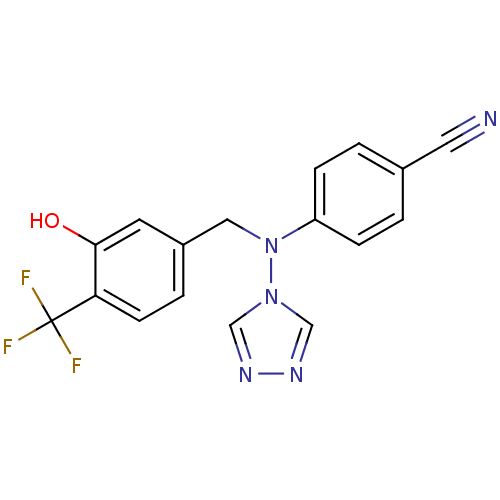 (4-({[3-hydroxy-4-(trifluoromethyl)phenyl]methyl}(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307883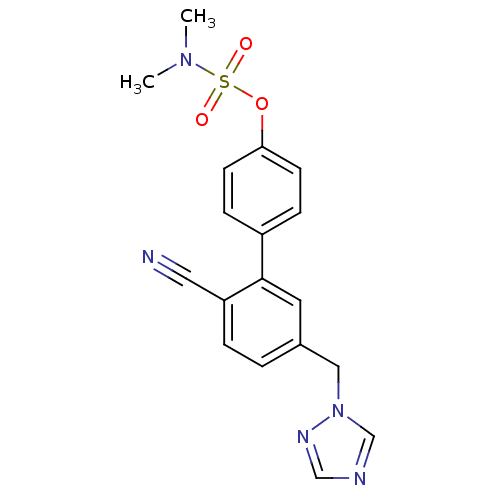 (5'-((1H-1,2,4-triazol-1-yl)methyl)-2'-cyanobipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10016 (4-{[(4-bromophenyl)methyl](4H-1,2,4-triazol-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10016 (4-{[(4-bromophenyl)methyl](4H-1,2,4-triazol-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 46: 3193-6 (2003) Article DOI: 10.1021/jm034033b BindingDB Entry DOI: 10.7270/Q2HT2MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24342 (4-{[(4-bromo-3-hydroxyphenyl)methyl](4H-1,2,4-tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307916 (2-(5-((1H-1,2,4-triazol-1-yl)methyl)biphenyl-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307884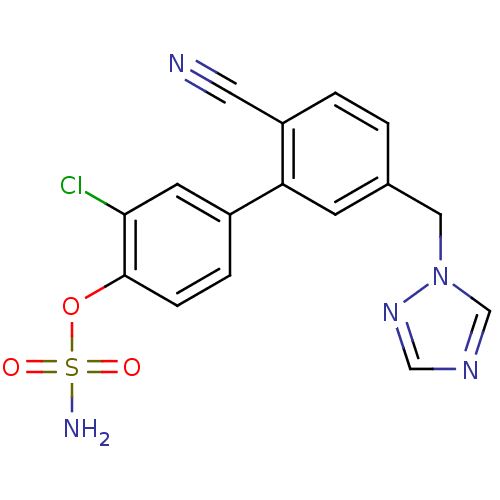 (3-CHLORO-2'-CYANO-5'-(1H-1,2,4-TRIAZOL-1-YLMETHYL)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24332 (4-{[(3-chloro-4-hydroxy-5-methoxyphenyl)methyl](4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24340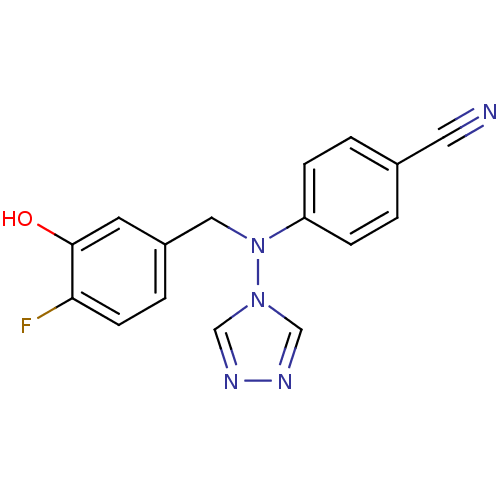 (4-{[(4-fluoro-3-hydroxyphenyl)methyl](4H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24317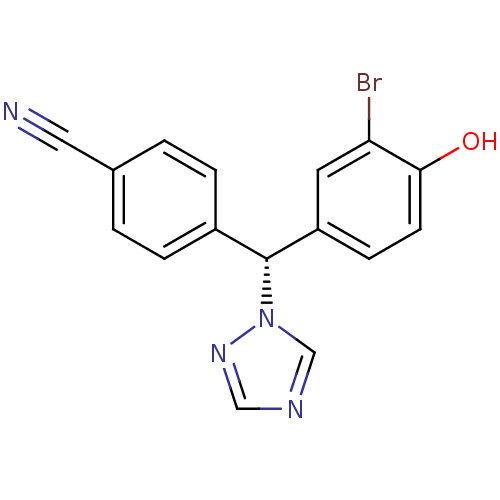 (4-[(R)-(3-bromo-4-hydroxyphenyl)(1H-1,2,4-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 51: 4226-38 (2008) Article