

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50260202 (CHEMBL451090 | myricetin 7-O-alpha-L-rhamnopyranos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50260201 (CHEMBL503182 | myricetin 7-O-beta-D-glucopyranosyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50022445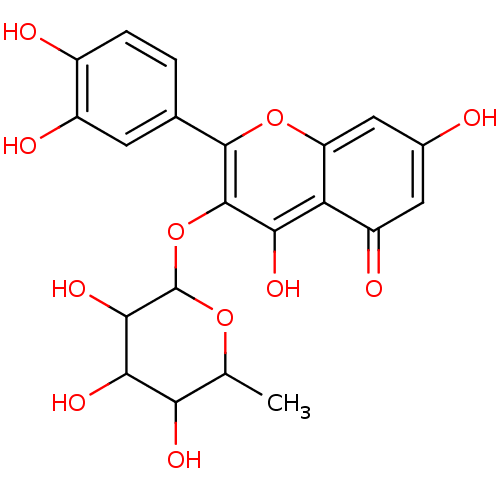 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50428820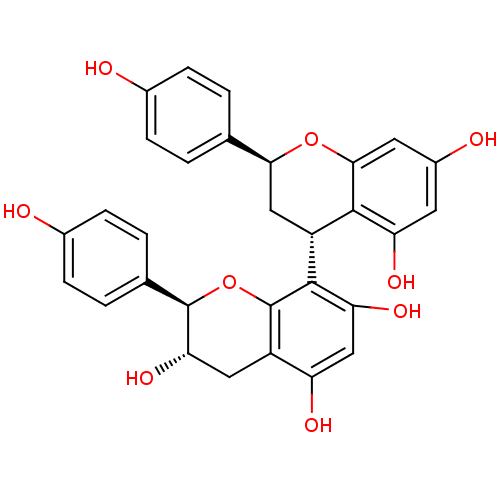 (CHEMBL2335722) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of recombinant PlGF-1/VEGFR-1 (unknown origin) interaction after 1 hr by ELISA | J Nat Prod 76: 29-35 (2013) Article DOI: 10.1021/np300614u BindingDB Entry DOI: 10.7270/Q24F1S3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50187133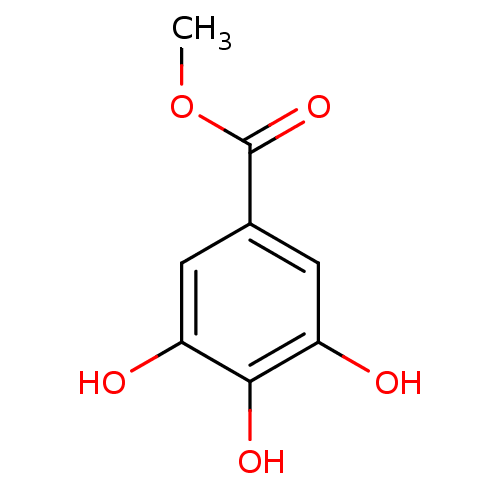 (3,4,5-Trihydroxy-benzoic acid methyl ester | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM23406 ((3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of hog pancreas alpha-amylase using starch as substrate preincubated for 10 mins followed by substrate addition measured after 10 mins by ... | J Nat Prod 79: 2104-12 (2016) Article DOI: 10.1021/acs.jnatprod.6b00484 BindingDB Entry DOI: 10.7270/Q2QN68R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50242283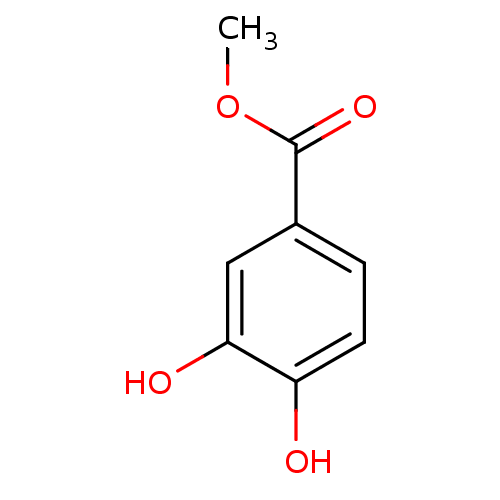 (3,4-dihydroxymethylbenzoate | CHEMBL486027 | Methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50240383 ((E)-3-(3,4-Dihydroxy-phenyl)-propenal | CHEMBL1729...