 Found 159 hits with Last Name = 'mitro' and Initial = 'n'
Found 159 hits with Last Name = 'mitro' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 74 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Consiglio Nazionale delle Ricerche
| Assay Description
The scintillation proximity assay was performed in 96-well plates containing polylysine-coated yttrium silicate beads, His-PPARgamma-LBD, and [3H]ros... |
J Biol Chem 282: 17314-24 (2007)
Article DOI: 10.1074/jbc.M702316200
BindingDB Entry DOI: 10.7270/Q27M0682 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28762
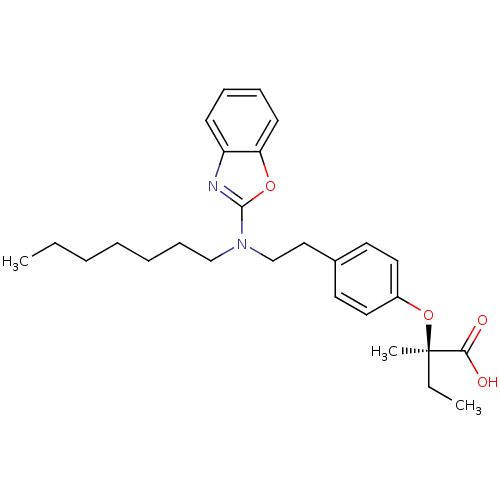
((2R)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 88 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Consiglio Nazionale delle Ricerche
| Assay Description
The scintillation proximity assay was performed in 96-well plates containing polylysine-coated yttrium silicate beads, His-PPARgamma-LBD, and [3H]ros... |
J Biol Chem 282: 17314-24 (2007)
Article DOI: 10.1074/jbc.M702316200
BindingDB Entry DOI: 10.7270/Q27M0682 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28762

((2R)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28763
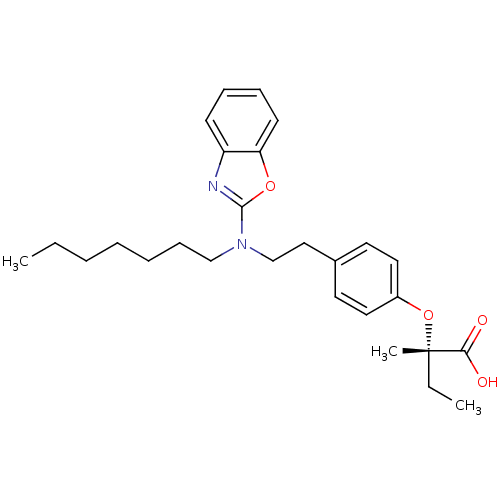
((2S)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 971 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Consiglio Nazionale delle Ricerche
| Assay Description
The scintillation proximity assay was performed in 96-well plates containing polylysine-coated yttrium silicate beads, His-PPARgamma-LBD, and [3H]ros... |
J Biol Chem 282: 17314-24 (2007)
Article DOI: 10.1074/jbc.M702316200
BindingDB Entry DOI: 10.7270/Q27M0682 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28763

