

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384793 (CHEMBL2037384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550232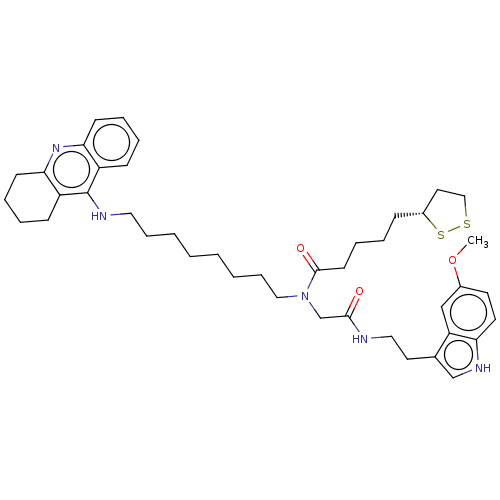 (CHEMBL4762083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550236 (CHEMBL4759893) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550234 (CHEMBL4784757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550235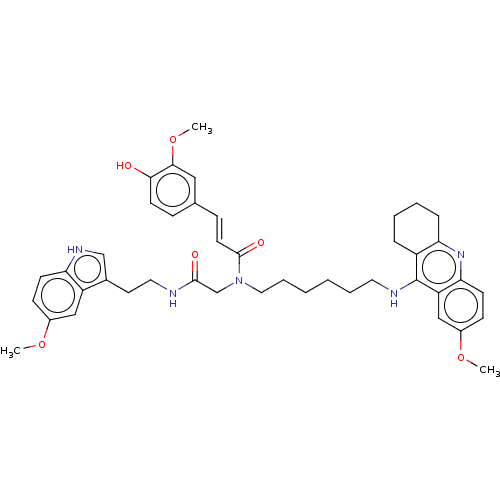 (CHEMBL4790426) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550232 (CHEMBL4762083) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550231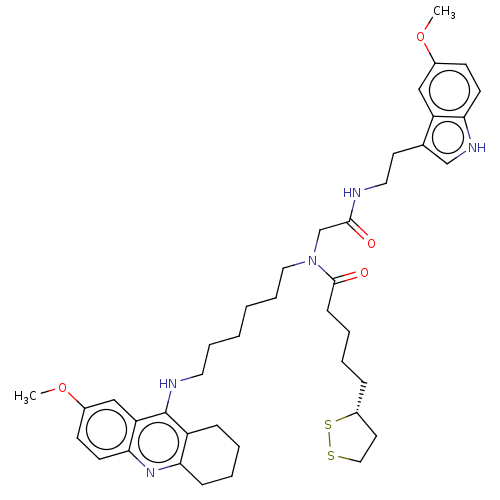 (CHEMBL4752488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50369070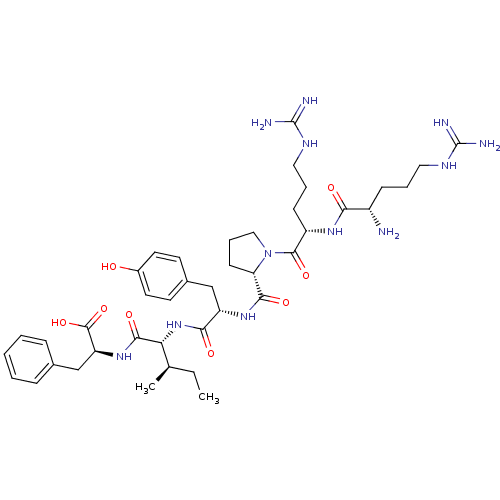 (CHEMBL1793839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550230 (CHEMBL4761802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550230 (CHEMBL4761802) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033671 ((S)-2-{(R)-2-[2-({1-[2-(2-Amino-5-guanidino-pentan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550229 (CHEMBL4755679) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550234 (CHEMBL4784757) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50240845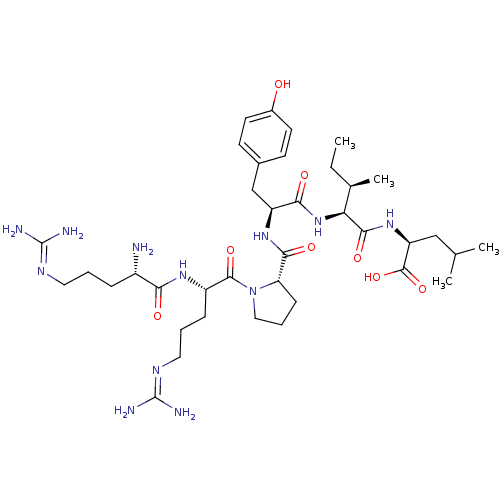 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550233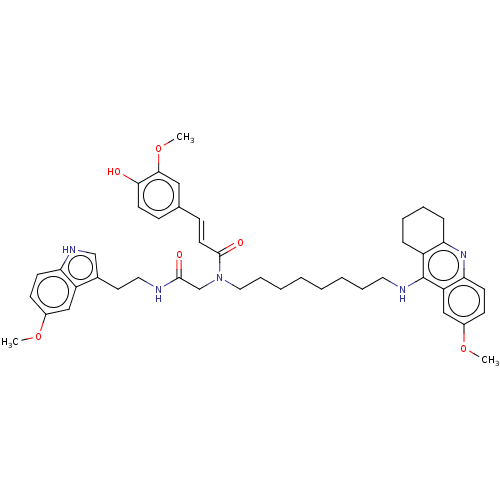 (CHEMBL4762095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550236 (CHEMBL4759893) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033670 ((S)-2-[(R)-5-({1-[2-(2-Amino-5-guanidino-pentanoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550229 (CHEMBL4755679) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550231 (CHEMBL4752488) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550235 (CHEMBL4790426) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550236 (CHEMBL4759893) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550233 (CHEMBL4762095) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50550236 (CHEMBL4759893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of acetylthiocholine iodide substrate by Ellman's metho... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthrax toxin receptor 2 (Homo sapiens) | BDBM175526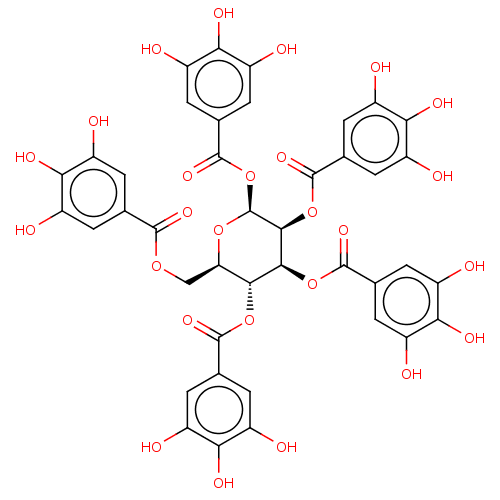 (US9120744, CDE-002) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM Curated by ChEMBL | Assay Description Inhibition of AF488-labelled PA binding to AF546- labelled GST-tagged CMG2 R40C/C178A double mutant (unknown origin) expressed in Escherichia coli BL... | J Med Chem 62: 3958-3970 (2019) Article DOI: 10.1021/acs.jmedchem.8b01988 BindingDB Entry DOI: 10.7270/Q2CZ3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384793 (CHEMBL2037384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthrax toxin receptor 2 (Homo sapiens) | BDBM50522442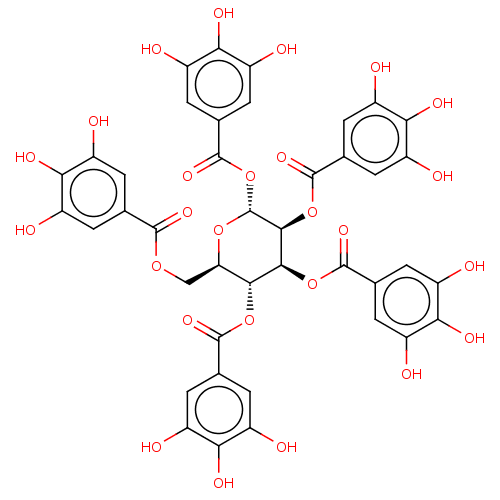 (CHEMBL383306) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM Curated by ChEMBL | Assay Description Inhibition of AF488-labelled PA binding to AF546- labelled GST-tagged CMG2 R40C/C178A double mutant (unknown origin) expressed in Escherichia coli BL... | J Med Chem 62: 3958-3970 (2019) Article DOI: 10.1021/acs.jmedchem.8b01988 BindingDB Entry DOI: 10.7270/Q2CZ3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033677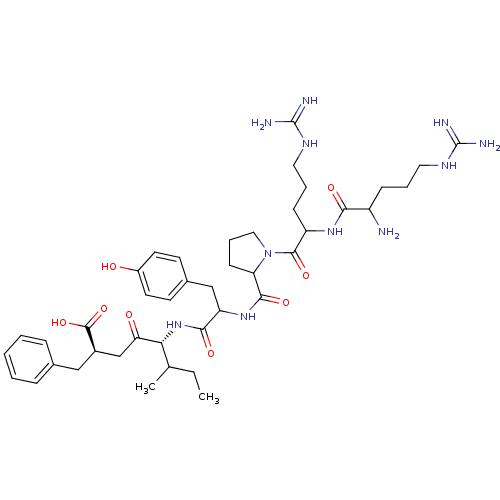 ((S,R)5-[1-{1-[2-[1-amino-4-amino(imino)methylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthrax toxin receptor 2 (Homo sapiens) | BDBM50522441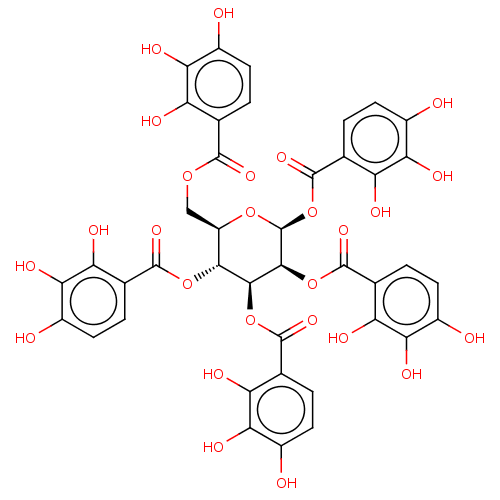 (CHEMBL4557794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM Curated by ChEMBL | Assay Description Inhibition of AF488-labelled PA binding to AF546- labelled GST-tagged CMG2 R40C/C178A double mutant (unknown origin) expressed in Escherichia coli BL... | J Med Chem 62: 3958-3970 (2019) Article DOI: 10.1021/acs.jmedchem.8b01988 BindingDB Entry DOI: 10.7270/Q2CZ3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthrax toxin receptor 2 (Homo sapiens) | BDBM50522452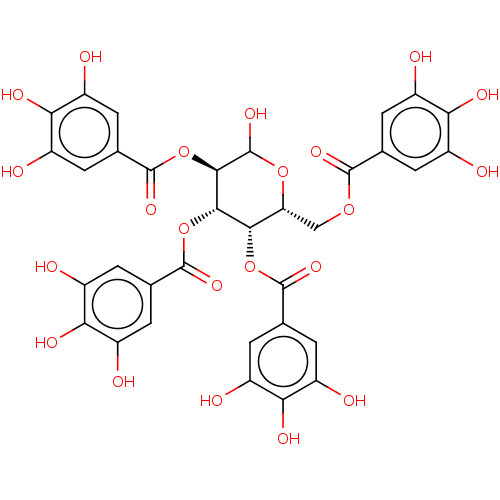 (CHEMBL4469305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM Curated by ChEMBL | Assay Description Inhibition of AF488-labelled PA binding to AF546- labelled GST-tagged CMG2 R40C/C178A double mutant (unknown origin) expressed in Escherichia coli BL... | J Med Chem 62: 3958-3970 (2019) Article DOI: 10.1021/acs.jmedchem.8b01988 BindingDB Entry DOI: 10.7270/Q2CZ3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033667 ((S,S)2-{1-[2-{1-[2-[1-amino-4-amino(imino)methylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT (neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50550232 (CHEMBL4762083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of acetylthiocholine iodide substrate by Ellman's metho... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550232 (CHEMBL4762083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384792 (CHEMBL2037385) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550234 (CHEMBL4784757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550233 (CHEMBL4762095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50550235 (CHEMBL4790426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 358 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BuChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of butyrylthiocholine iodide substrate by Ellman's met... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of acetylthiocholine iodide substrate by Ellman's metho... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384794 (CHEMBL2037380) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384796 (CHEMBL2037382) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthrax toxin receptor 2 (Homo sapiens) | BDBM50241052 (1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 607 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM Curated by ChEMBL | Assay Description Inhibition of AF488-labelled PA binding to AF546- labelled GST-tagged CMG2 R40C/C178A double mutant (unknown origin) expressed in Escherichia coli BL... | J Med Chem 62: 3958-3970 (2019) Article DOI: 10.1021/acs.jmedchem.8b01988 BindingDB Entry DOI: 10.7270/Q2CZ3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033668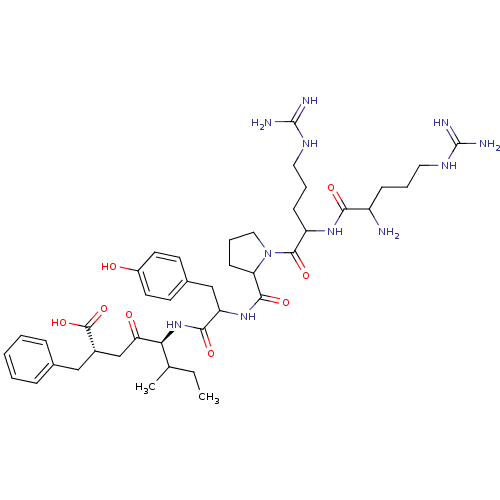 ((R,S)5-[1-{1-[2-[1-amino-4-amino(imino)methylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthrax toxin receptor 2 (Homo sapiens) | BDBM50522447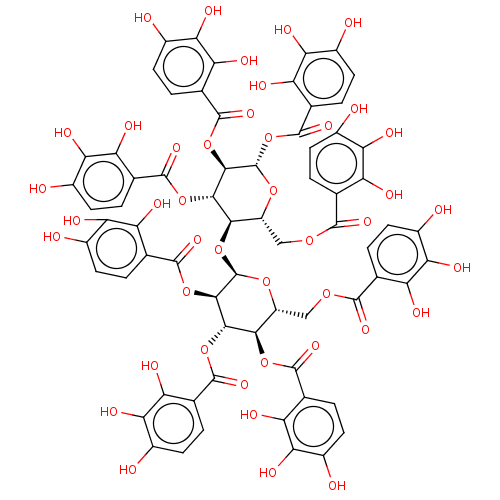 (CHEMBL4440070) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (IQM Curated by ChEMBL | Assay Description Inhibition of AF488-labelled PA binding to AF546- labelled GST-tagged CMG2 R40C/C178A double mutant (unknown origin) expressed in Escherichia coli BL... | J Med Chem 62: 3958-3970 (2019) Article DOI: 10.1021/acs.jmedchem.8b01988 BindingDB Entry DOI: 10.7270/Q2CZ3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033678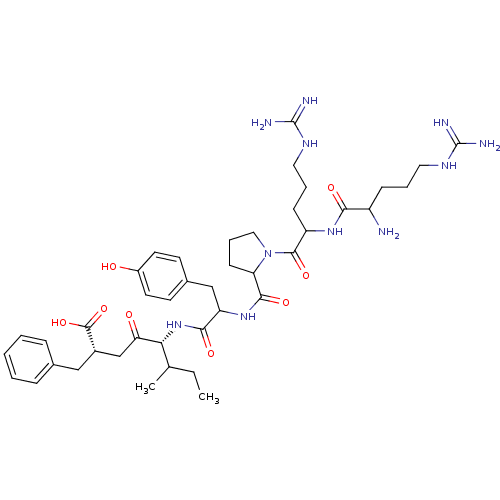 ((S,S)5-[1-{1-[2-[1-amino-4-amino(imino)methylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Rattus norvegicus) | BDBM50033675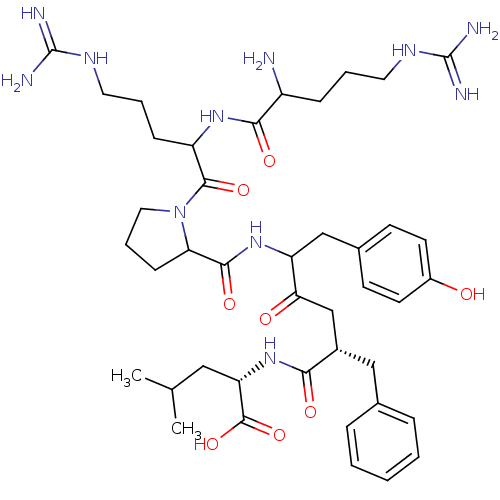 ((S)-2-[(S)-5-({1-[2-(2-Amino-5-guanidino-pentanoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description IC50 was measured as binding to rat cortex membranes using [3H]- NT(neurotensin) as tracer | J Med Chem 38: 1015-21 (1995) BindingDB Entry DOI: 10.7270/Q2DZ08ZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50550234 (CHEMBL4784757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocyte hemo-lyzates pre-incubated for 5 mins before addition of acetylthiocholine iodide substrate by Ellman's metho... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |