

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187686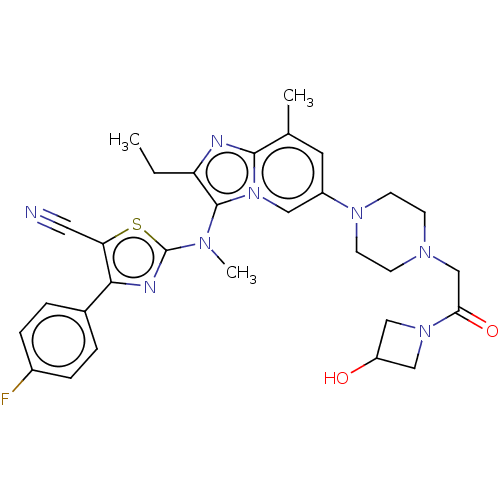 (CHEMBL3828074 | US10526329, Compound 2 | US1107261...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU Curated by ChEMBL | Assay Description Competitive inhibition of human ATX using LPC (16:0) as substrate after 30 mins by Michaelis-Menten plot analysis | J Med Chem 60: 3580-3590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00032 BindingDB Entry DOI: 10.7270/Q25141PD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090718 (CHEMBL327528 | N-(1-{1-[1-Carboxy-2-(1H-indol-3-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090717 (15,18-Di-sec-butyl-9,12-bis-(1H-indol-3-ylmethyl)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090721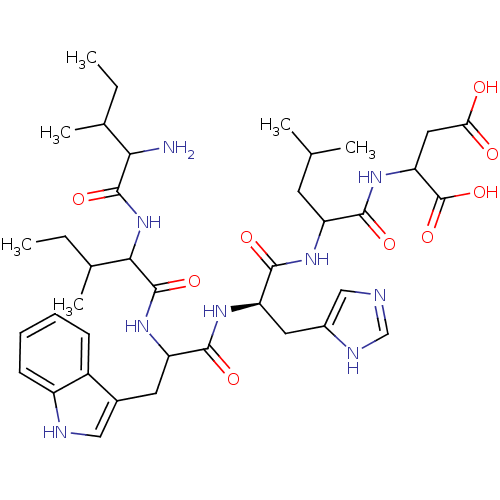 (2-{2-[2-[2-[2-(2-Amino-3-methyl-pentanoylamino)-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090712 (2-{2-[2-[2-[2-(2-Amino-3-methyl-pentanoylamino)-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090722 (CHEMBL329695 | [5,8-Di-sec-butyl-11,14-bis-(1H-ind...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50001220 (3-{2-[2-Acetylamino-3-(1H-indol-3-yl)-propionylami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090719 (2-{2-[2-(2-{2-[2-Acetylamino-3-(1H-indol-3-yl)-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090716 (2-{2-[2-[2-[2-(2-Amino-3-methyl-pentanoylamino)-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090720 (2-(2-{2-[2-[2-(2-Amino-3-methyl-pentanoylamino)-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090715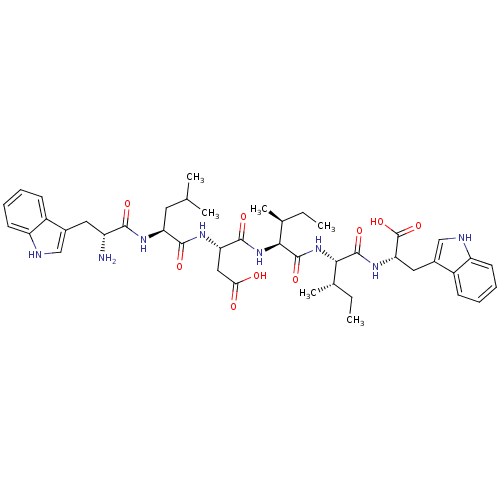 (3-{2-[2-Amino-3-(1H-indol-3-yl)-propionylamino]-4-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090714 (2-(2-{2-[2-[2-(2-Amino-3-methyl-pentanoylamino)-3-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition constant for the displacement of specific binding of [125I]-[D-Lys6]-GnRH (Kd=177 pM) bound to rat pituitary membranes | J Med Chem 43: 2824-30 (2000) BindingDB Entry DOI: 10.7270/Q2FF3RK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50105862 (CHEMBL410632 | pGlu-His-Trp-Ser-Tyr-D-Lys(2-(hydro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Evaluated for gonadotropin-releasing hormone receptor binding by displacing the 50% of the bound tracer [125I]-[D-Lys6]-GnRH radioligand in rat pitui... | J Med Chem 44: 3645-52 (2001) BindingDB Entry DOI: 10.7270/Q2W66K2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50105860 (CHEMBL385043 | pGlu-His-Trp-Ser-Tyr-D-Lys-Leu-Arg-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Evaluated for gonadotropin-releasing hormone receptor binding by displacing the 50% of the bound tracer [125I]-[D-Lys6]-GnRH radioligand in rat pitui... | J Med Chem 44: 3645-52 (2001) BindingDB Entry DOI: 10.7270/Q2W66K2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50105861 (CHEMBL414239 | pGlu-His-Trp-Ser-Tyr-D-Lys(N-(2 chl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Evaluated for gonadotropin-releasing hormone receptor binding by displacing the 50% of the bound tracer [125I]-[D-Lys6]-GnRH radioligand in rat pitui... | J Med Chem 44: 3645-52 (2001) BindingDB Entry DOI: 10.7270/Q2W66K2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50105863 (CHEMBL438099 | pGlu-His-Trp-Ser-Tyr-D-Lys(emodic a...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Evaluated for gonadotropin-releasing hormone receptor binding by displacing the 50% of the bound tracer [125I]-[D-Lys6]-GnRH radioligand in rat pitui... | J Med Chem 44: 3645-52 (2001) BindingDB Entry DOI: 10.7270/Q2W66K2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50422274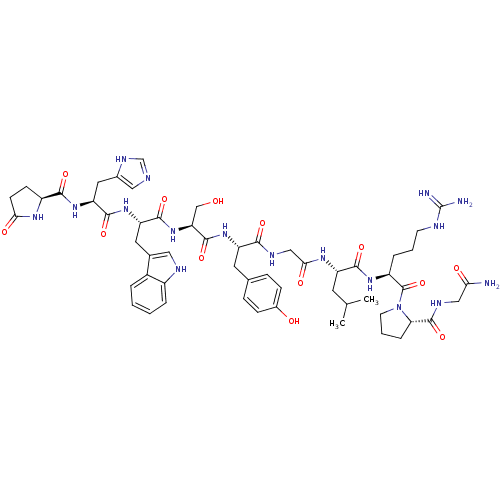 (GONADORELIN | Human gonadoliberin-i) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50257775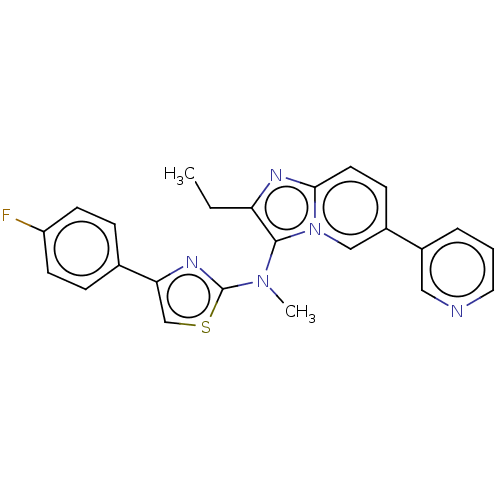 (CHEMBL4100462) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU , 102 