((2S)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 971 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597817
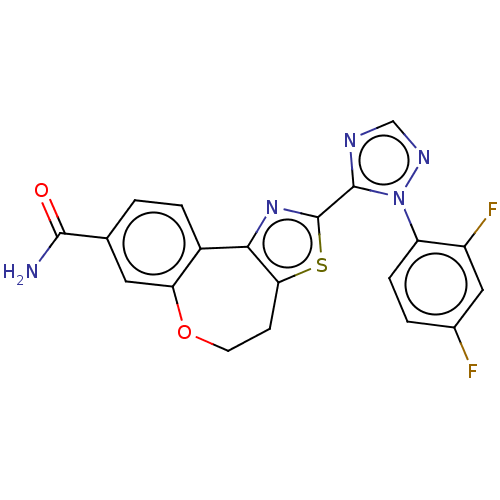
(CHEMBL5192406)Show SMILES NC(=O)c1ccc2-c3nc(sc3CCOc2c1)-c1ncnn1-c1ccc(F)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597812
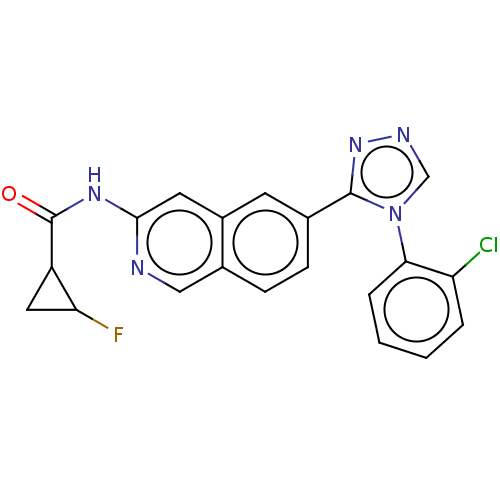
(CHEMBL5192215)Show SMILES FC1CC1C(=O)Nc1cc2cc(ccc2cn1)-c1nncn1-c1ccccc1Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50597817

(CHEMBL5192406)Show SMILES NC(=O)c1ccc2-c3nc(sc3CCOc2c1)-c1ncnn1-c1ccc(F)cc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597813

(CHEMBL5183804)Show SMILES CC(C)[C@@H]1CNC(=O)c2cc(nn12)-c1ccnc(NC(=O)C2CC2)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597807
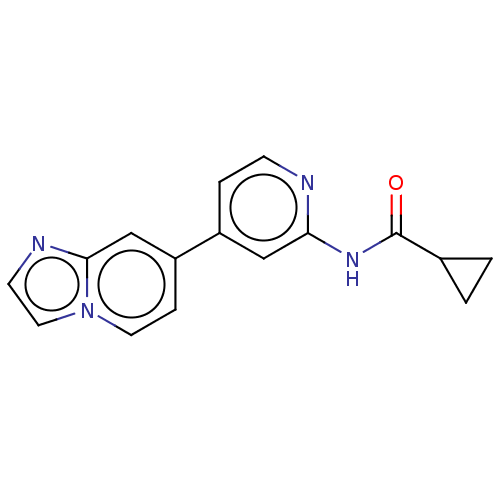
(CHEMBL5190688) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19992

(2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...)Show SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1 Show InChI InChI=1S/C33H31ClF3NO3/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548269
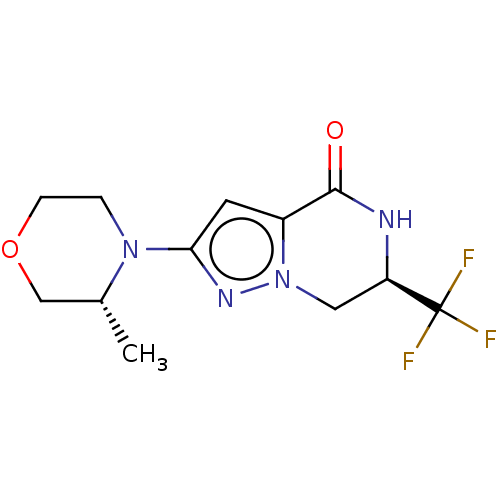
((R)-2-((R)-3-methylmorpholino)-6-(trifluoromethyl)...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)N[C@H](Cn2n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548239
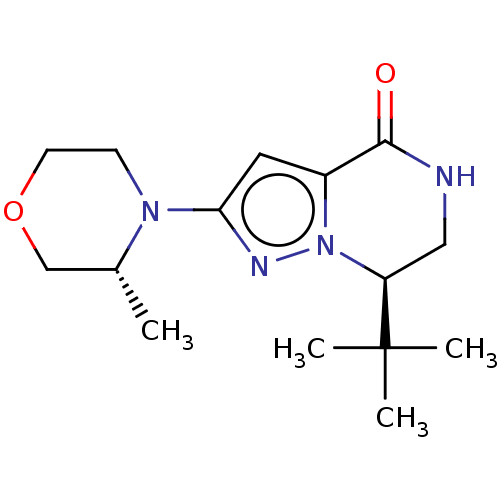
((R)-7-(tert-butyl)-2-((R)-3-methylmorpholino)-6,7-...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC[C@H](n2n1)C(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597814
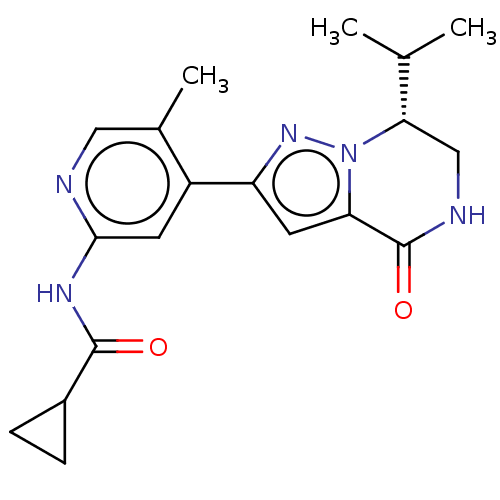
(CHEMBL5200373)Show SMILES CC(C)[C@@H]1CNC(=O)c2cc(nn12)-c1cc(NC(=O)C2CC2)ncc1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597809
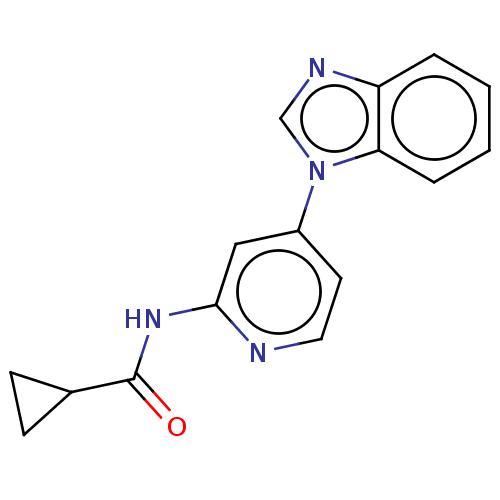
