 Found 160 hits with Last Name = 'chadha' and Initial = 'n'
Found 160 hits with Last Name = 'chadha' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50613673

(CHEMBL1964259)Show SMILES OCCOCCNS(=O)(=O)c1ccc(N\C=C2/C(=O)Nc3ccc4ncsc4c23)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50318884

(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Reversible inhibition of NH2-terminal 6His-tagged FAK catalytic domain (410 to 689 residues) (unknown origin) expressed in Sf9 insect cells using p(G... |
Eur J Med Chem 134: 159-184 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.003
BindingDB Entry DOI: 10.7270/Q23X88SH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM10112
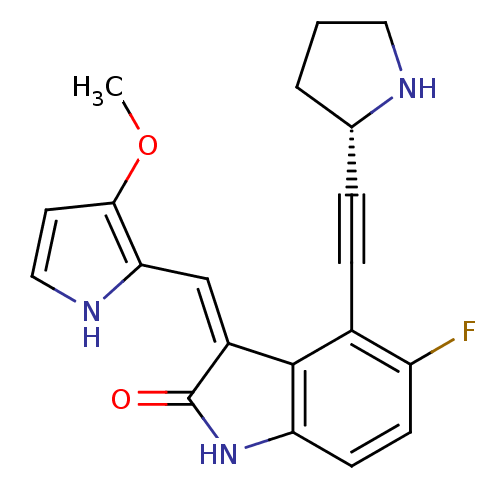
((3Z)-5-fluoro-3-[(3-methoxy-1H-pyrrol-2-yl)methyli...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@@H]3CCCN3)c12 |r| Show InChI InChI=1S/C20H18FN3O2/c1-26-18-8-10-23-17(18)11-14-19-13(5-4-12-3-2-9-22-12)15(21)6-7-16(19)24-20(14)25/h6-8,10-12,22-23H,2-3,9H2,1H3,(H,24,25)/b14-11-/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM10109
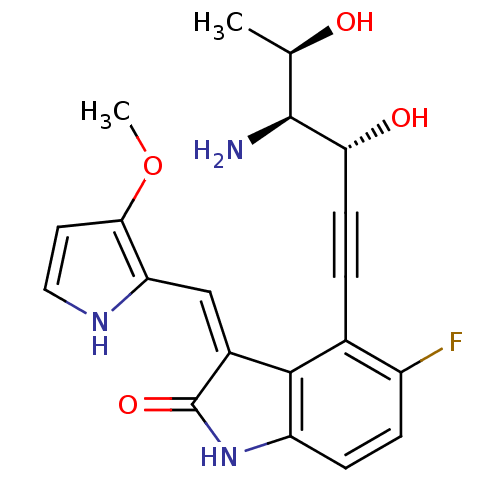
((3Z)-4-[(3R,4S,5R)-4-amino-3,5-dihydroxyhex-1-yn-1...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@@H](O)[C@@H](N)[C@@H](C)O)c12 |r| Show InChI InChI=1S/C20H20FN3O4/c1-10(25)19(22)16(26)6-3-11-13(21)4-5-14-18(11)12(20(27)24-14)9-15-17(28-2)7-8-23-15/h4-5,7-10,16,19,23,25-26H,22H2,1-2H3,(H,24,27)/b12-9-/t10-,16-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132450

(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C19H17N3O4S/c1-22-10-11(14-8-12(26-2)3-6-18(14)22)7-16-15-9-13(27(20,24)25)4-5-17(15)21-19(16)23/h3-10H,1-2H3,(H,21,23)(H2,20,24,25)/b16-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50366929
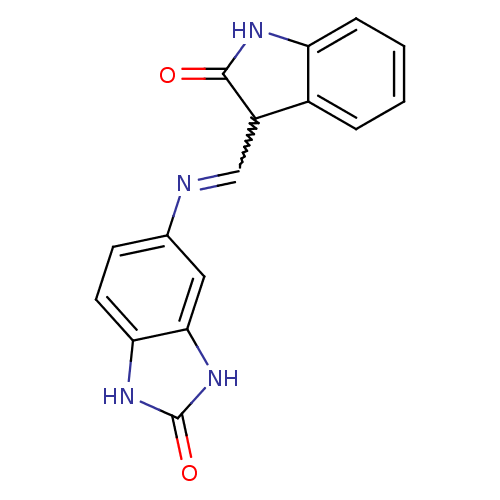
(CHEMBL1794057)Show SMILES O=C1Nc2ccccc2C1C=Nc1ccc2[nH]c(=O)[nH]c2c1 |w:10.11| Show InChI InChI=1S/C16H12N4O2/c21-15-11(10-3-1-2-4-12(10)18-15)8-17-9-5-6-13-14(7-9)20-16(22)19-13/h1-8,11H,(H,18,21)(H2,19,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50613675
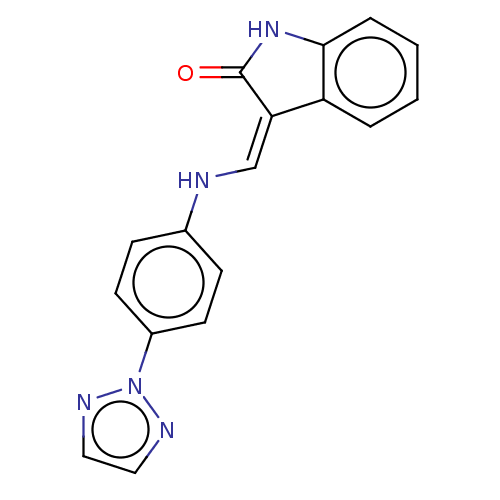
(CHEMBL5275659) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50085415
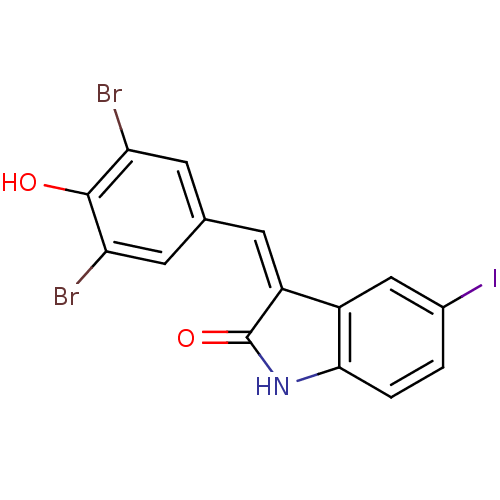
(3-(3,5-Dibromo-4-hydroxy-benzylidene)-5-iodo-1,3-d...)Show InChI InChI=1S/C15H8Br2INO2/c16-11-4-7(5-12(17)14(11)20)3-10-9-6-8(18)1-2-13(9)19-15(10)21/h1-6,20H,(H,19,21)/b10-3- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50324735