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50428820 (CHEMBL2335722) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of recombinant VEGF-A/VEGFR-1 (unknown origin) interaction after 1 hr by ELISA | J Nat Prod 76: 29-35 (2013) Article DOI: 10.1021/np300614u BindingDB Entry DOI: 10.7270/Q24F1S3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50240367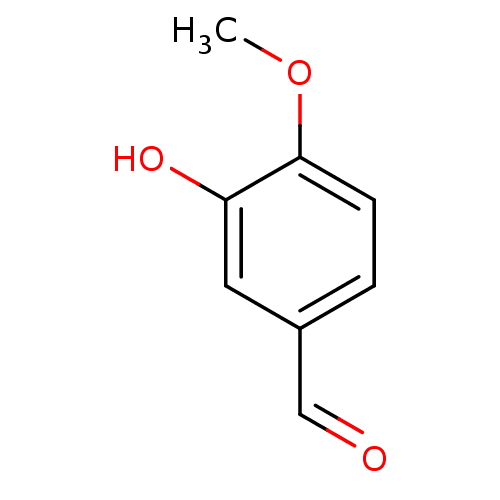 (3-Hydroxy-4-methoxy-benzaldehyde | 3-hydroxy-4-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50241354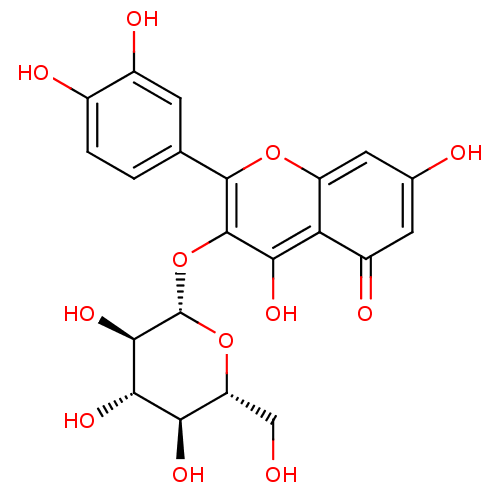 (2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-(3,4,5-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50209100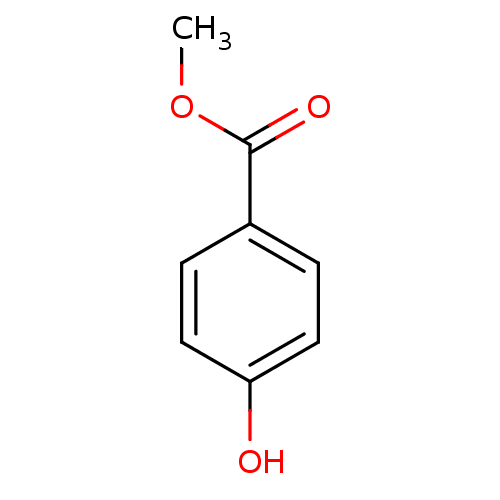 (4-hydroxymethylbenzoate | CHEMBL325372 | Methyl pa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Salerno Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase (unknown origin)-mediated formation of uric acid by spectrophotometry | J Nat Prod 65: 1526-9 (2002) BindingDB Entry DOI: 10.7270/Q2959H9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50428819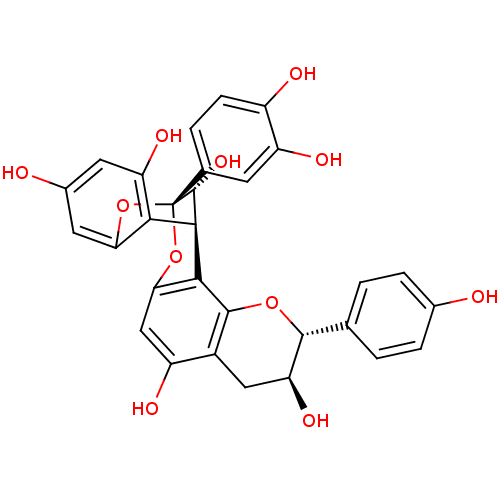 (GERANIN B) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of recombinant VEGF-A/VEGFR-1 (unknown origin) interaction after 1 hr by ELISA | J Nat Prod 76: 29-35 (2013) Article DOI: 10.1021/np300614u BindingDB Entry DOI: 10.7270/Q24F1S3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM50202632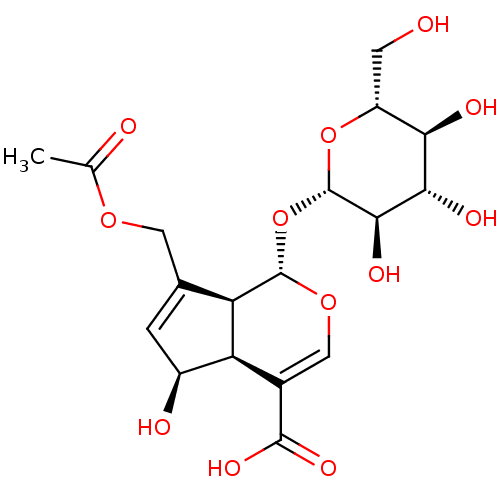 (CHEMBL460030) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of hog pancreas alpha-amylase using starch as substrate preincubated for 10 mins followed by substrate addition measured after 10 mins by ... | J Nat Prod 79: 2104-12 (2016) Article DOI: 10.1021/acs.jnatprod.6b00484 BindingDB Entry DOI: 10.7270/Q2QN68R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic alpha-amylase (Sus scrofa (Pig)) | BDBM23410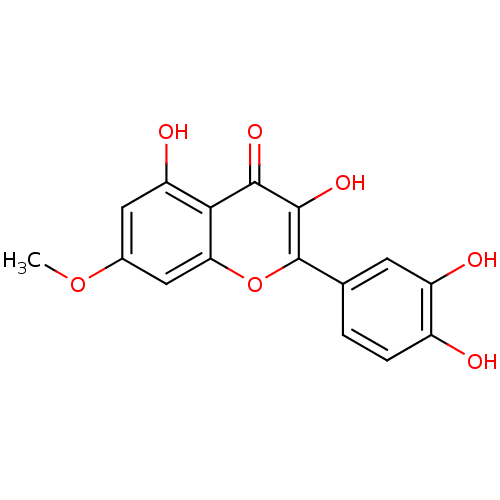 (2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-7-methoxy-4H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Basilicata Curated by ChEMBL | Assay Description Inhibition of hog pancreas alpha-amylase using starch as substrate preincubated for 10 mins followed by substrate addition measured after 10 mins by ... | J Nat Prod 79: 2104-12 (2016) Article DOI: 10.1021/acs.jnatprod.6b00484 BindingDB Entry DOI: 10.7270/Q2QN68R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456573 (CHEMBL3581233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456574 (CHEMBL4217921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456576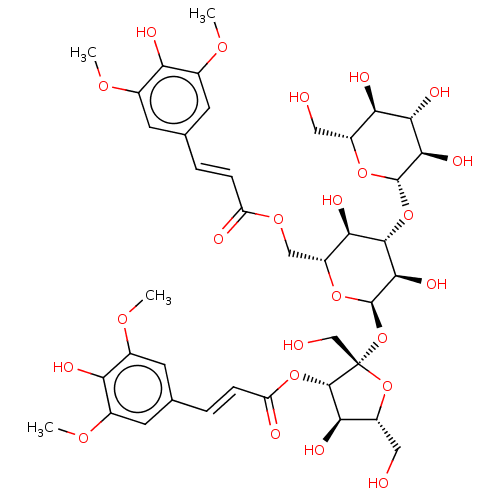 (CHEMBL4210558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456579 (CHEMBL4217513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456580 (CHEMBL4212672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50217942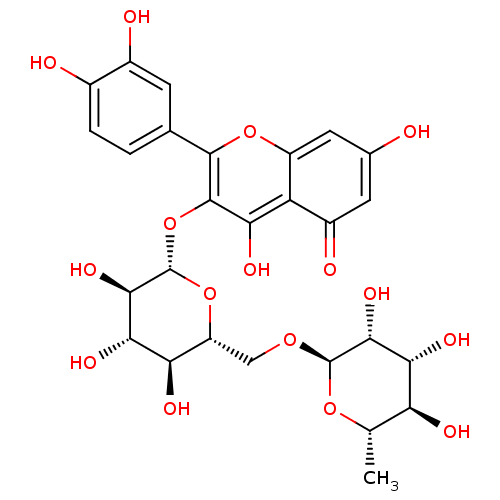 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456581 (CHEMBL4214983) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456578 (CHEMBL4213100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456575 (CHEMBL4205695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456572 (CHEMBL4218023) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456582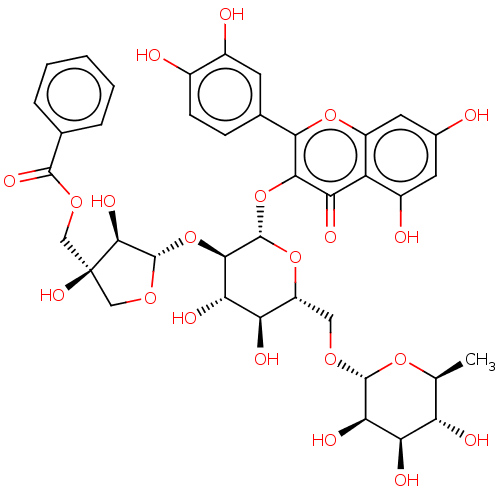 (CHEMBL4205812) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50456577 (CHEMBL4207766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human liver LDH5 using pyruvate as substrate and NADH as cofactor measured after 15 mins by fluorescence assay | J Nat Prod 80: 2077-2087 (2017) Article DOI: 10.1021/acs.jnatprod.7b00295 BindingDB