(CHEMBL5181274) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM20132
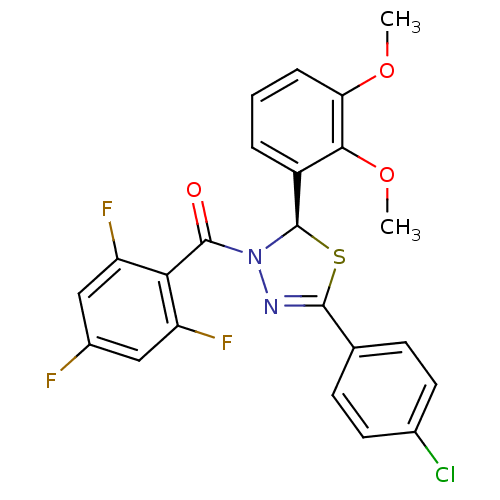
((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...)Show SMILES COc1cccc([C@H]2SC(=NN2C(=O)c2c(F)cc(F)cc2F)c2ccc(Cl)cc2)c1OC |r,c:9| Show InChI InChI=1S/C23H16ClF3N2O3S/c1-31-18-5-3-4-15(20(18)32-2)23-29(22(30)19-16(26)10-14(25)11-17(19)27)28-21(33-23)12-6-8-13(24)9-7-12/h3-11,23H,1-2H3/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548235
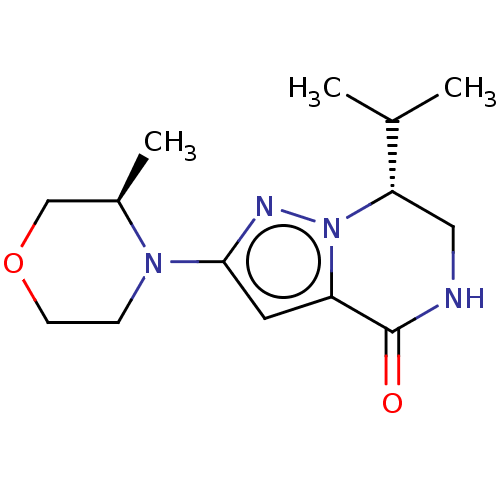
((R)-7-isopropyl-2-((R)-3-methylmorpholino)-6,7-dih...)Show SMILES CC(C)[C@@H]1CNC(=O)c2cc(nn12)N1CCOC[C@H]1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548243
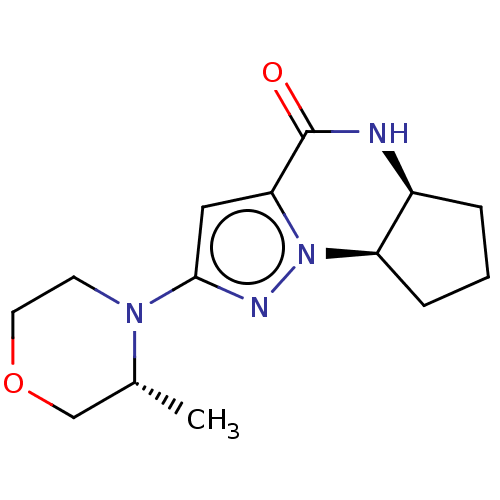
((5aS,8aR)-2-((R)-3-methylmorpholino)-5,5a,6,7,8,8a...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)N[C@H]3CCC[C@H]3n2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548265
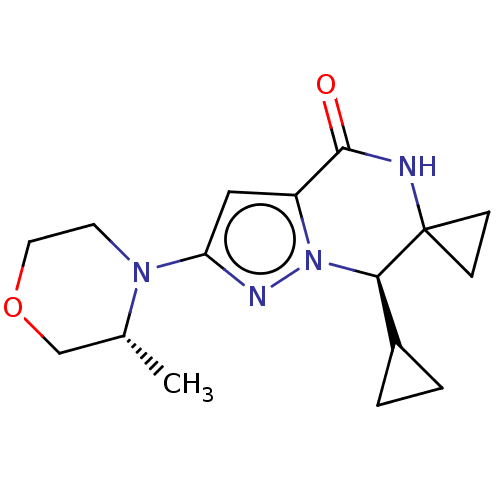
((R)-7'-cyclopropyl-2'-((R)-3-methylmorpholino)-7'H...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC3(CC3)[C@@H](C3CC3)n2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548251
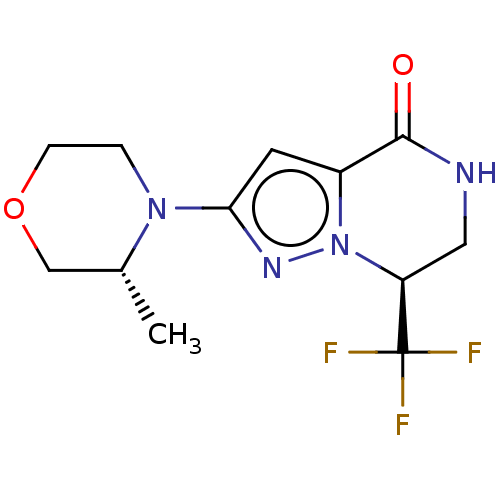
((S)-2-((R)-3-methylmorpholino)-7-(trifluoromethyl)...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC[C@H](n2n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM19992

(2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...)Show SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1 Show InChI InChI=1S/C33H31ClF3NO3/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597811
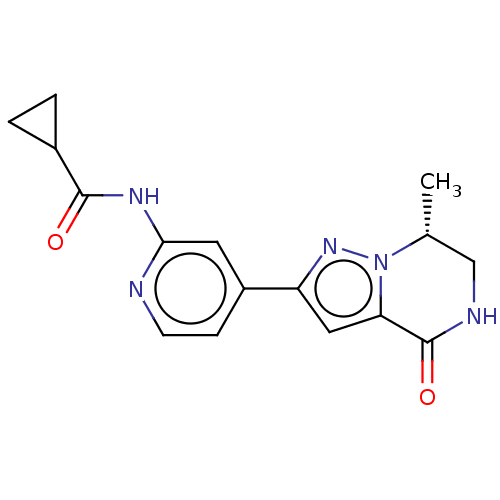
(CHEMBL5195258)Show SMILES C[C@@H]1CNC(=O)c2cc(nn12)-c1ccnc(NC(=O)C2CC2)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597818
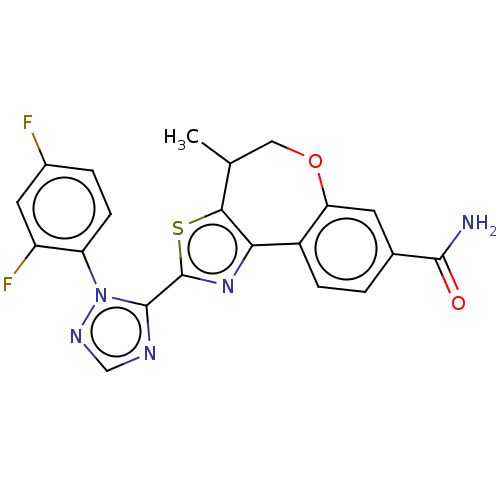