Avenue Gaston Roussel, 93230 Romainville, France. Curated by ChEMBL | Assay Description Inhibition of glycosylated human ATX using LPC 16:0 as substrate after 30 mins by luminescence assay | J Med Chem 60: 7371-7392 (2017) Article DOI: 10.1021/acs.jmedchem.7b00647 BindingDB Entry DOI: 10.7270/Q2R213VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50257807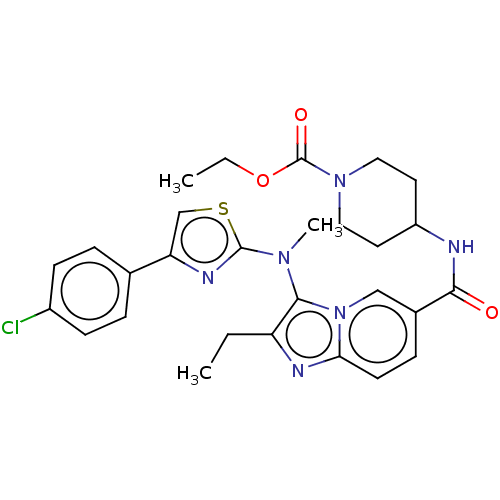 (CHEMBL4073638) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU , 102 Avenue Gaston Roussel, 93230 Romainville, France. Curated by ChEMBL | Assay Description Inhibition of glycosylated human ATX using LPC 16:0 as substrate after 30 mins by luminescence assay | J Med Chem 60: 7371-7392 (2017) Article DOI: 10.1021/acs.jmedchem.7b00647 BindingDB Entry DOI: 10.7270/Q2R213VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090745 (1-[2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50257776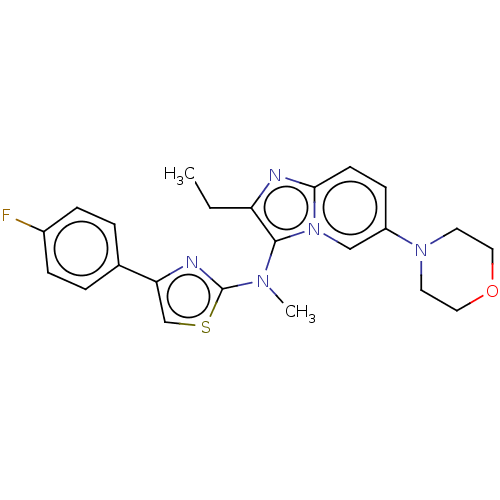 (CHEMBL4084005) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU , 102 Avenue Gaston Roussel, 93230 Romainville, France. Curated by ChEMBL | Assay Description Inhibition of glycosylated human ATX using LPC 16:0 as substrate after 30 mins by luminescence assay | J Med Chem 60: 7371-7392 (2017) Article DOI: 10.1021/acs.jmedchem.7b00647 BindingDB Entry DOI: 10.7270/Q2R213VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM50399411 (CHEMBL2178304 | PT405) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins | J Med Chem 55: 9914-28 (2012) Article DOI: 10.1021/jm301113w BindingDB Entry DOI: 10.7270/Q2H99699 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50257813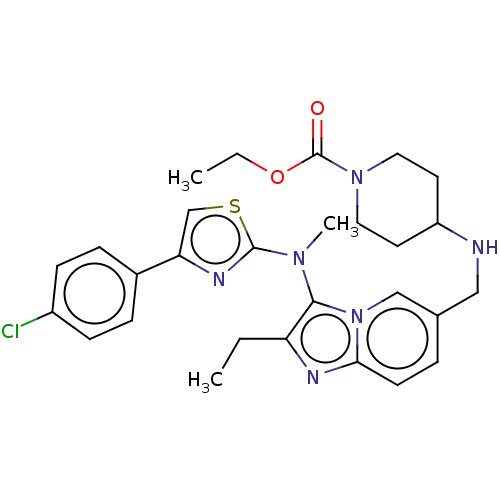 (CHEMBL4062291) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU , 102 Avenue Gaston Roussel, 93230 Romainville, France. Curated by ChEMBL | Assay Description Inhibition of glycosylated human ATX using FS-3 as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by