Entry DOI: 10.7270/Q22N54W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50241990 (CHEMBL502489 | Camboginol | Garcinol | Garcinol, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM84873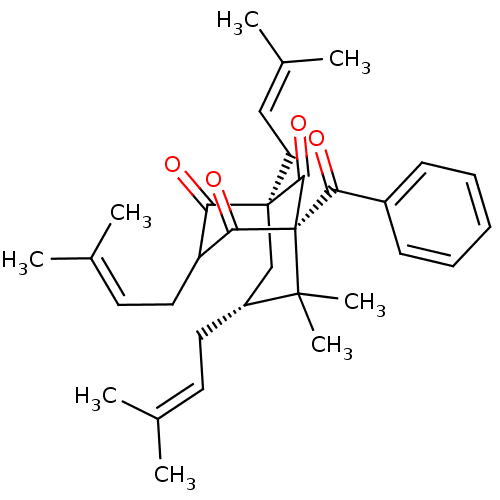 (Nemorosone, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM84874 (Guttiferone A, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM84875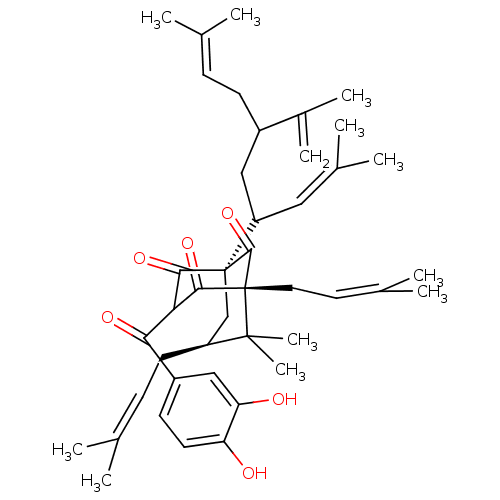 (Guttiferone E, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM84877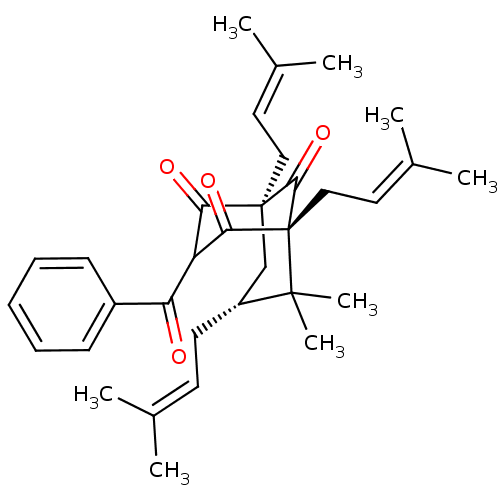 (Clusianone, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | 37 |
Universitá degli Studi di Salerno | Assay Description The p300 activity assays were performed by a colorimetric kit (JM-K322-100, MBL) using active recombinant p300/HAT as positive control and acetyl-CoA... | Chembiochem 11: 818-27 (2010) Article DOI: 10.1002/cbic.200900721 BindingDB Entry DOI: 10.7270/Q2S1810J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293285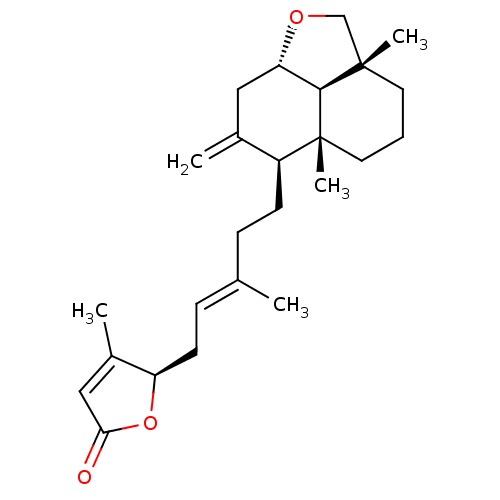 (23,6alpha-epoxy-labd-8,13(14),17-trien-16(R),19-ol...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293286 (8alpha-23-dihydroxy-23,6alpha-epoxy-labd-13(14),15...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293287 (8alpha-hydroxylabd-13(14),15,17-trien-6alpha,23-16...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293288 (6alpha,8alpha-dihydroxy-23-oxo-13(14),15,17-trien-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293289 (6alpha,8alpha-dihydroxy-23-carbossi-labd-13(14),15...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293290 (6alpha,8alpha,23-trihydroxy-labd-13(14),15,17-trie...