(CHEMBL5197817)Show SMILES CC1COc2cc(ccc2-c2nc(sc12)-c1ncnn1-c1ccc(F)cc1F)C(N)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548253
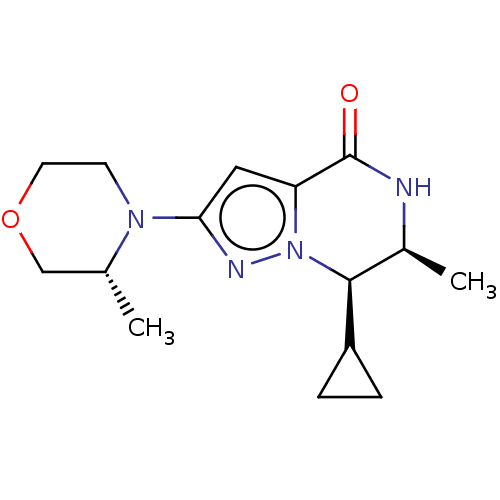
((6S,7R)-7-cyclopropyl-6-methyl-2-((R)-3-methylmorp...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)N[C@@H](C)[C@@H](C3CC3)n2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548247
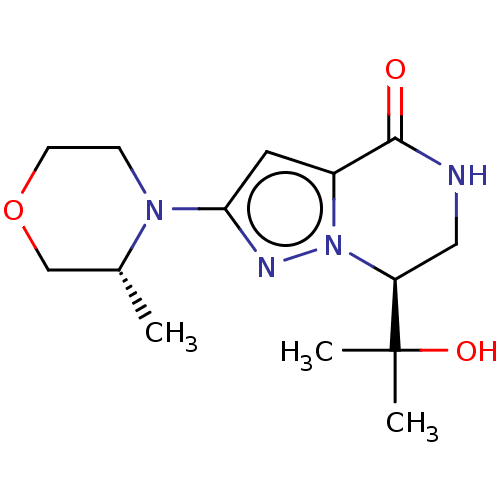
((S)-7-(2-hydroxypropan-2-yl)-2-((R)-3-methylmorpho...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC[C@H](n2n1)C(C)(C)O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548237
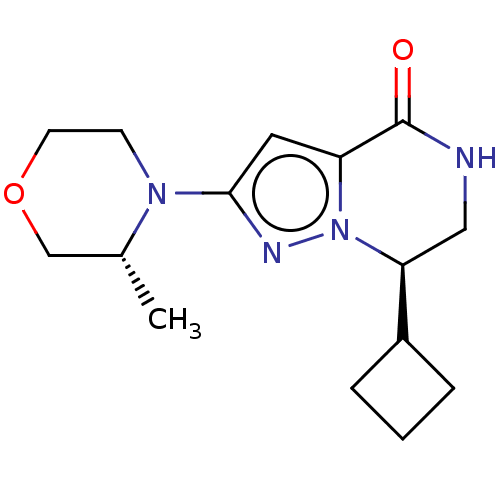
((R)-7-cyclobutyl-2-((R)-3-methylmorpholino)-6,7-di...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC[C@@H](C3CCC3)n2n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597816
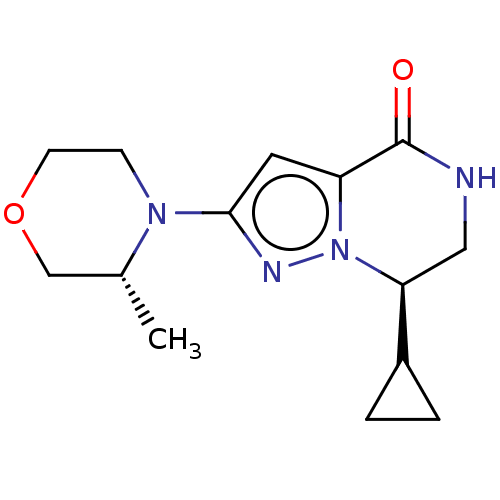
(CHEMBL5202968)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC[C@@H](C3CC3)n2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM20131

(5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...)Show SMILES COc1cccc(C2SC(=NN2C(=O)c2c(F)cc(F)cc2F)c2ccc(Cl)cc2)c1OC |c:9| Show InChI InChI=1S/C23H16ClF3N2O3S/c1-31-18-5-3-4-15(20(18)32-2)23-29(22(30)19-16(26)10-14(25)11-17(19)27)28-21(33-23)12-6-8-13(24)9-7-12/h3-11,23H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597808
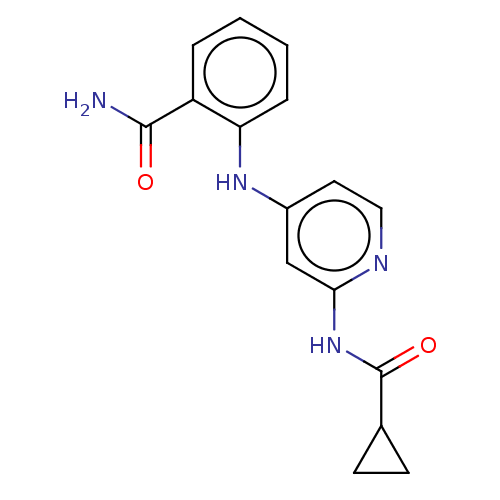
(CHEMBL5190274) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50597818

(CHEMBL5197817)Show SMILES CC1COc2cc(ccc2-c2nc(sc12)-c1ncnn1-c1ccc(F)cc1F)C(N)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548241
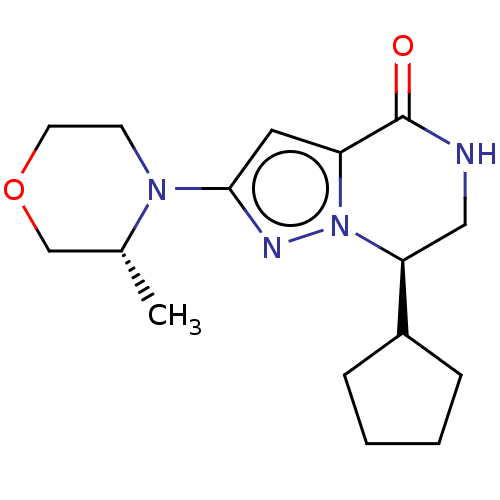