fluore... | J Med Chem 60: 7371-7392 (2017) Article DOI: 10.1021/acs.jmedchem.7b00647 BindingDB Entry DOI: 10.7270/Q2R213VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090748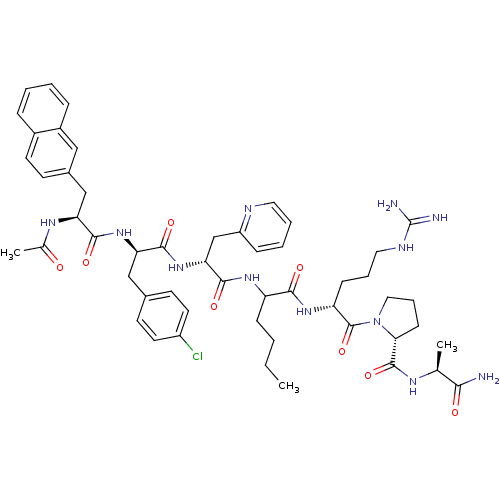 (1-[2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM50399407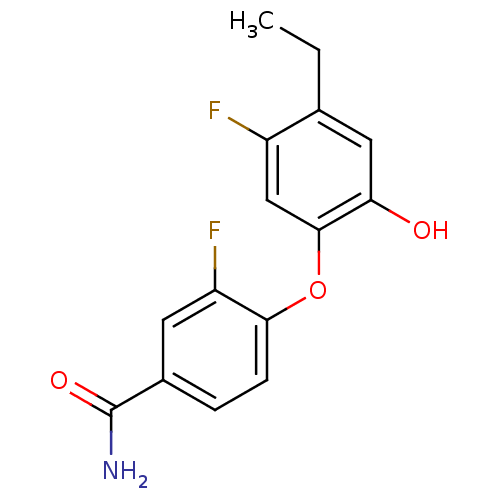 (CHEMBL2178284 | MUT056399) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins | J Med Chem 55: 9914-28 (2012) Article DOI: 10.1021/jm301113w BindingDB Entry DOI: 10.7270/Q2H99699 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM193000 (US10526329, Compound 180 | US11072611, Compound 18...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU , 102 Avenue Gaston Roussel, 93230 Romainville, France. Curated by ChEMBL | Assay Description Inhibition of glycosylated human ATX using FS-3 as substrate preincubated for 30 mins followed by substrate addition measured after 30 mins by fluore... | J Med Chem 60: 7371-7392 (2017) Article DOI: 10.1021/acs.jmedchem.7b00647 BindingDB Entry DOI: 10.7270/Q2R213VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM50399400 (CHEMBL2178291) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins | J Med Chem 55: 9914-28 (2012) Article DOI: 10.1021/jm301113w BindingDB Entry DOI: 10.7270/Q2H99699 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090736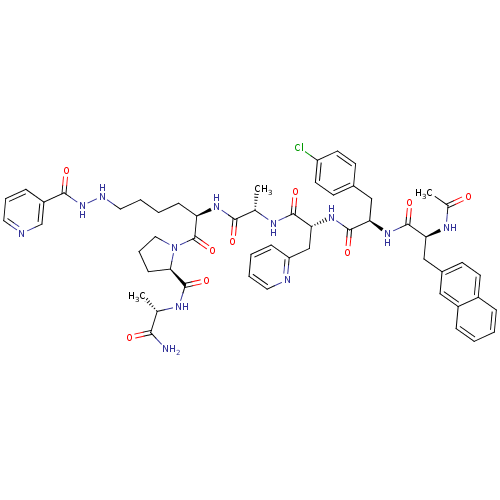 (1-{2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM50399404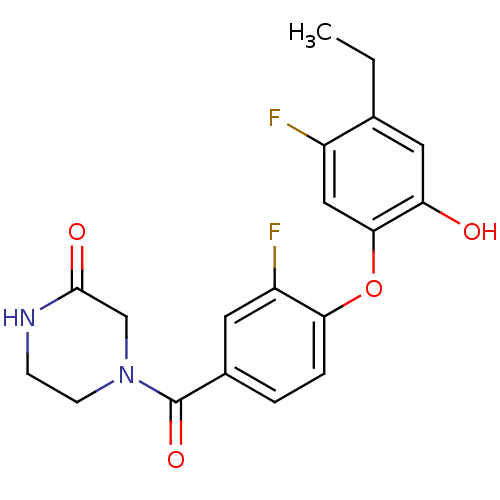 (CHEMBL2178287 | US8623865, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins | J Med Chem 55: 9914-28 (2012) Article DOI: 10.1021/jm301113w BindingDB Entry DOI: 10.7270/Q2H99699 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090732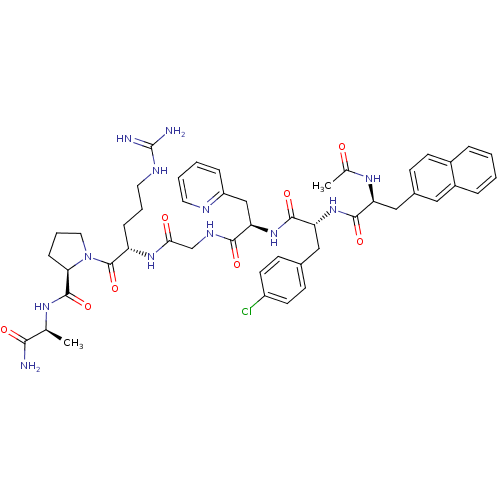 (1-[2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090724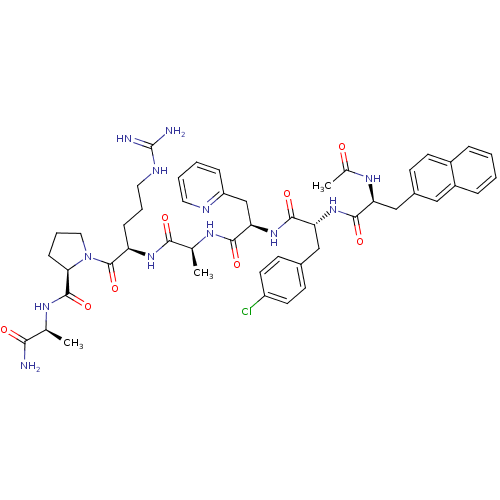 (1-[2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090730 (1-[2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50133022 (CHEMBL413854 | Compound GnRH-PpIX) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Ability to bind to Gonadotropin-releasing hormone receptor was evaluated invitro by displacement assay using [125I]-[D-Lys6]-GnRH as radioligand | J Med Chem 46: 3965-74 (2003) Article DOI: 10.1021/jm020535y BindingDB Entry DOI: 10.7270/Q2N58KR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM192946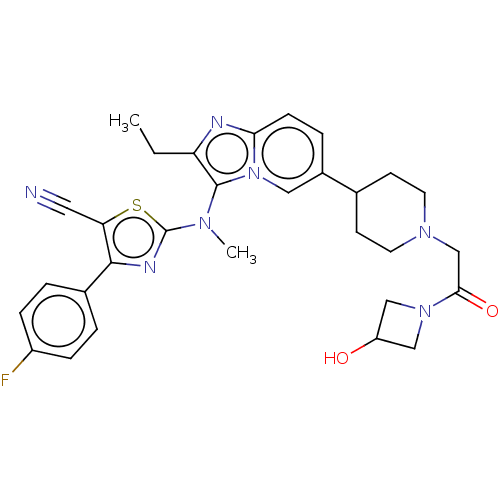 (US11072611, Compound 141 | US9670204, 141 2-((2-et...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU Curated by ChEMBL | Assay Description Inhibition of ATX in rat plasma assessed as reduction in LPA 18:2 production after 2 hrs by LC-MS/MS analysis | J Med Chem 60: 3580-3590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00032 BindingDB Entry DOI: 10.7270/Q25141PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Rattus norvegicus) | BDBM192946 (US11072611, Compound 141 | US9670204, 141 2-((2-et...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU , 102 Avenue Gaston Roussel, 93230 Romainville, France. Curated by ChEMBL | Assay Description Inhibition of ATX in rat plasma assessed as reduction in plasma lysophosphatidic acid 18:2 levels after 2 hrs by LC-MS/MS method | J Med Chem 60: 7371-7392 (2017) Article DOI: 10.1021/acs.jmedchem.7b00647 BindingDB Entry DOI: 10.7270/Q2R213VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090734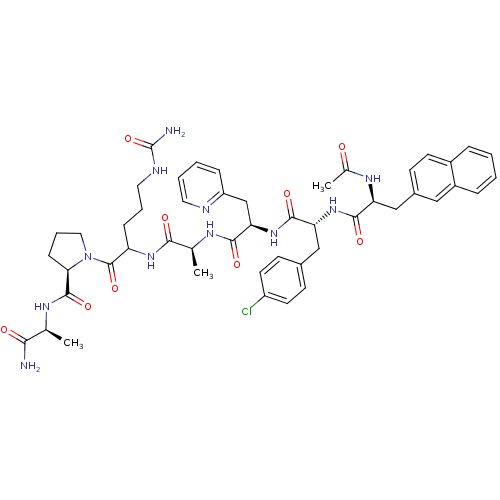 (1-[2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090723 (1-{2-[2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM192943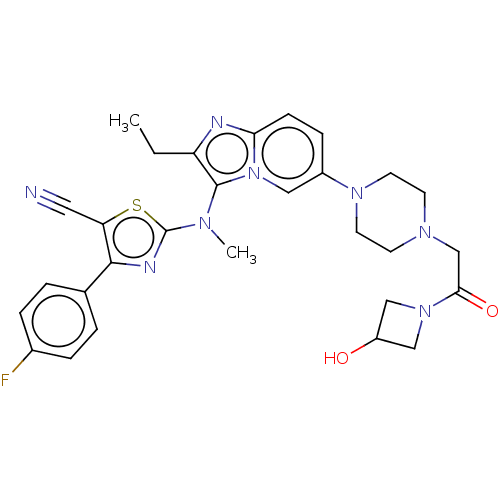 (US10526329, Compound 139 | US9670204, 138 2-((2-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU Curated by ChEMBL | Assay Description Inhibition of human ATX using LPC (16:0) as substrate after 30 mins by horseradish peroxidase/choline oxidase coupled enzyme