((5E)-5-(QUINOXALIN-6-YLMETHYLENE)-1,3-THIAZOLIDINE...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc2nccnc2c1 |w:7.8,t:1| Show InChI InChI=1S/C12H7N3O2S/c16-11-10(18-12(17)15-11)6-7-1-2-8-9(5-7)14-4-3-13-8/h1-6H,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged PI3Kgamma incubated for 180 mins using [33P]gamma-ATP by scintillation proximity assay |
Bioorg Med Chem 23: 2953-74 (2015)
Article DOI: 10.1016/j.bmc.2015.03.071
BindingDB Entry DOI: 10.7270/Q2F76F9R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50613673

(CHEMBL1964259)Show SMILES OCCOCCNS(=O)(=O)c1ccc(N\C=C2/C(=O)Nc3ccc4ncsc4c23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50121167

(5-fluoro-3-(2'-oxo-1',2'-dihydrospiro[cyclopentane...)Show InChI InChI=1S/C19H15FN2O/c20-15-8-12(11-21)7-14(9-15)13-3-4-17-16(10-13)19(18(23)22-17)5-1-2-6-19/h3-4,7-10H,1-2,5-6H2,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50318884

(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Reversible inhibition of PYK2 (unknown origin) |
Eur J Med Chem 134: 159-184 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.003
BindingDB Entry DOI: 10.7270/Q23X88SH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223693
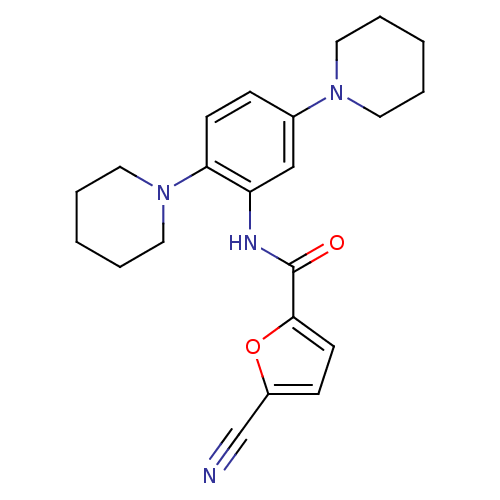
(5-cyano-N-(2,5-di(piperidin-1-yl)phenyl)furan-2-ca...)Show SMILES O=C(Nc1cc(ccc1N1CCCCC1)N1CCCCC1)c1ccc(o1)C#N Show InChI InChI=1S/C22H26N4O2/c23-16-18-8-10-21(28-18)22(27)24-19-15-17(25-11-3-1-4-12-25)7-9-20(19)26-13-5-2-6-14-26/h7-10,15H,1-6,11-14H2,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223698
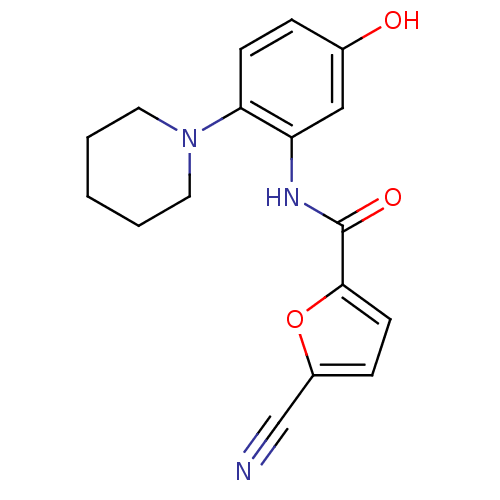
(5-cyano-N-(5-hydroxy-2-(piperidin-1-yl)phenyl)fura...)Show InChI InChI=1S/C17H17N3O3/c18-11-13-5-7-16(23-13)17(22)19-14-10-12(21)4-6-15(14)20-8-2-1-3-9-20/h4-7,10,21H,1-3,8-9H2,(H,19,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50162972
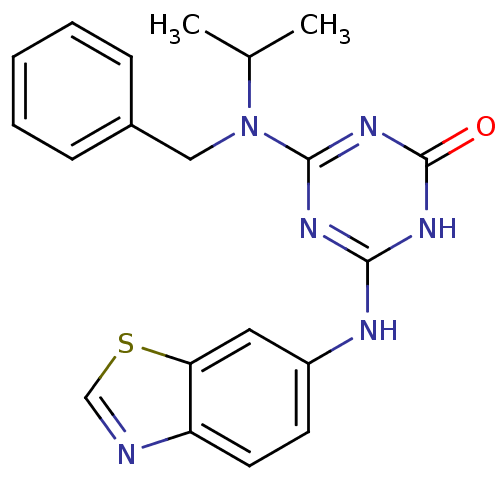
(4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...)Show SMILES CC(C)N(Cc1ccccc1)c1nc(Nc2ccc3ncsc3c2)[nH]c(=O)n1 Show InChI InChI=1S/C20H20N6OS/c1-13(2)26(11-14-6-4-3-5-7-14)19-23-18(24-20(27)25-19)22-15-8-9-16-17(10-15)28-12-21-16/h3-10,12-13H,11H2,1-2H3,(H2,22,23,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP |
J Med Chem 48: 1717-20 (2005)
Article DOI: 10.1021/jm049372z
BindingDB Entry DOI: 10.7270/Q2GM86TF |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223670
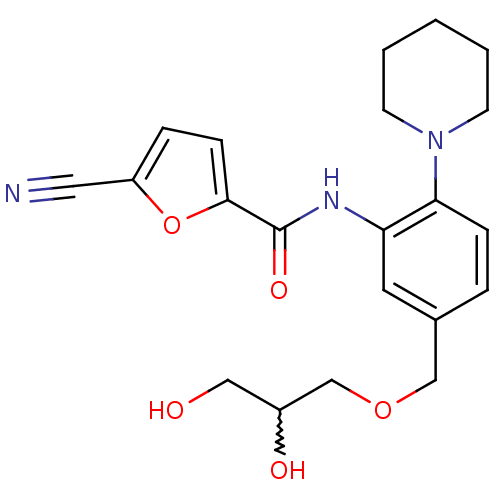
(5-cyano-N-(5-((2,3-dihydroxypropoxy)methyl)-2-(pip...)Show SMILES OCC(O)COCc1ccc(N2CCCCC2)c(NC(=O)c2ccc(o2)C#N)c1 |w:2.2| Show InChI InChI=1S/C21H25N3O5/c22-11-17-5-7-20(29-17)21(27)23-18-10-15(13-28-14-16(26)12-25)4-6-19(18)24-8-2-1-3-9-24/h4-7,10,16,25-26H,1-3,8-9,12-14H2,(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM17750

(5-cyano-N-[5-(hydroxymethyl)-2-(4-methylpiperidin-...)Show InChI InChI=1S/C19H21N3O3/c1-13-6-8-22(9-7-13)17-4-2-14(12-23)10-16(17)21-19(24)18-5-3-15(11-20)25-18/h2-5,10,13,23H,6-9,12H2,1H3,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223700
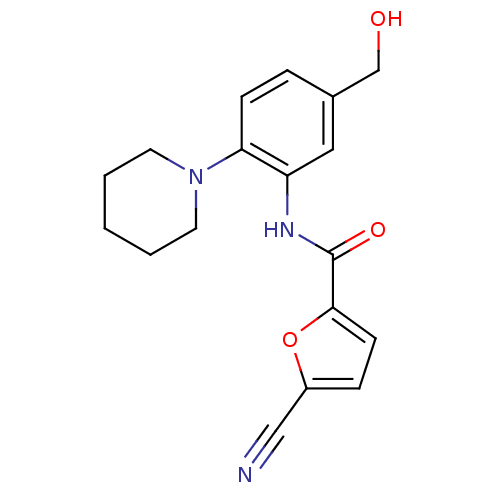
(5-cyano-N-(5-(hydroxymethyl)-2-(piperidin-1-yl)phe...)Show InChI InChI=1S/C18H19N3O3/c19-11-14-5-7-17(24-14)18(23)20-15-10-13(12-22)4-6-16(15)21-8-2-1-3-9-21/h4-7,10,22H,1-3,8-9,12H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50121177
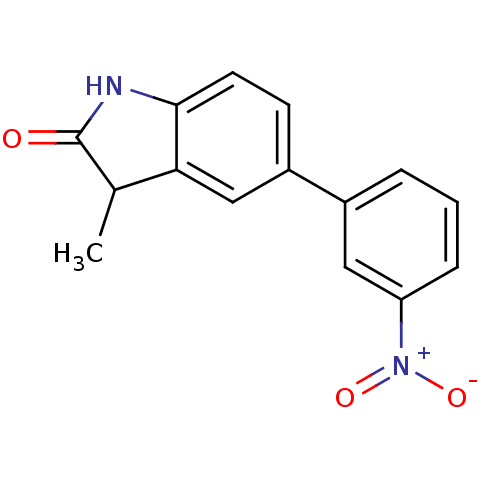
(3-Methyl-5-(3-nitro-phenyl)-1,3-dihydro-indol-2-on...)Show SMILES CC1C(=O)Nc2ccc(cc12)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C15H12N2O3/c1-9-13-8-11(5-6-14(13)16-15(9)18)10-3-2-4-12(7-10)17(19)20/h2-9H,1H3,(H,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50121158
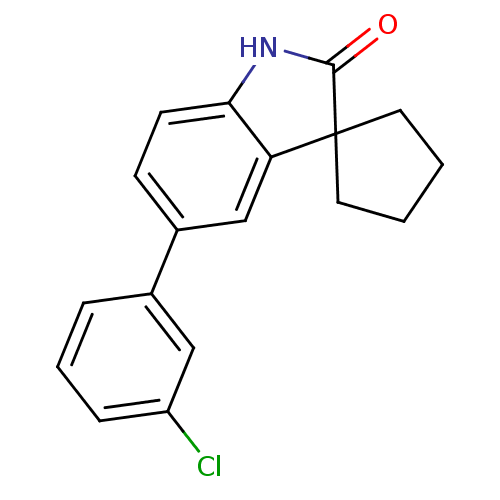
(5'-(3-chlorophenyl)spiro[cyclopentane-1,3'-indol]-...)Show InChI InChI=1S/C18H16ClNO/c19-14-5-3-4-12(10-14)13-6-7-16-15(11-13)18(17(21)20-16)8-1-2-9-18/h3-7,10-11H,1-2,8-9H2,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223680
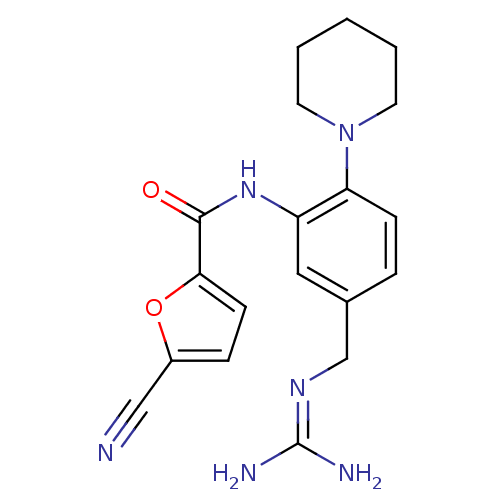
(5-cyano-furan-2-carboxylic acid (5-guanidinomethyl...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1ccc(-[#7]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2)c(-[#7]-[#6](=O)-c2ccc(o2)C#N)c1 Show InChI InChI=1S/C19H22N6O2/c20-11-14-5-7-17(27-14)18(26)24-15-10-13(12-23-19(21)22)4-6-16(15)25-8-2-1-3-9-25/h4-7,10H,1-3,8-9,12H2,(H,24,26)(H4,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50162976

(4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...)Show SMILES CC(C)(Nc1nc(Nc2ccc3ncsc3c2)[nH]c(=O)n1)c1ccccc1 Show InChI InChI=1S/C19H18N6OS/c1-19(2,12-6-4-3-5-7-12)25-17-22-16(23-18(26)24-17)21-13-8-9-14-15(10-13)27-11-20-14/h3-11H,1-2H3,(H3,21,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP |
J Med Chem 48: 1717-20 (2005)
Article DOI: 10.1021/jm049372z
BindingDB Entry DOI: 10.7270/Q2GM86TF |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223699
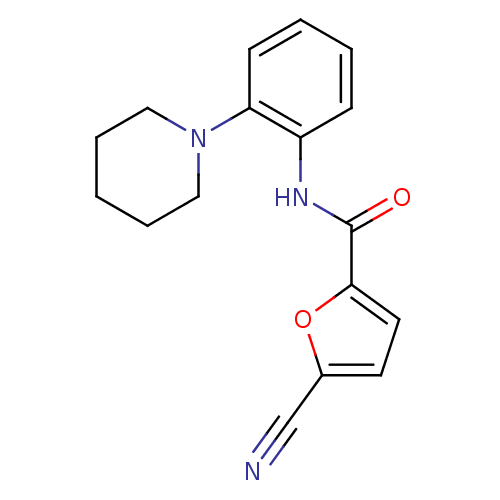
(5-cyano-N-(2-(piperidin-1-yl)phenyl)furan-2-carbox...)Show InChI InChI=1S/C17H17N3O2/c18-12-13-8-9-16(22-13)17(21)19-14-6-2-3-7-15(14)20-10-4-1-5-11-20/h2-3,6-9H,1,4-5,10-11H2,(H,19,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223674
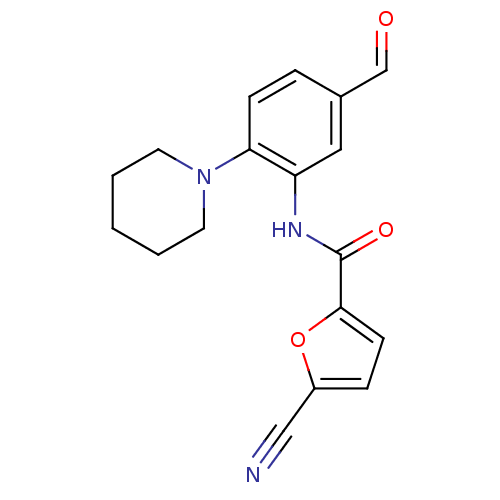
(5-cyano-N-(5-formyl-2-(piperidin-1-yl)phenyl)furan...)Show InChI InChI=1S/C18H17N3O3/c19-11-14-5-7-17(24-14)18(23)20-15-10-13(12-22)4-6-16(15)21-8-2-1-3-9-21/h4-7,10,12H,1-3,8-9H2,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223681
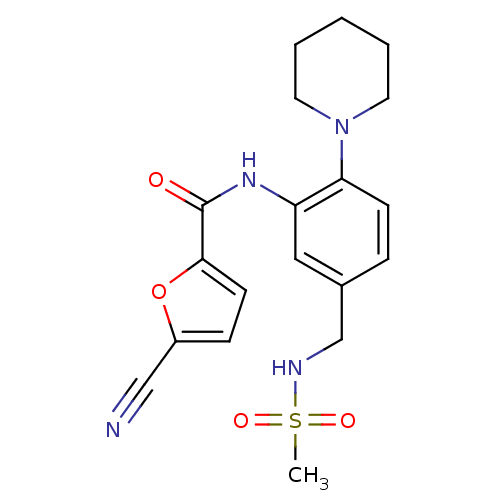
(5-cyano-N-(5-(methylsulfonamidomethyl)-2-(piperidi...)Show SMILES CS(=O)(=O)NCc1ccc(N2CCCCC2)c(NC(=O)c2ccc(o2)C#N)c1 Show InChI InChI=1S/C19H22N4O4S/c1-28(25,26)21-13-14-5-7-17(23-9-3-2-4-10-23)16(11-14)22-19(24)18-8-6-15(12-20)27-18/h5-8,11,21H,2-4,9-10,13H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50162973

(CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...)Show SMILES CC(C)(Nc1nc(NO)nc(Nc2ccc3ncsc3c2)n1)c1ccccc1 Show InChI InChI=1S/C19H19N7OS/c1-19(2,12-6-4-3-5-7-12)25-17-22-16(23-18(24-17)26-27)21-13-8-9-14-15(10-13)28-11-20-14/h3-11,27H,1-2H3,(H3,21,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP |
J Med Chem 48: 1717-20 (2005)
Article DOI: 10.1021/jm049372z
BindingDB Entry DOI: 10.7270/Q2GM86TF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50162973

(CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...)Show SMILES CC(C)(Nc1nc(NO)nc(Nc2ccc3ncsc3c2)n1)c1ccccc1 Show InChI InChI=1S/C19H19N7OS/c1-19(2,12-6-4-3-5-7-12)25-17-22-16(23-18(24-17)26-27)21-13-8-9-14-15(10-13)28-11-20-14/h3-11,27H,1-2H3,(H3,21,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP |
J Med Chem 48: 1717-20 (2005)
Article DOI: 10.1021/jm049372z
BindingDB Entry DOI: 10.7270/Q2GM86TF |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223706
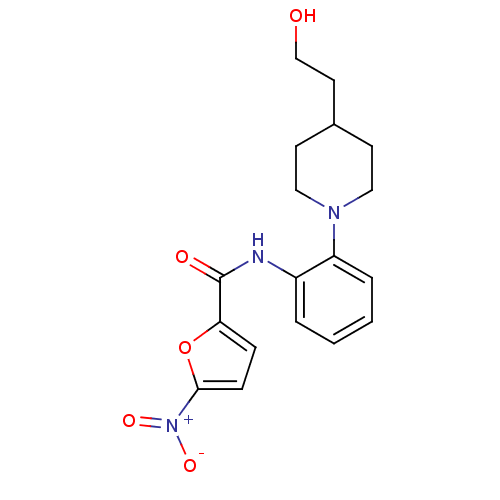
(CHEMBL251351 | N-(2-(4-(2-hydroxyethyl)piperidin-1...)Show SMILES OCCC1CCN(CC1)c1ccccc1NC(=O)c1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C18H21N3O5/c22-12-9-13-7-10-20(11-8-13)15-4-2-1-3-14(15)19-18(23)16-5-6-17(26-16)21(24)25/h1-6,13,22H,7-12H2,(H,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223701
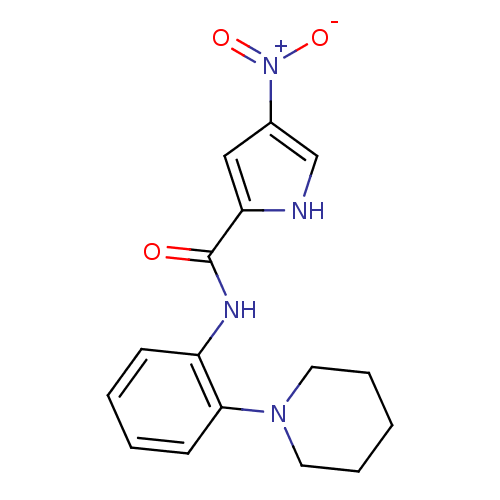
(4-nitro-N-(2-(piperidin-1-yl)phenyl)-1H-pyrrole-2-...)Show SMILES [O-][N+](=O)c1c[nH]c(c1)C(=O)Nc1ccccc1N1CCCCC1 Show InChI InChI=1S/C16H18N4O3/c21-16(14-10-12(11-17-14)20(22)23)18-13-6-2-3-7-15(13)19-8-4-1-5-9-19/h2-3,6-7,10-11,17H,1,4-5,8-9H2,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223665
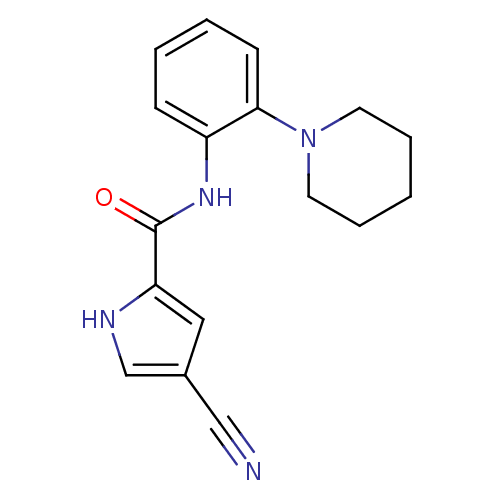
(4-cyano-N-(2-(piperidin-1-yl)phenyl)-1H-pyrrole-2-...)Show InChI InChI=1S/C17H18N4O/c18-11-13-10-15(19-12-13)17(22)20-14-6-2-3-7-16(14)21-8-4-1-5-9-21/h2-3,6-7,10,12,19H,1,4-5,8-9H2,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50162978

(4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...)Show SMILES CCN(Cc1ccccc1)c1nc(Nc2ccc3ncsc3c2)[nH]c(=O)n1 Show InChI InChI=1S/C19H18N6OS/c1-2-25(11-13-6-4-3-5-7-13)18-22-17(23-19(26)24-18)21-14-8-9-15-16(10-14)27-12-20-15/h3-10,12H,2,11H2,1H3,(H2,21,22,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP |
J Med Chem 48: 1717-20 (2005)
Article DOI: 10.1021/jm049372z
BindingDB Entry DOI: 10.7270/Q2GM86TF |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223683
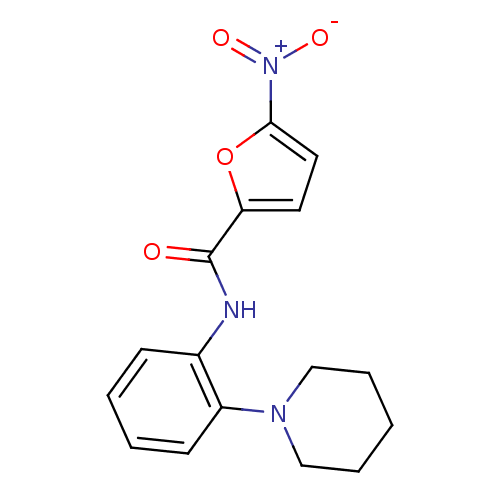
(5-nitro-N-(2-(piperidin-1-yl)phenyl)furan-2-carbox...)Show SMILES [O-][N+](=O)c1ccc(o1)C(=O)Nc1ccccc1N1CCCCC1 Show InChI InChI=1S/C16H17N3O4/c20-16(14-8-9-15(23-14)19(21)22)17-12-6-2-3-7-13(12)18-10-4-1-5-11-18/h2-3,6-9H,1,4-5,10-11H2,(H,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50366930
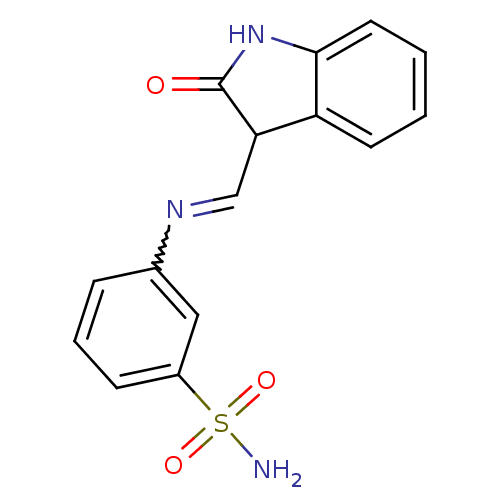
(CHEMBL1794059)Show SMILES NS(=O)(=O)c1cccc(c1)N=CC1C(=O)Nc2ccccc12 |w:10.10| Show InChI InChI=1S/C15H13N3O3S/c16-22(20,21)11-5-3-4-10(8-11)17-9-13-12-6-1-2-7-14(12)18-15(13)19/h1-9,13H,(H,18,19)(H2,16,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223693

(5-cyano-N-(2,5-di(piperidin-1-yl)phenyl)furan-2-ca...)Show SMILES O=C(Nc1cc(ccc1N1CCCCC1)N1CCCCC1)c1ccc(o1)C#N Show InChI InChI=1S/C22H26N4O2/c23-16-18-8-10-21(28-18)22(27)24-19-15-17(25-11-3-1-4-12-25)7-9-20(19)26-13-5-2-6-14-26/h7-10,15H,1-6,11-14H2,(H,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase in HEK 293 cells |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223668
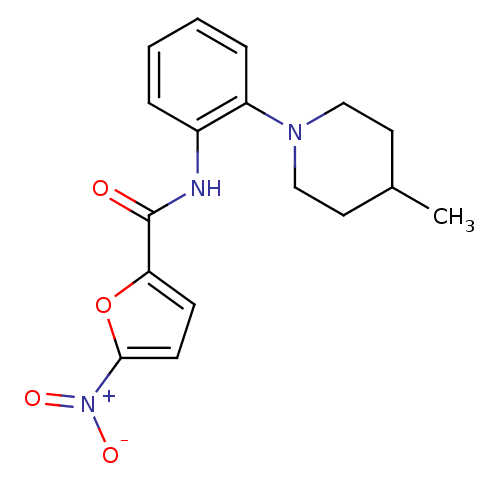
(CHEMBL251602 | N-(2-(4-methylpiperidin-1-yl)phenyl...)Show SMILES CC1CCN(CC1)c1ccccc1NC(=O)c1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C17H19N3O4/c1-12-8-10-19(11-9-12)14-5-3-2-4-13(14)18-17(21)15-6-7-16(24-15)20(22)23/h2-7,12H,8-11H2,1H3,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223682
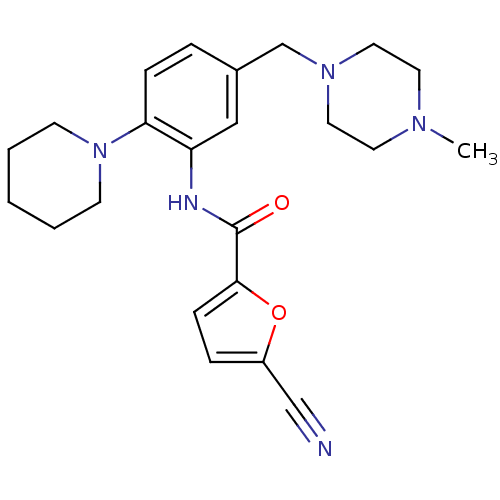
(5-cyano-N-(5-((4-methylpiperazin-1-yl)methyl)-2-(p...)Show SMILES CN1CCN(Cc2ccc(N3CCCCC3)c(NC(=O)c3ccc(o3)C#N)c2)CC1 Show InChI InChI=1S/C23H29N5O2/c1-26-11-13-27(14-12-26)17-18-5-7-21(28-9-3-2-4-10-28)20(15-18)25-23(29)22-8-6-19(16-24)30-22/h5-8,15H,2-4,9-14,17H2,1H3,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50121162
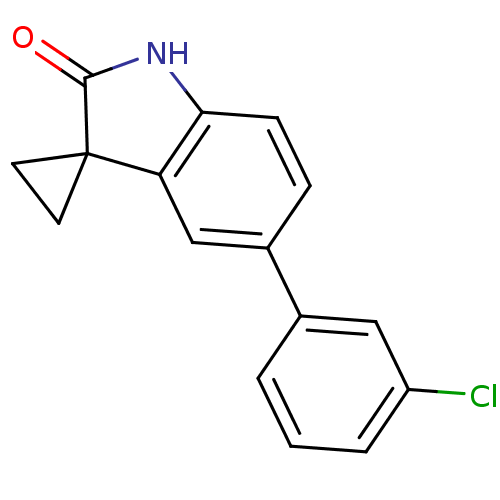
(5'-(3-chlorophenyl)spiro[cyclopropane-1,3'-indol]-...)Show InChI InChI=1S/C16H12ClNO/c17-12-3-1-2-10(8-12)11-4-5-14-13(9-11)16(6-7-16)15(19)18-14/h1-5,8-9H,6-7H2,(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223686
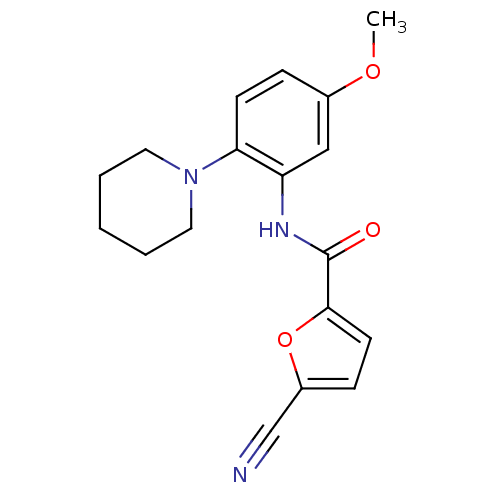
(5-cyano-N-(5-methoxy-2-(piperidin-1-yl)phenyl)fura...)Show InChI InChI=1S/C18H19N3O3/c1-23-13-5-7-16(21-9-3-2-4-10-21)15(11-13)20-18(22)17-8-6-14(12-19)24-17/h5-8,11H,2-4,9-10H2,1H3,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50613676
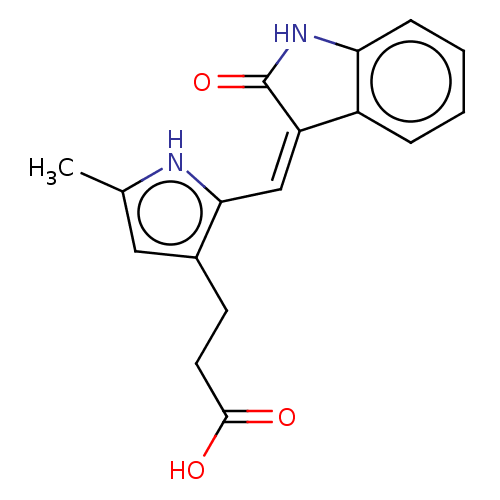
(CHEMBL5278067)Show SMILES Cc1cc(CCC(O)=O)c(\C=C2/C(=O)Nc3ccccc23)[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50121168
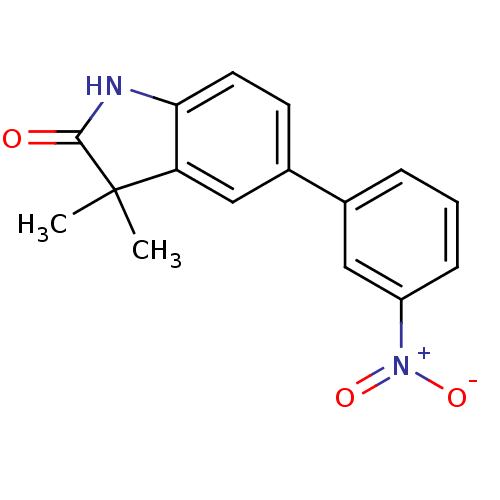
(3,3-Dimethyl-5-(3-nitro-phenyl)-1,3-dihydro-indol-...)Show SMILES CC1(C)C(=O)Nc2ccc(cc12)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C16H14N2O3/c1-16(2)13-9-11(6-7-14(13)17-15(16)19)10-4-3-5-12(8-10)18(20)21/h3-9H,1-2H3,(H,17,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223685
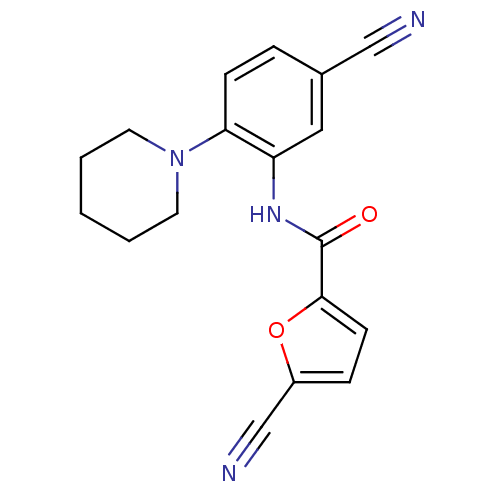
(5-cyano-N-(5-cyano-2-(piperidin-1-yl)phenyl)furan-...)Show InChI InChI=1S/C18H16N4O2/c19-11-13-4-6-16(22-8-2-1-3-9-22)15(10-13)21-18(23)17-7-5-14(12-20)24-17/h4-7,10H,1-3,8-9H2,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223676
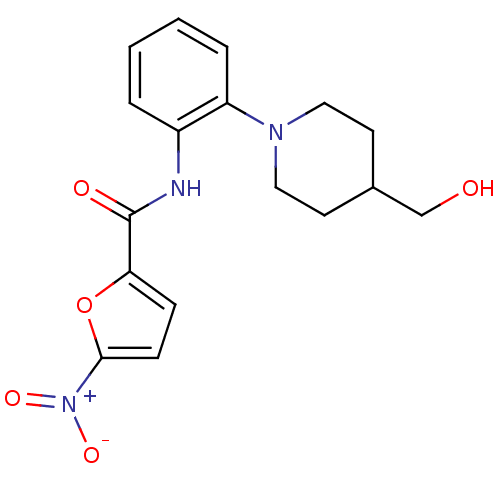
(CHEMBL249330 | N-(2-(4-(hydroxymethyl)piperidin-1-...)Show SMILES OCC1CCN(CC1)c1ccccc1NC(=O)c1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C17H19N3O5/c21-11-12-7-9-19(10-8-12)14-4-2-1-3-13(14)18-17(22)15-5-6-16(25-15)20(23)24/h1-6,12,21H,7-11H2,(H,18,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Homo sapiens (Human)) | BDBM50334175
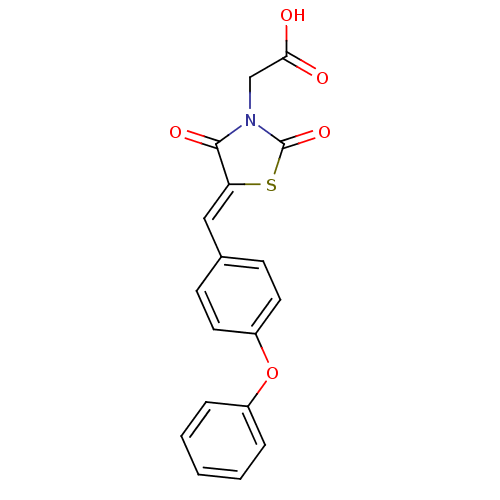
(2-(2,4-dioxo-5-(4-phenoxybenzylidene)thiazolidin-3...)Show SMILES OC(=O)CN1C(=O)S\C(=C/c2ccc(Oc3ccccc3)cc2)C1=O Show InChI InChI=1S/C18H13NO5S/c20-16(21)11-19-17(22)15(25-18(19)23)10-12-6-8-14(9-7-12)24-13-4-2-1-3-5-13/h1-10H,11H2,(H,20,21)/b15-10- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of aldose reductase (unknown origin) |
Bioorg Med Chem 23: 2953-74 (2015)
Article DOI: 10.1016/j.bmc.2015.03.071
BindingDB Entry DOI: 10.7270/Q2F76F9R |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223688
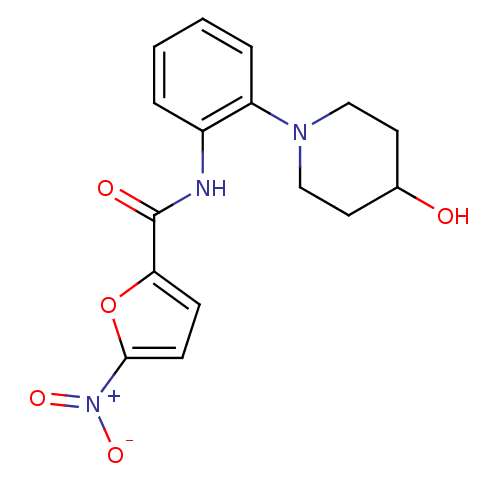
(CHEMBL249124 | N-(2-(4-hydroxypiperidin-1-yl)pheny...)Show SMILES OC1CCN(CC1)c1ccccc1NC(=O)c1ccc(o1)[N+]([O-])=O Show InChI InChI=1S/C16H17N3O5/c20-11-7-9-18(10-8-11)13-4-2-1-3-12(13)17-16(21)14-5-6-15(24-14)19(22)23/h1-6,11,20H,7-10H2,(H,17,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132451
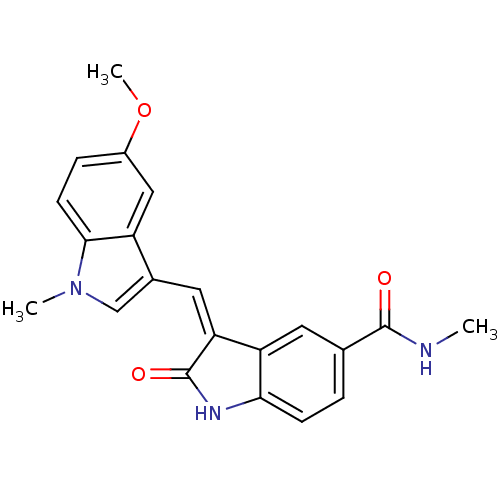
(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)-N-...)Show SMILES CNC(=O)c1ccc2NC(=O)\C(=C/c3cn(C)c4ccc(OC)cc34)c2c1 Show InChI InChI=1S/C21H19N3O3/c1-22-20(25)12-4-6-18-16(8-12)17(21(26)23-18)9-13-11-24(2)19-7-5-14(27-3)10-15(13)19/h4-11H,1-3H3,(H,22,25)(H,23,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223665

(4-cyano-N-(2-(piperidin-1-yl)phenyl)-1H-pyrrole-2-...)Show InChI InChI=1S/C17H18N4O/c18-11-13-10-15(19-12-13)17(22)20-14-6-2-3-7-16(14)21-8-4-1-5-9-21/h2-3,6-7,10,12,19H,1,4-5,8-9H2,(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase in HEK 293 cells |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223670

(5-cyano-N-(5-((2,3-dihydroxypropoxy)methyl)-2-(pip...)Show SMILES OCC(O)COCc1ccc(N2CCCCC2)c(NC(=O)c2ccc(o2)C#N)c1 |w:2.2| Show InChI InChI=1S/C21H25N3O5/c22-11-17-5-7-20(29-17)21(27)23-18-10-15(13-28-14-16(26)12-25)4-6-19(18)24-8-2-1-3-9-24/h4-7,10,16,25-26H,1-3,8-9,12-14H2,(H,23,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase in HEK 293 cells |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50223701

(4-nitro-N-(2-(piperidin-1-yl)phenyl)-1H-pyrrole-2-...)Show SMILES [O-][N+](=O)c1c[nH]c(c1)C(=O)Nc1ccccc1N1CCCCC1 Show InChI InChI=1S/C16H18N4O3/c21-16(14-10-12(11-17-14)20(22)23)18-13-6-2-3-7-15(13)19-8-4-1-5-9-19/h2-3,6-7,10-11,17H,1,4-5,8-9H2,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of ATP-induced autophosphorylation of cFMS tyrosine kinase in HEK 293 cells |
Bioorg Med Chem Lett 17: 6070-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.057
BindingDB Entry DOI: 10.7270/Q2028R9G |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50162972

(4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...)Show SMILES CC(C)N(Cc1ccccc1)c1nc(Nc2ccc3ncsc3c2)[nH]c(=O)n1 Show InChI InChI=1S/C20H20N6OS/c1-13(2)26(11-14-6-4-3-5-7-14)19-23-18(24-20(27)25-19)22-15-8-9-16-17(10-15)28-12-21-16/h3-10,12-13H,11H2,1-2H3,(H2,22,23,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of tyrosine protein kinase receptor TIE-2 |
J Med Chem 48: 1717-20 (2005)
Article DOI: 10.1021/jm049372z
BindingDB Entry DOI: 10.7270/Q2GM86TF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50093478
(CHEMBL3585578)Show SMILES C\C(=N\OCCOc1cccc(CC2SC(=O)NC2=O)c1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C13H20N6O4/c1-2-3-15-13-17-10(14)7-11(18-13)19(5-16-7)12-9(22)8(21)6(4-20)23-12/h5-6,8-9,12,20-22H,2-4H2,1H3,(H3,14,15,17,18)/t6?,8-,9-,12?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) |
Bioorg Med Chem 23: 2953-74 (2015)
Article DOI: 10.1016/j.bmc.2015.03.071
BindingDB Entry DOI: 10.7270/Q2F76F9R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data