((R)-7-cyclopentyl-2-((R)-3-methylmorpholino)-6,7-d...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC[C@@H](C3CCCC3)n2n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597810
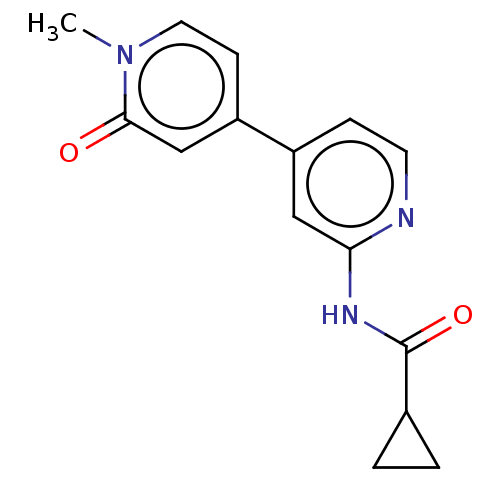
(CHEMBL5204287) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548271
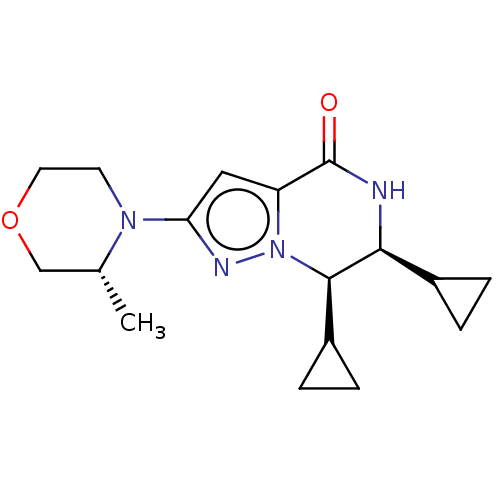
((6S,7R)-6,7-dicyclopropyl-2-((R)-3-methylmorpholin...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)N[C@@H](C3CC3)[C@@H](C3CC3)n2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50597815
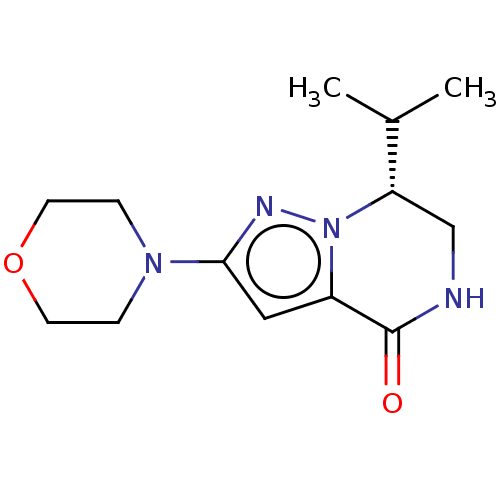
(CHEMBL5182171) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM548273
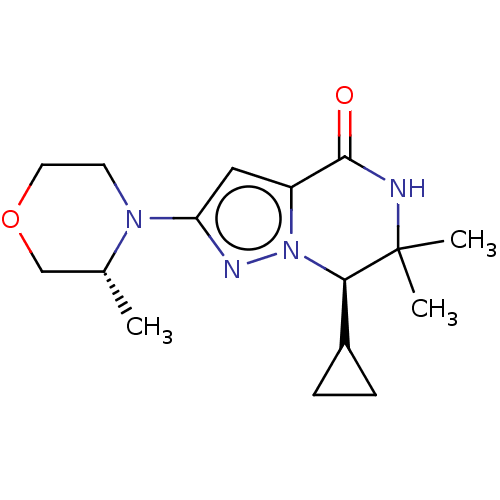
((R)-7-cyclopropyl-6,6-dimethyl-2-((R)-3-methylmorp...)Show SMILES C[C@@H]1COCCN1c1cc2C(=O)NC(C)(C)[C@@H](C3CC3)n2n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01180
BindingDB Entry DOI: 10.7270/Q2ZC86XQ |
More data for this
Ligand-Target Pair | |
Peptidoglycan D,D-transpeptidase FtsI
(Pseudomonas aeruginosa) | BDBM50538667
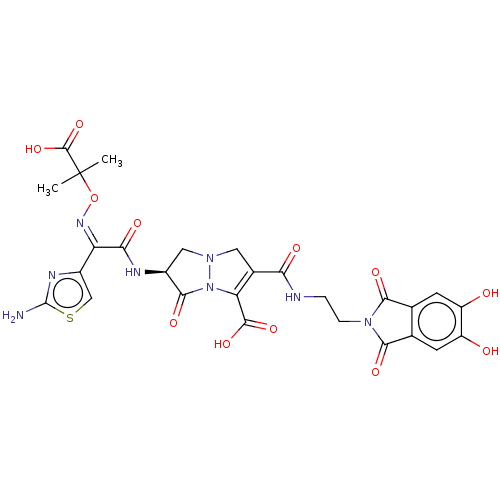
(CHEMBL4634190)Show SMILES CC(C)(O\N=C(/C(=O)N[C@H]1CN2CC(C(=O)NCCN3C(=O)c4cc(O)c(O)cc4C3=O)=C(N2C1=O)C(O)=O)c1csc(N)n1)C(O)=O |r,c:33| Show InChI InChI=1S/C27H26N8O12S/c1-27(2,25(45)46)47-32-17(14-9-48-26(28)31-14)20(39)30-13-8-33-7-12(18(24(43)44)35(33)23(13)42)19(38)29-3-4-34-21(40)10-5-15(36)16(37)6-11(10)22(34)41/h5-6,9,13,36-37H,3-4,7-8H2,1-2H3,(H2,28,31)(H,29,38)(H,30,39)(H,43,44)(H,45,46)/b32-17-/t13-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Louis Stokes Cleveland Department of Veterans Affairs Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa PBP3 assessed as reduction in fluorescence intensity of bocillin labeled protein pre-incubated for 20 mins befor... |
J Med Chem 63: 5990-6002 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00255
BindingDB Entry DOI: 10.7270/Q25142S5 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20132

((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...)Show SMILES COc1cccc([C@H]2SC(=NN2C(=O)c2c(F)cc(F)cc2F)c2ccc(Cl)cc2)c1OC |r,c:9| Show InChI InChI=1S/C23H16ClF3N2O3S/c1-31-18-5-3-4-15(20(18)32-2)23-29(22(30)19-16(26)10-14(25)11-17(19)27)28-21(33-23)12-6-8-13(24)9-7-12/h3-11,23H,1-2H3/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20131

(5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...)Show SMILES COc1cccc(C2SC(=NN2C(=O)c2c(F)cc(F)cc2F)c2ccc(Cl)cc2)c1OC |c:9| Show InChI InChI=1S/C23H16ClF3N2O3S/c1-31-18-5-3-4-15(20(18)32-2)23-29(22(30)19-16(26)10-14(25)11-17(19)27)28-21(33-23)12-6-8-13(24)9-7-12/h3-11,23H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF
| Assay Description
SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM19992

(2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...)Show SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1 Show InChI InChI=1S/C33H31ClF3NO3/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 97 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20131

(5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...)Show SMILES COc1cccc(C2SC(=NN2C(=O)c2c(F)cc(F)cc2F)c2ccc(Cl)cc2)c1OC |c:9| Show InChI InChI=1S/C23H16ClF3N2O3S/c1-31-18-5-3-4-15(20(18)32-2)23-29(22(30)19-16(26)10-14(25)11-17(19)27)28-21(33-23)12-6-8-13(24)9-7-12/h3-11,23H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20132

((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...)Show SMILES COc1cccc([C@H]2SC(=NN2C(=O)c2c(F)cc(F)cc2F)c2ccc(Cl)cc2)c1OC |r,c:9| Show InChI InChI=1S/C23H16ClF3N2O3S/c1-31-18-5-3-4-15(20(18)32-2)23-29(22(30)19-16(26)10-14(25)11-17(19)27)28-21(33-23)12-6-8-13(24)9-7-12/h3-11,23H,1-2H3/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20133
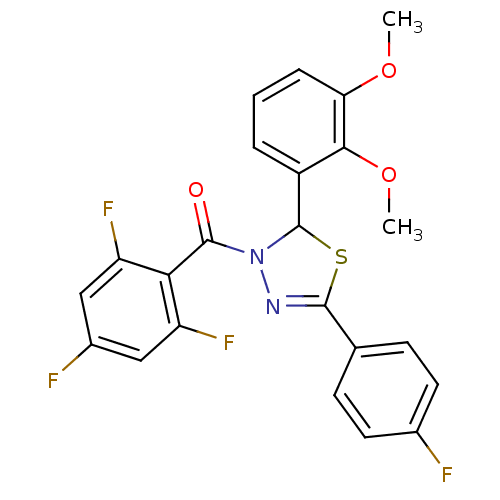
(2-(2,3-dimethoxyphenyl)-5-(4-fluorophenyl)-3-[(2,4...)Show SMILES COc1cccc(C2SC(=NN2C(=O)c2c(F)cc(F)cc2F)c2ccc(F)cc2)c1OC |c:9| Show InChI InChI=1S/C23H16F4N2O3S/c1-31-18-5-3-4-15(20(18)32-2)23-29(22(30)19-16(26)10-14(25)11-17(19)27)28-21(33-23)12-6-8-13(24)9-7-12/h3-11,23H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20134
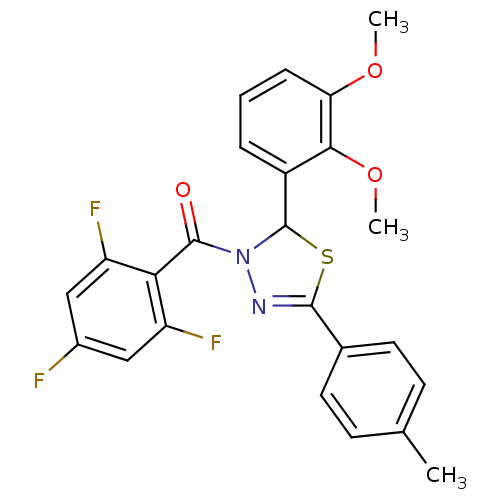
(2-(2,3-dimethoxyphenyl)-5-(4-methylphenyl)-3-[(2,4...)Show SMILES COc1cccc(C2SC(=NN2C(=O)c2c(F)cc(F)cc2F)c2ccc(C)cc2)c1OC |c:9| Show InChI InChI=1S/C24H19F3N2O3S/c1-13-7-9-14(10-8-13)22-28-29(23(30)20-17(26)11-15(25)12-18(20)27)24(33-22)16-5-4-6-19(31-2)21(16)32-3/h4-12,24H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
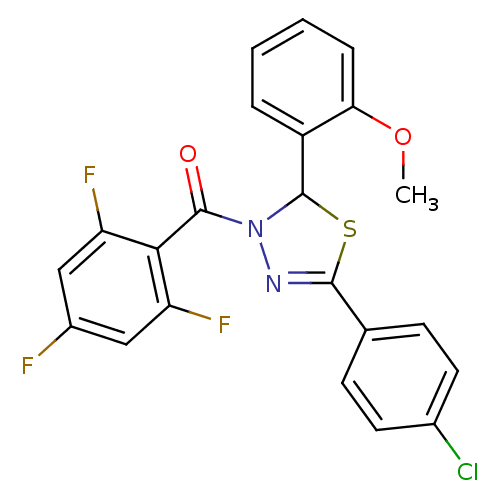
(Homo sapiens (Human)) | BDBM20135
(5-(4-chlorophenyl)-2-(2-methoxyphenyl)-3-[(2,4,6-t...)Show SMILES COc1ccccc1C1SC(=NN1C(=O)c1c(F)cc(F)cc1F)c1ccc(Cl)cc1 |c:11| Show InChI InChI=1S/C22H14ClF3N2O2S/c1-30-18-5-3-2-4-15(18)22-28(21(29)19-16(25)10-14(24)11-17(19)26)27-20(31-22)12-6-8-13(23)9-7-12/h2-11,22H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
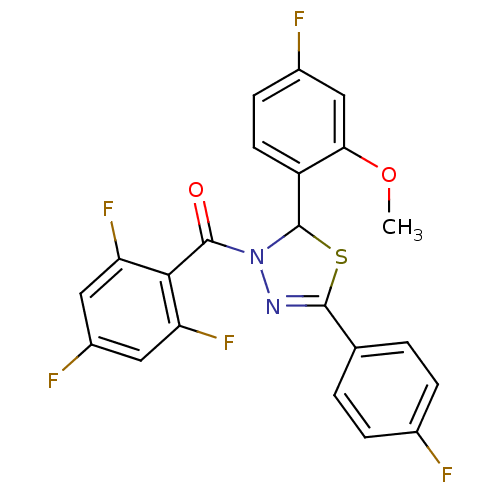
(Homo sapiens (Human)) | BDBM20136
(2-(4-fluoro-2-methoxyphenyl)-5-(4-fluorophenyl)-3-...)Show SMILES COc1cc(F)ccc1C1SC(=NN1C(=O)c1c(F)cc(F)cc1F)c1ccc(F)cc1 |c:12| Show InChI InChI=1S/C22H13F5N2O2S/c1-31-18-10-13(24)6-7-15(18)22-29(21(30)19-16(26)8-14(25)9-17(19)27)28-20(32-22)11-2-4-12(23)5-3-11/h2-10,22H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
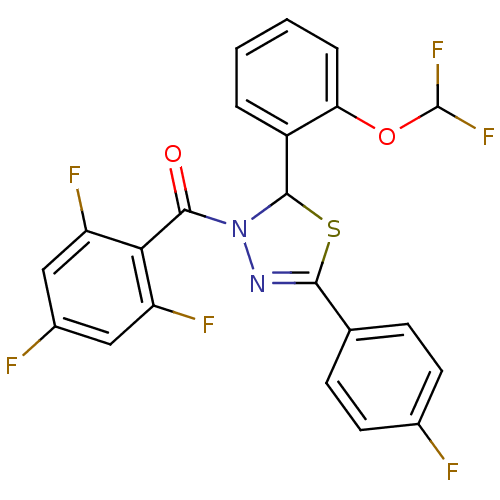
(Homo sapiens (Human)) | BDBM20137
(2-[2-(difluoromethoxy)phenyl]-5-(4-fluorophenyl)-3...)Show SMILES FC(F)Oc1ccccc1C1SC(=NN1C(=O)c1c(F)cc(F)cc1F)c1ccc(F)cc1 |c:13| Show InChI InChI=1S/C22H12F6N2O2S/c23-12-7-5-11(6-8-12)19-29-30(20(31)18-15(25)9-13(24)10-16(18)26)21(33-19)14-3-1-2-4-17(14)32-22(27)28/h1-10,21-22H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20138
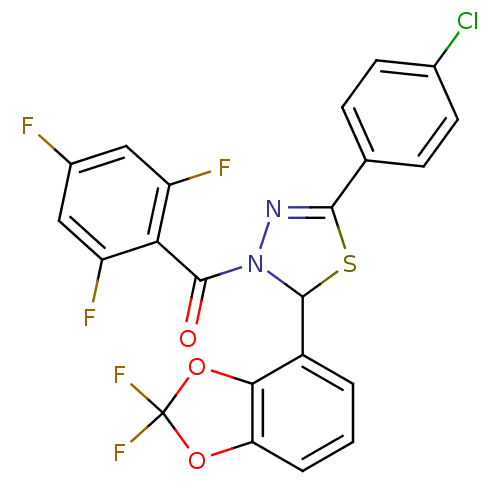
(5-(4-chlorophenyl)-2-(2,2-difluoro-2H-1,3-benzodio...)Show SMILES Fc1cc(F)c(C(=O)N2N=C(SC2c2cccc3OC(F)(F)Oc23)c2ccc(Cl)cc2)c(F)c1 |c:9| Show InChI InChI=1S/C22H10ClF5N2O3S/c23-11-6-4-10(5-7-11)19-29-30(20(31)17-14(25)8-12(24)9-15(17)26)21(34-19)13-2-1-3-16-18(13)33-22(27,28)32-16/h1-9,21H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20139
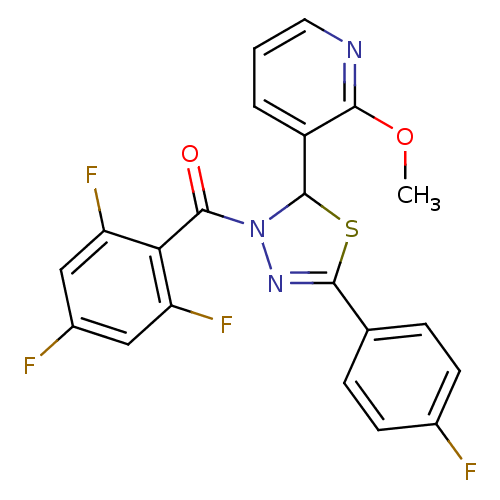
(5-(4-fluorophenyl)-2-(2-methoxypyridin-3-yl)-3-[(2...)Show SMILES COc1ncccc1C1SC(=NN1C(=O)c1c(F)cc(F)cc1F)c1ccc(F)cc1 |c:11| Show InChI InChI=1S/C21H13F4N3O2S/c1-30-18-14(3-2-8-26-18)21-28(20(29)17-15(24)9-13(23)10-16(17)25)27-19(31-21)11-4-6-12(22)7-5-11/h2-10,21H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20140
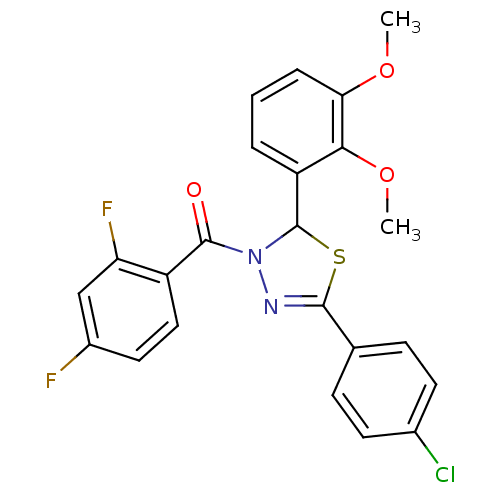
(5-(4-chlorophenyl)-3-[(2,4-difluorophenyl)carbonyl...)Show SMILES COc1cccc(C2SC(=NN2C(=O)c2ccc(F)cc2F)c2ccc(Cl)cc2)c1OC |c:9| Show InChI InChI=1S/C23H17ClF2N2O3S/c1-30-19-5-3-4-17(20(19)31-2)23-28(22(29)16-11-10-15(25)12-18(16)26)27-21(32-23)13-6-8-14(24)9-7-13/h3-12,23H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | 7.2 | 37 |
GNF
| Assay Description
FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... |
J Med Chem 50: 4255-9 (2007)
Article DOI: 10.1021/jm070453f
BindingDB Entry DOI: 10.7270/Q2BK19N6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data