

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAD protein (Homo sapiens (Human)) | BDBM50010599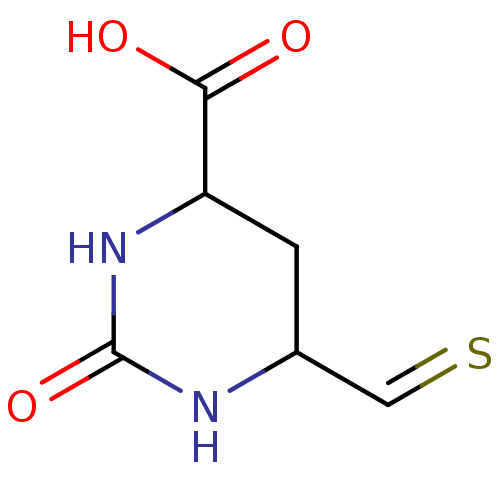 (6-Mercaptomethyl-2-oxo-2,3,4,5-tetrahydro-pyrimidi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Mayo Clinic & Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Dihydroorotase (DHO) at pH 7.4 and 8.5 | J Med Chem 33: 819-23 (1990) BindingDB Entry DOI: 10.7270/Q2ZC81VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CAD protein (Homo sapiens (Human)) | BDBM50010600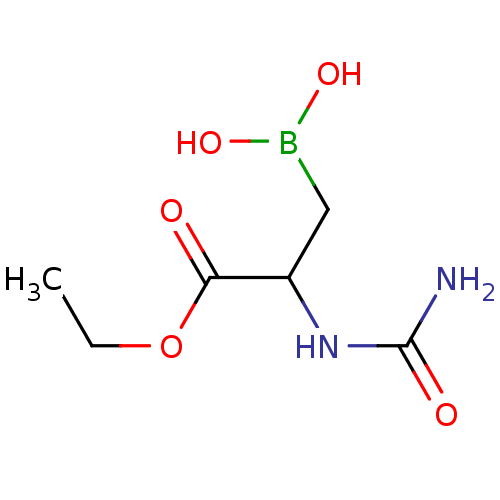 (CHEMBL168878 | Ethyl-3-borono-2-(carbamylamino)pro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Mayo Clinic & Foundation Curated by ChEMBL | Assay Description Inhibitory activity against Dihydroorotase (DHO) at pH 6.0 | J Med Chem 33: 819-23 (1990) BindingDB Entry DOI: 10.7270/Q2ZC81VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatic-L-amino-acid decarboxylase (Mus musculus) | BDBM50046351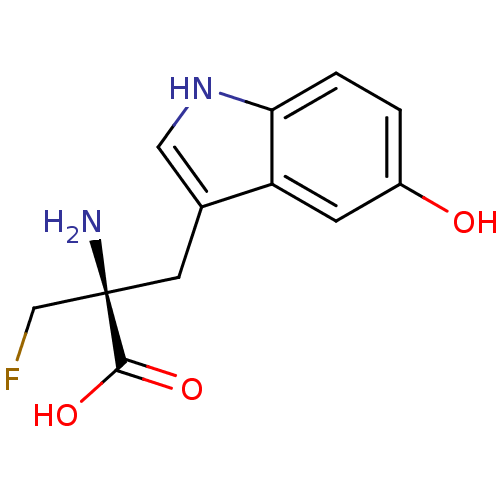 (2-Amino-2-fluoromethyl-3-(5-hydroxy-1H-indol-3-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation Curated by ChEMBL | Assay Description Dissociation constant (KI) of the compound was evaluated as inhibitor of murine liver aromatic L-amino acid decarboxylase (AADC) | J Med Chem 36: 305-13 (1993) BindingDB Entry DOI: 10.7270/Q2DV1J0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50073857 (3,9-dihydroxy-12-(3,4,5-trihydroxy-6-hydroxymethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human DNA topoisomerase I (Topo I) | Bioorg Med Chem Lett 9: 145-50 (1999) BindingDB Entry DOI: 10.7270/Q2571B6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50073854 (2,10-dihydroxy-12-(3,4,5-trihydroxy-6-hydroxymethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human DNA topoisomerase I (Topo I) | Bioorg Med Chem Lett 9: 145-50 (1999) BindingDB Entry DOI: 10.7270/Q2571B6S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50073855 (1,11-dihydroxy-12-(3,4,5-trihydroxy-6-hydroxymethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human DNA topoisomerase I (Topo I) | Bioorg Med Chem Lett 9: 145-50 (1999) BindingDB Entry DOI: 10.7270/Q2571B6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50073856 (4,8-dihydroxy-12-(3,4,5-trihydroxy-6-hydroxymethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human DNA topoisomerase I (Topo I) | Bioorg Med Chem Lett 9: 145-50 (1999) BindingDB Entry DOI: 10.7270/Q2571B6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||