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293291 (6alpha,15(S)-dihydroxy-23-oxo-labd-8(22),13(14),17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293292 (6alpha,15(S),23-trihydroxy-labd-8(22),13(14),17-tr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293293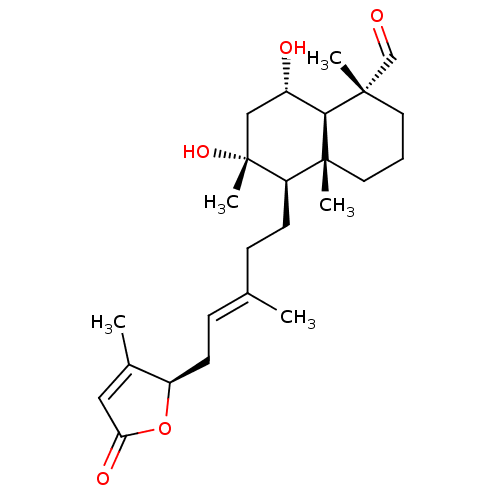 (6alpha,8alpha-dihydroxy-23-oxo-labd-13(14),17-dien...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293294 (6alpha,8alpha,15(S)-trihydroxy-23-oxo-labd-13(14),...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293295 (6alpha,8alpha,15(S)-trihydroxy-23-carbossimethylla...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293296 (6alpha,8alpha-dihydroxy-23-carbossi-labd-13(14),17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293297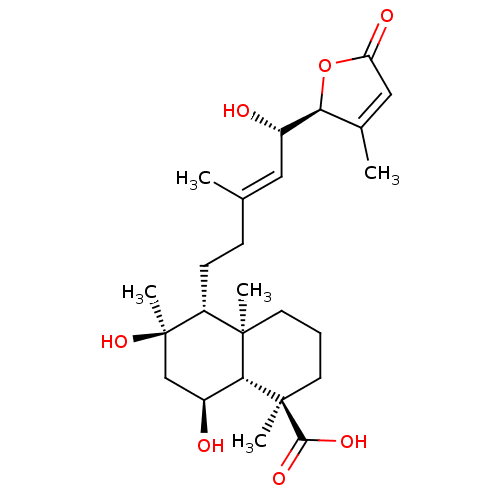 (6alpha,8alpha,15(S)-trihydroxy-23-carbossi-labd-13...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293298 (6alpha,8alpha,23-trihydroxy-labd-13(14),17-dien-16...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293299 (6alpha,8alpha,15(S),23-tetrahydroxy-labd-13(14),17...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293300 (8alpha-hydroxy,23alpha-O-ethyl-23,6alpha-epoxy-lab...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin--tyrosine ligase (Homo sapiens (Human)) | BDBM50293301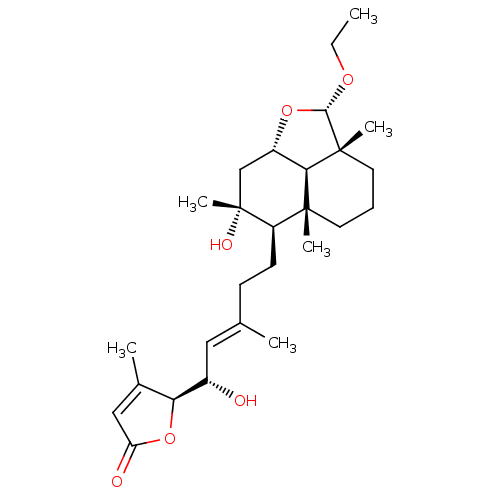 (8alpha,15(S)-dihydroxy,23alpha-O-ethyl-23,6alpha-e...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a |
Universita di Salerno Curated by ChEMBL | Assay Description Binding affinity to TTL by surface plasmon resonance | J Med Chem 52: 3814-28 (2009) Article DOI: 10.1021/jm801637f BindingDB Entry DOI: 10.7270/Q2JD4WQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |