

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arginase (Leishmania amazonensis) | BDBM50521944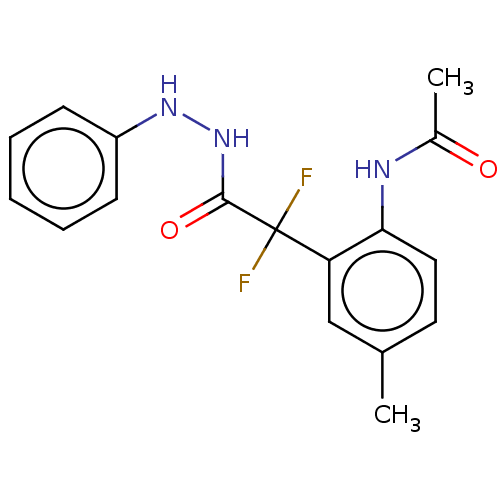 (CHEMBL4204512) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521942 (CHEMBL4216180) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510706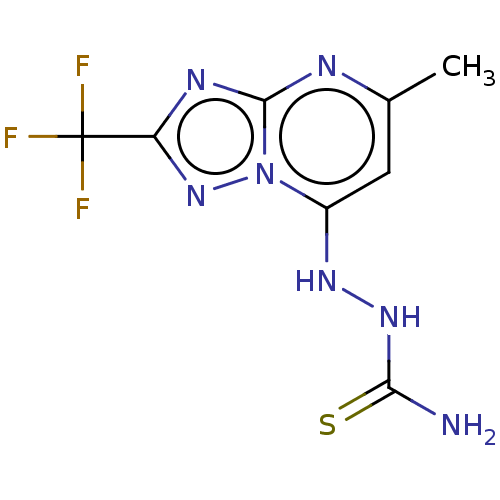 (CHEMBL4515068) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Non-competitive inhibition of Leishmania amazonensis arginase using L-arginine as substrate incubated for 15 mins | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521943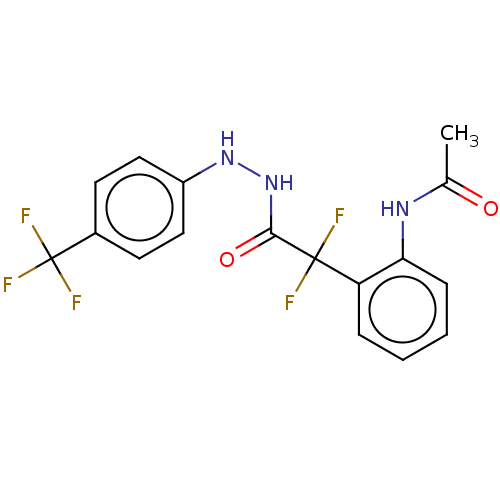 (CHEMBL4574177) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521947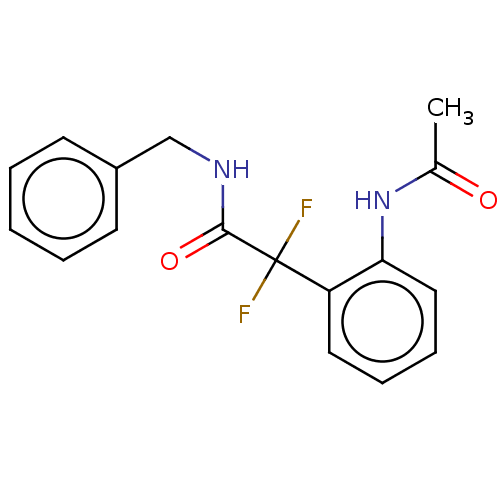 (CHEMBL4530523) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137118 (CHEMBL3753575) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137117 (CHEMBL3754535) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137116 (CHEMBL378856) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137113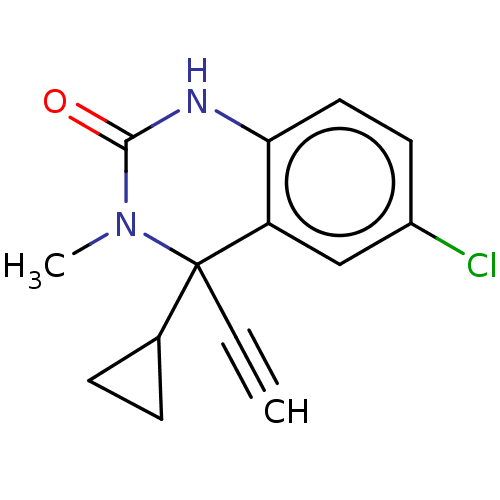 (CHEMBL3753096) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137112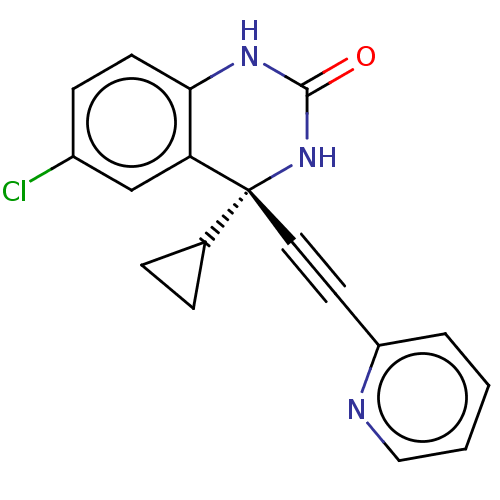 (CHEMBL309896) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2899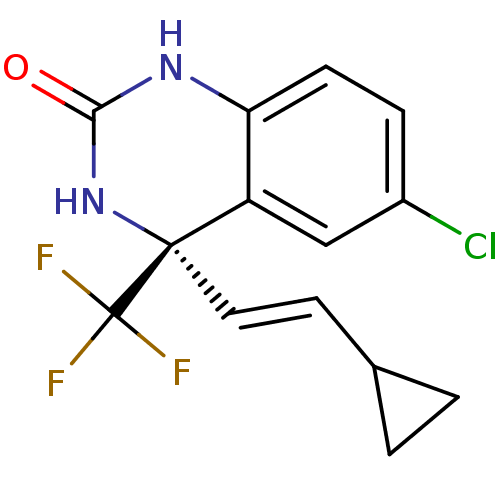 ((4S)-6-chloro-4-[(E)-2-cyclopropylethenyl]-4-(trif...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM27577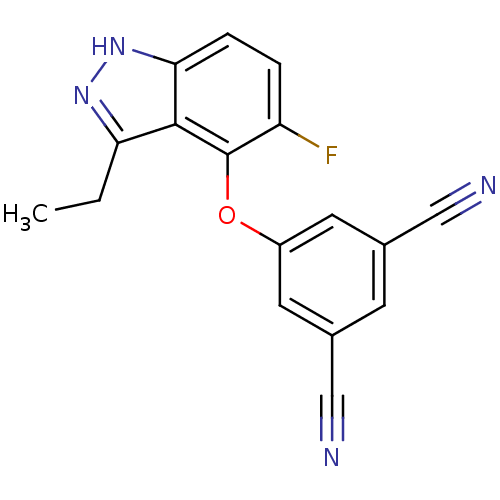 (5-[(3-ethyl-5-fluoro-1H-indazol-4-yl)oxy]benzene-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2898 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2955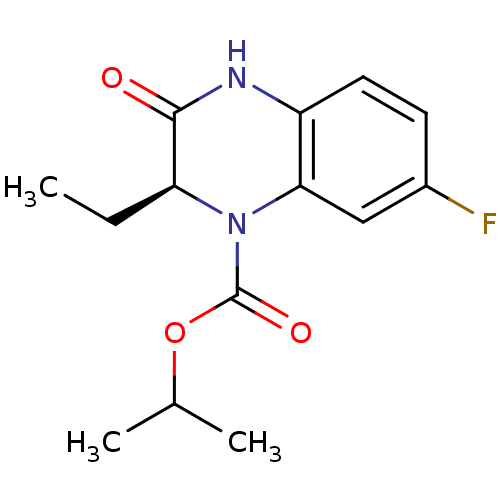 (GW420867X | HBY1293 | propan-2-yl (2S)-2-ethyl-7-f...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2912 ((3S,5S)-7-chloro-5-[(E)-2-cyclopropylethenyl]-3-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM27576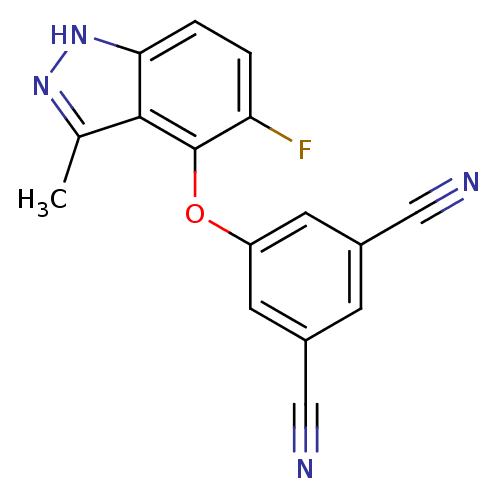 (5-[(5-fluoro-3-methyl-1H-indazol-4-yl)oxy]benzene-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137114 (CHEMBL3753238) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137119 (CHEMBL3753009) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2906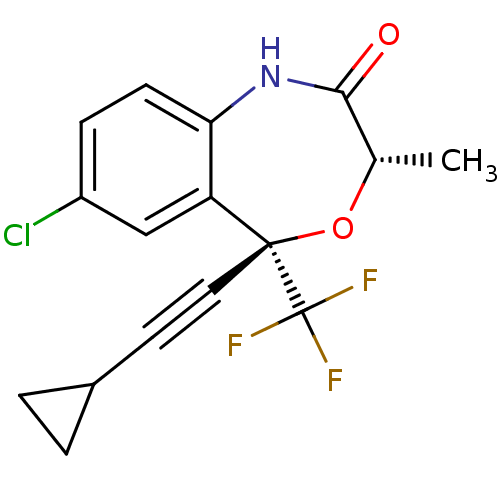 ((3S,5S)-7-chloro-5-(2-cyclopropylethynyl)-3-methyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137120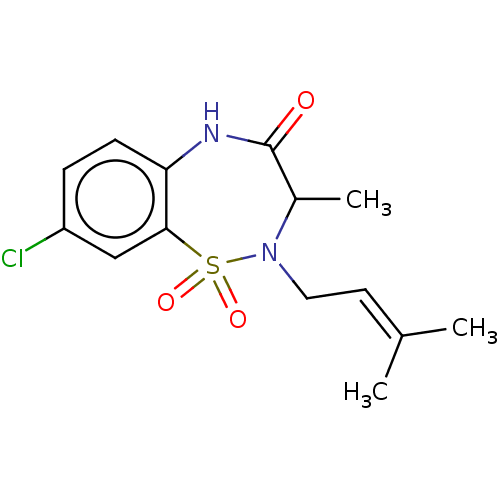 (CHEMBL3753006) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50137115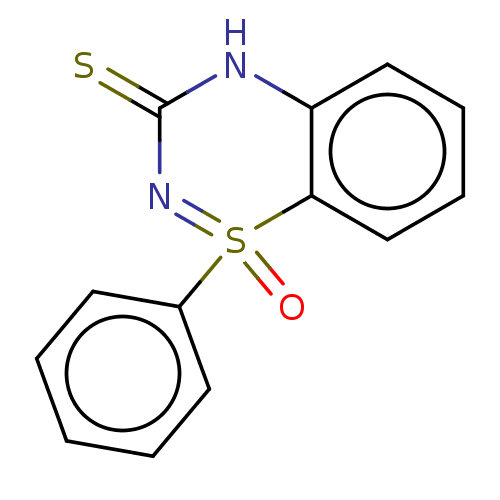 (CHEMBL3754170) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93122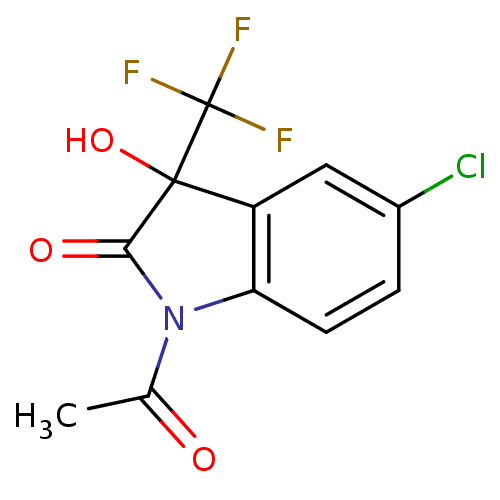 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 25) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93122 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 25) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93121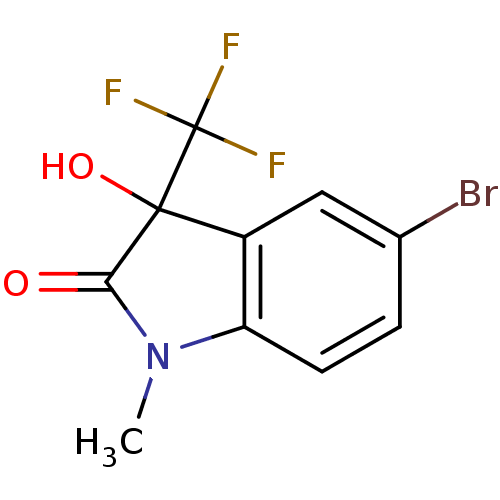 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 21) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93121 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 21) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93119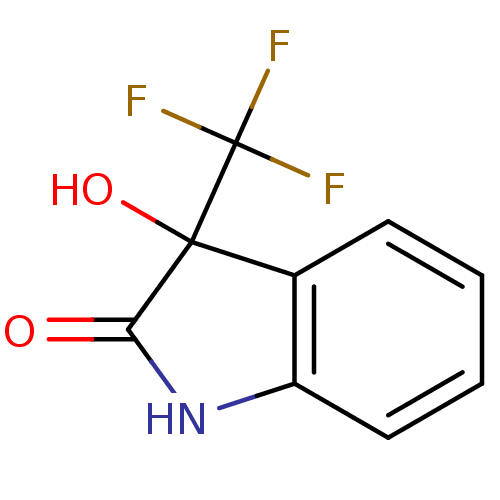 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 16) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93119 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 16) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93120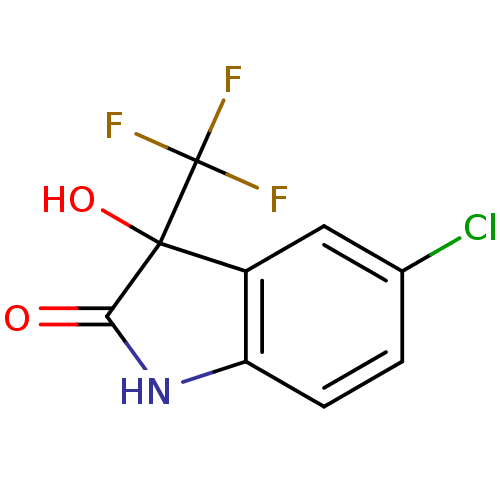 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 18) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93120 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 18) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93123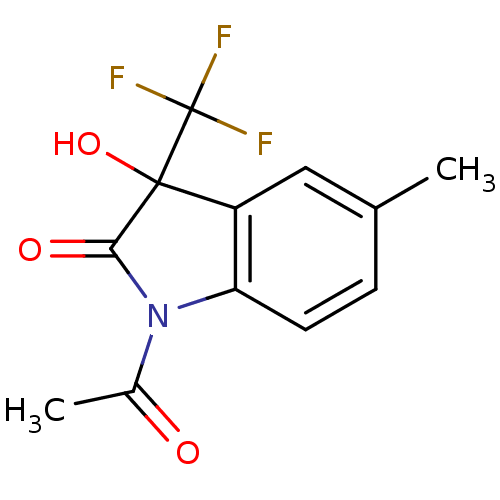 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 26) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
Instituto de Tecnologia em Fa´rmacos | Assay Description Biological assay using HIV-1 reverse transcriptase. | Medicinal Chemistry Research 15: 492-510 (2007) BindingDB Entry DOI: 10.7270/Q2DZ06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM93123 (3-Hydroxy-2-oxo-3-trifluoromethylindole, 26) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Eur J Med Chem 108: 455-65 (2016) Article DOI: 10.1016/j.ejmech.2015.11.025 BindingDB Entry DOI: 10.7270/Q2G162NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510709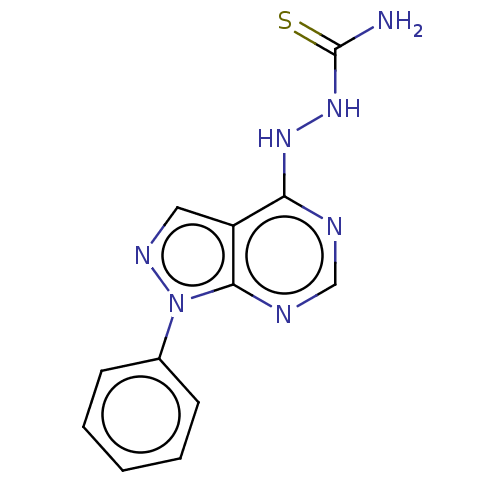 (CHEMBL4571367) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrate | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521944 (CHEMBL4204512) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521942 (CHEMBL4216180) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510706 (CHEMBL4515068) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Non-competitive inhibition of Leishmania amazonensis arginase using L-arginine as substrate incubated for 15 mins | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510706 (CHEMBL4515068) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrate | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521947 (CHEMBL4530523) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521943 (CHEMBL4574177) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510711 (CHEMBL4467463) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrate | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521945 (CHEMBL4216693) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50521946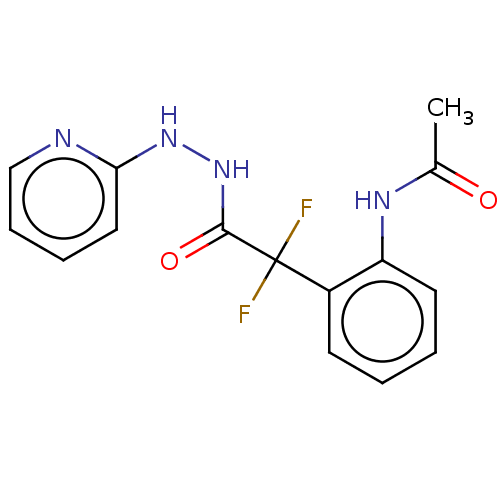 (CHEMBL4211729) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ... | Bioorg Med Chem 27: 3853-3859 (2019) Article DOI: 10.1016/j.bmc.2019.07.022 BindingDB Entry DOI: 10.7270/Q27D2ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510710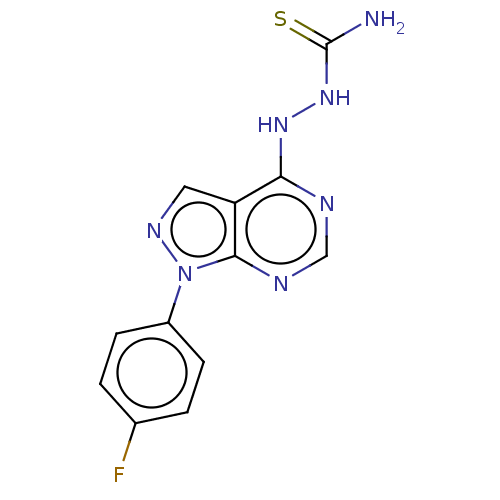 (CHEMBL4438412) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrate | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510707 (CHEMBL4541649) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrate | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510708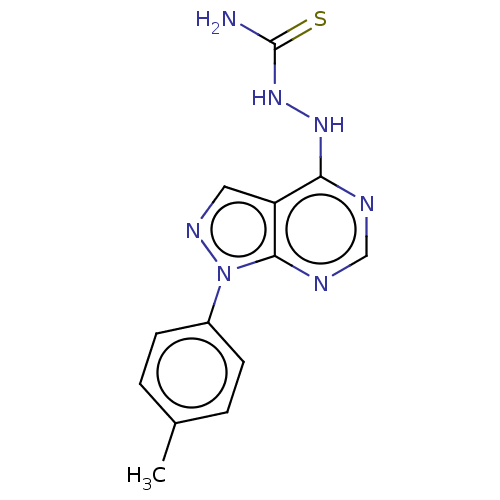 (CHEMBL4456185) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrate | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase (Leishmania amazonensis) | BDBM50510712 (CHEMBL4570250) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em Farmacos Curated by ChEMBL | Assay Description Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrate | Bioorg Med Chem 27: 3061-3069 (2019) Article DOI: 10.1016/j.bmc.2019.05.026 BindingDB Entry DOI: 10.7270/Q2SX6HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||