DOI: 10.1021/jm800168s BindingDB Entry DOI: 10.7270/Q26W98DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24335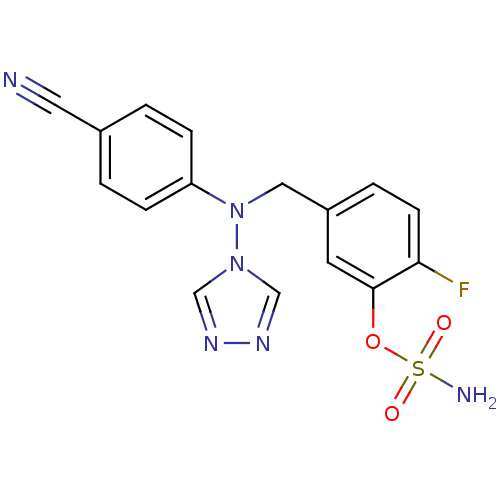 ((5-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307915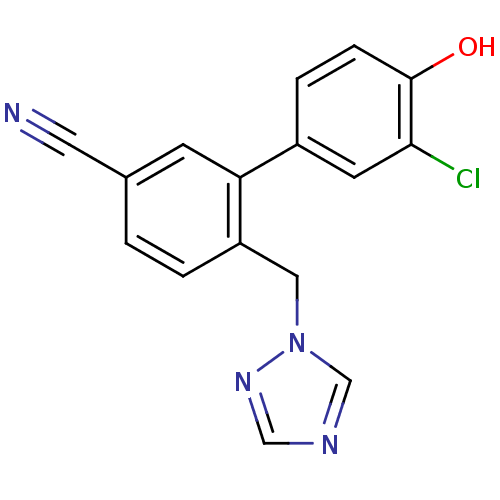 (6-((1H-1,2,4-triazol-1-yl)methyl)-3'-chloro-4'-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10020 ((2-bromo-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 46: 3193-6 (2003) Article DOI: 10.1021/jm034033b BindingDB Entry DOI: 10.7270/Q2HT2MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10020 ((2-bromo-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24331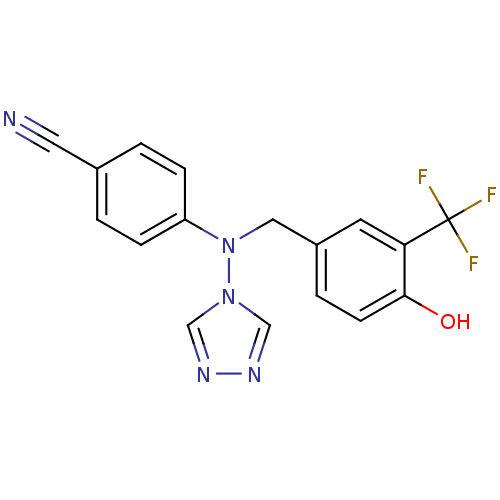 (4-({[4-hydroxy-3-(trifluoromethyl)phenyl]methyl}(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM13061 (4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 51: 4226-38 (2008) Article DOI: 10.1021/jm800168s BindingDB Entry DOI: 10.7270/Q26W98DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24336 ((2-chloro-5-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24327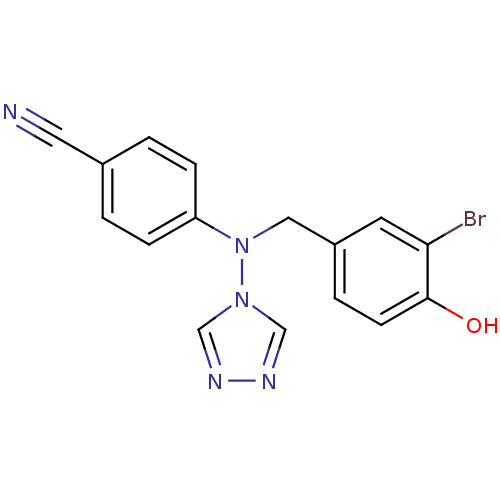 (4-{[(3-bromo-4-hydroxyphenyl)methyl](4H-1,2,4-tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24343 (4-{[(3-hydroxy-4-methoxyphenyl)methyl](4H-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM13058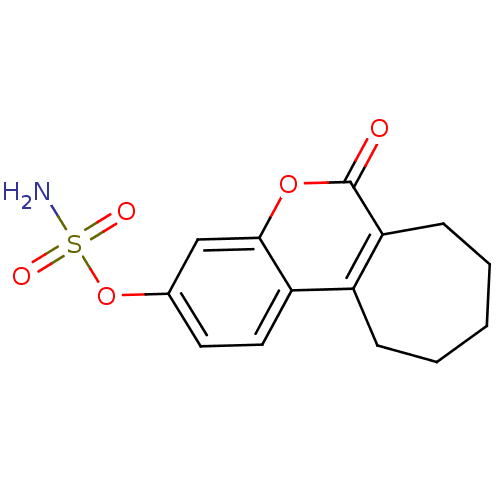 (6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | J Med Chem 51: 4226-38 (2008) Article DOI: 10.1021/jm800168s BindingDB Entry DOI: 10.7270/Q26W98DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10015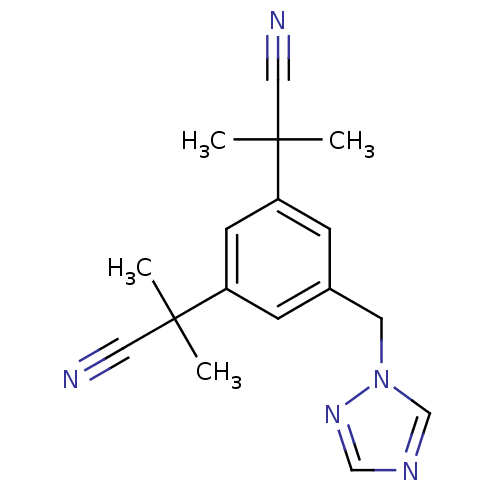 (2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM13058 (6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of steroid sulfatase in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24320 ((4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl)amino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM13058 (6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath | Assay Description The extent of in vitro inhibition of sulfatase activities was assessed using intact monolayers of JEG-3 cells. Sulfatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307914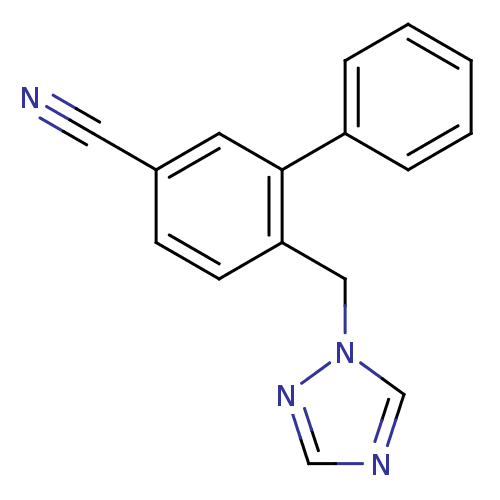 (6-((1H-1,2,4-triazol-1-yl)methyl)biphenyl-3-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24316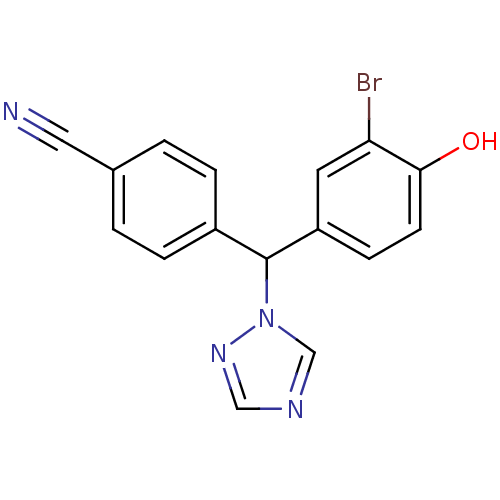 (4-[(3-bromo-4-hydroxyphenyl)(1H-1,2,4-triazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 51: 4226-38 (2008) Article DOI: 10.1021/jm800168s BindingDB Entry DOI: 10.7270/Q26W98DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307885 (2'-((1H-1,2,4-triazol-1-yl)methyl)-3-chloro-5'-cya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307886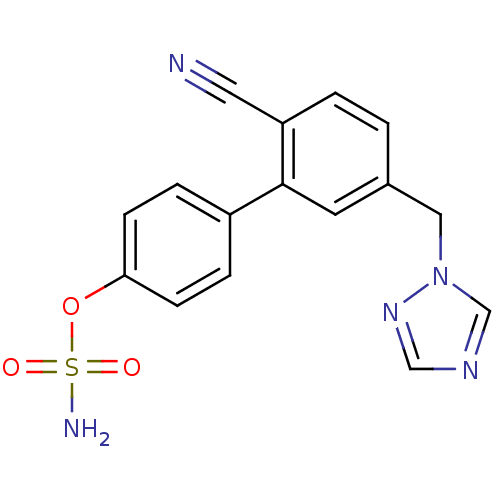 (5'-((1H-1,2,4-triazol-1-yl)methyl)-2'-cyanobipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10019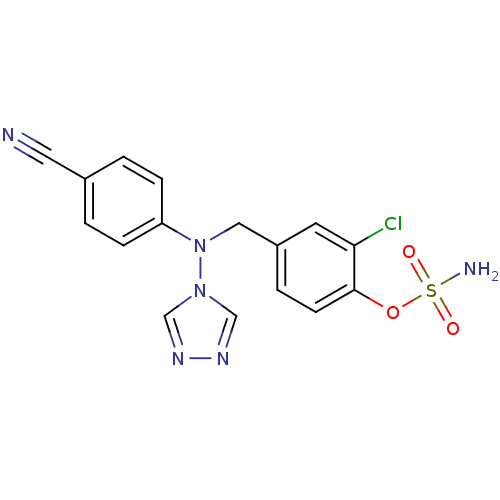 ((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10019 ((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 46: 3193-6 (2003) Article DOI: 10.1021/jm034033b BindingDB Entry DOI: 10.7270/Q2HT2MHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50307887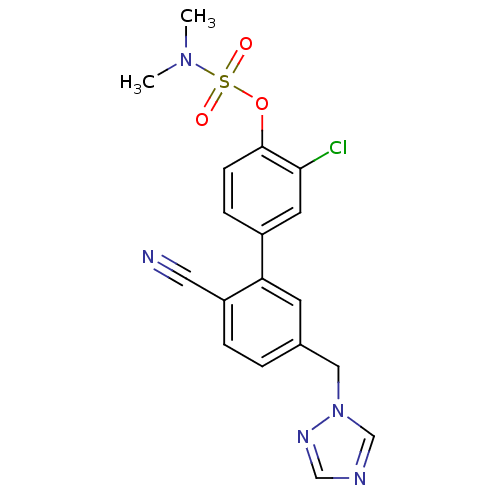 (5'-((1H-1,2,4-triazol-1-yl)methyl)-3-chloro-2'-cya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of aromatse in human JEG3 cells by scintillation spectrometry | J Med Chem 53: 2155-70 (2010) Article DOI: 10.1021/jm901705h BindingDB Entry DOI: 10.7270/Q2959JGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24326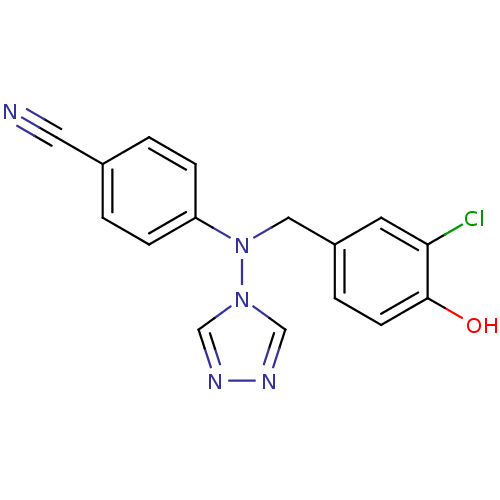 (4-{[(3-chloro-4-hydroxyphenyl)methyl](4H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24339 (4-{[(3-hydroxyphenyl)methyl](4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24329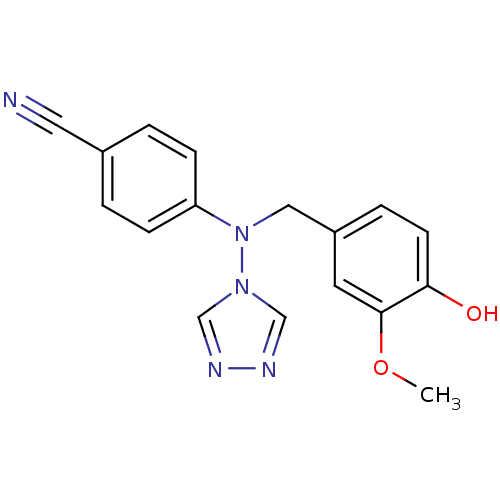 (4-{[(4-hydroxy-3-methoxyphenyl)methyl](4H-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24323 ((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24325 (4-{[(3-fluoro-4-hydroxyphenyl)methyl](4H-1,2,4-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 50: 3540-60 (2007) Article DOI: 10.1021/jm061462b BindingDB Entry DOI: 10.7270/Q2348HP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 281 total ) | Next | Last >> |