based spectrophotometric... | J Med Chem 60: 3580-3590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00032 BindingDB Entry DOI: 10.7270/Q25141PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM192946 (US11072611, Compound 141 | US9670204, 141 2-((2-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU , 102 Avenue Gaston Roussel, 93230 Romainville, France. Curated by ChEMBL | Assay Description Inhibition of glycosylated human ATX using LPC 16:0 as substrate after 30 mins by luminescence assay | J Med Chem 60: 7371-7392 (2017) Article DOI: 10.1021/acs.jmedchem.7b00647 BindingDB Entry DOI: 10.7270/Q2R213VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM192946 (US11072611, Compound 141 | US9670204, 141 2-((2-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU , 102 Avenue Gaston Roussel, 93230 Romainville, France. Curated by ChEMBL | Assay Description Inhibition of glycosylated human ATX using LPC 16:0 as substrate after 30 mins by luminescence assay | J Med Chem 60: 7371-7392 (2017) Article DOI: 10.1021/acs.jmedchem.7b00647 BindingDB Entry DOI: 10.7270/Q2R213VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM192946 (US11072611, Compound 141 | US9670204, 141 2-((2-et...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Galapagos SASU Curated by ChEMBL | Assay Description Inhibition of human ATX using LPC (16:0) as substrate after 30 mins by horseradish peroxidase/choline oxidase coupled enzyme based spectrophotometric... | J Med Chem 60: 3580-3590 (2017) Article DOI: 10.1021/acs.jmedchem.7b00032 BindingDB Entry DOI: 10.7270/Q25141PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM50399405 (CHEMBL2178286 | US8623865, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins | J Med Chem 55: 9914-28 (2012) Article DOI: 10.1021/jm301113w BindingDB Entry DOI: 10.7270/Q2H99699 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090752 (1-[2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090728 (1-[2-(2-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM50399410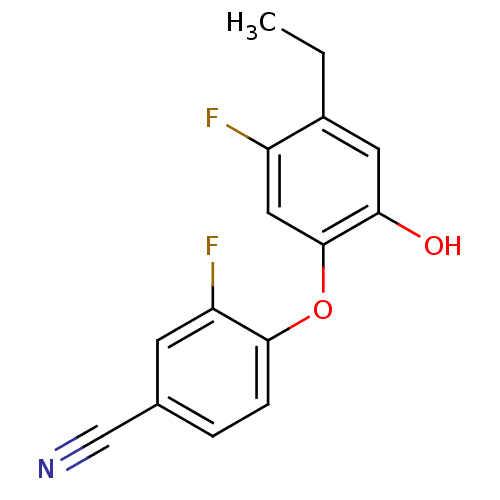 (CHEMBL2178305) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins | J Med Chem 55: 9914-28 (2012) Article DOI: 10.1021/jm301113w BindingDB Entry DOI: 10.7270/Q2H99699 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090725 (1-{2-[2-({2-[2-(2-Acetylamino-3-naphthalen-2-yl-pr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM50399412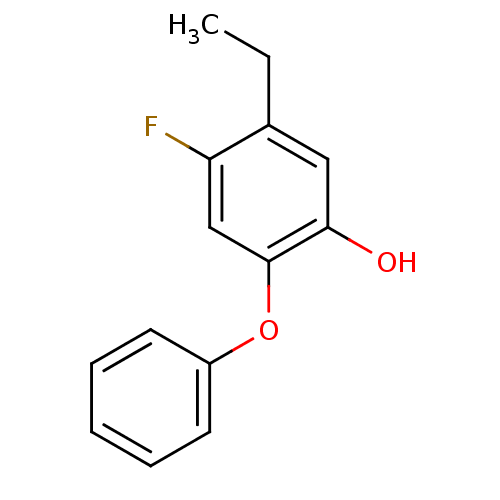 (CHEMBL2178303 | PT411) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Mutabilis Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus recombinant FabI using trans-2-octenoyl N-acetylcysteamine thioester as substrate preincubated for 60 mins | J Med Chem 55: 9914-28 (2012) Article DOI: 10.1021/jm301113w BindingDB Entry DOI: 10.7270/Q2H99699 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090737 (1-[2-(3-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystic fibrosis transmembrane conductance regulator (Homo sapiens (Human)) | BDBM300426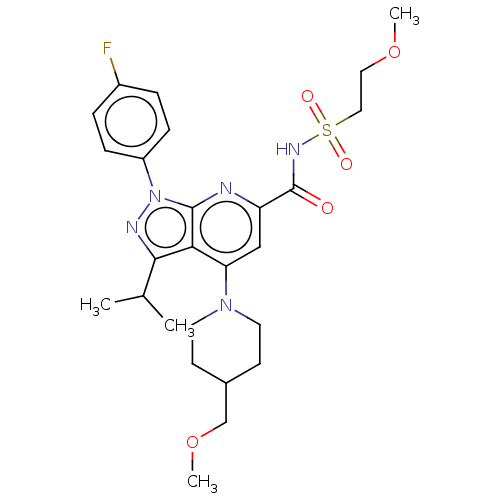 (US10130622, Example 00005) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie S.á.r.l.; Galapagos NV US Patent | Assay Description For this purpose, HEK293 cells are transfected with plasmid DNA containing WT CFTR and seeded in 96 well plates (70,000 HEK cells/well). Two days aft... | US Patent US10130622 (2018) BindingDB Entry DOI: 10.7270/Q2KD20ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50090727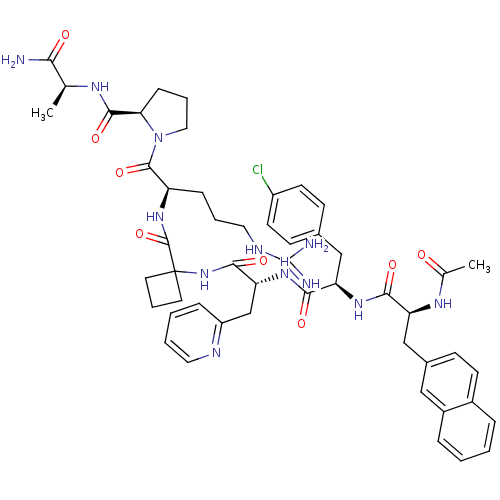 (1-{2-[(1-{2-[2-(2-Acetylamino-3-naphthalen-2-yl-pr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Concentration of the peptide which displaces 50% of 125 I-[D-Lys6] gonadotropin-releasing hormone bound to rat pituitary membranes | J Med Chem 43: 2831-6 (2000) BindingDB Entry DOI: 10.7270/Q29P30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1592 total ) | Next | Last >> |