

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (Cavia porcellus) | BDBM50406750 (Firazyr | HOE-140 | Icatibant) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. | J Med Chem 36: 1450-60 (1993) BindingDB Entry DOI: 10.7270/Q2PG1QS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50406751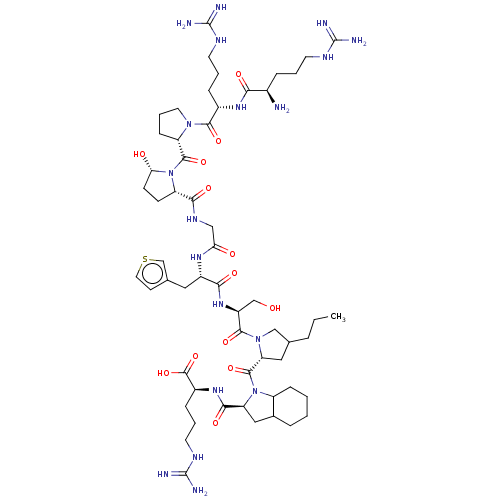 (CHEMBL2369941) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. | J Med Chem 36: 1450-60 (1993) BindingDB Entry DOI: 10.7270/Q2PG1QS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-U69,593 from KOR in guinea pig brain membranes incubated for 30 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50007838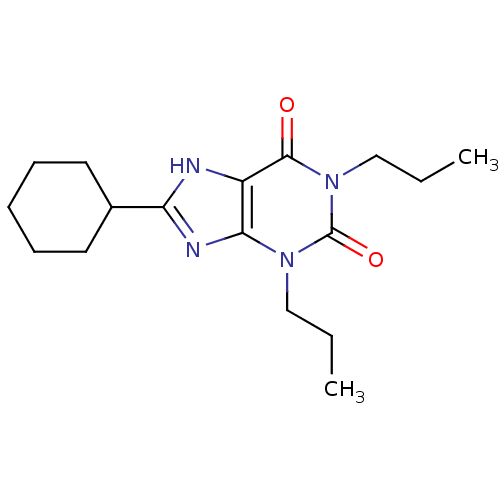 (8-Cyclohexyl-1,3-dipropyl-3,7-dihydro-purine-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82010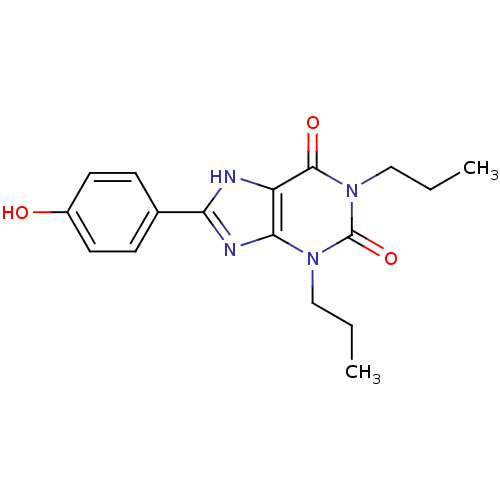 (1,3-Dipropyl-8-(4-hydroxyphenyl)xanthine | 8-(4-Hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50406749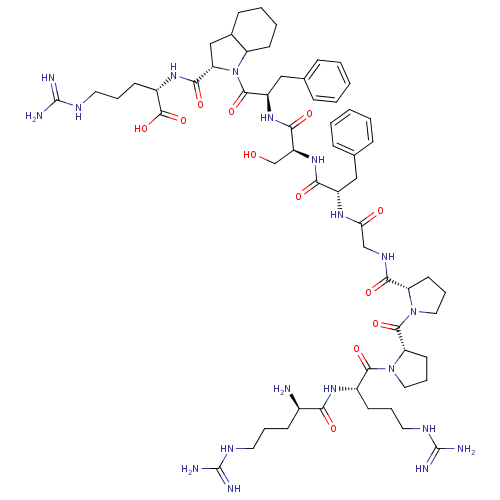 (CHEMBL2028979) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. | J Med Chem 36: 1450-60 (1993) BindingDB Entry DOI: 10.7270/Q2PG1QS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50370067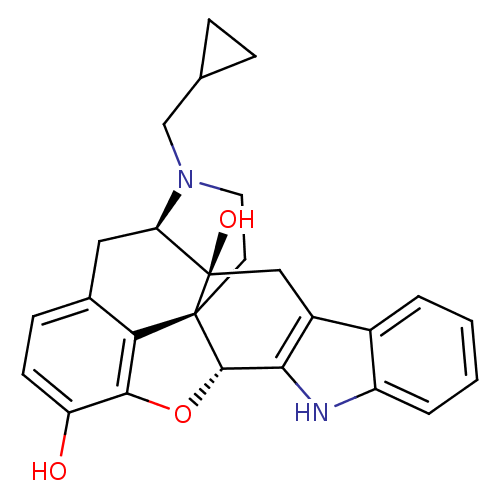 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from DOR in rat brain membranes incubated for 45 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011824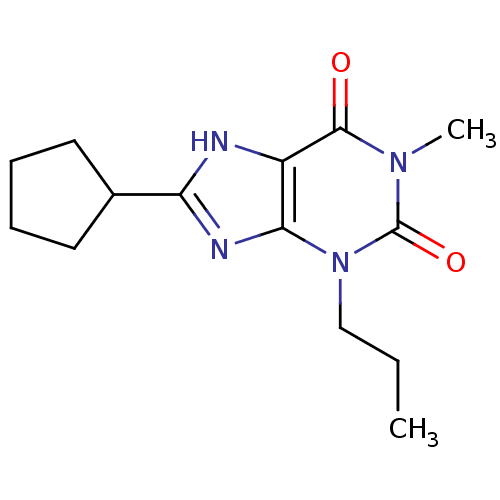 (8-Cyclopentyl-1-methyl-3-propyl-3,7-dihydro-purine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from MOR in rat brain membranes incubated for 45 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011842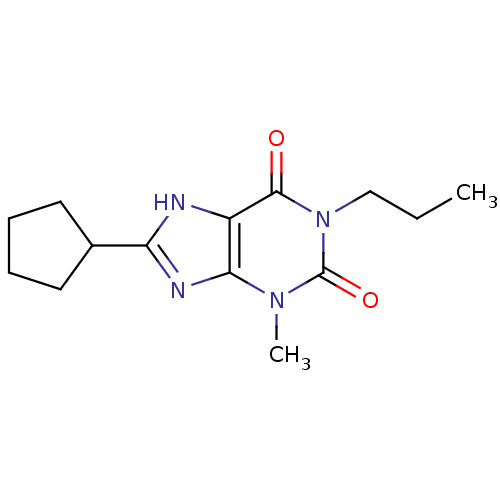 (8-Cyclopentyl-3-methyl-1-propyl-3,7-dihydro-purine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011838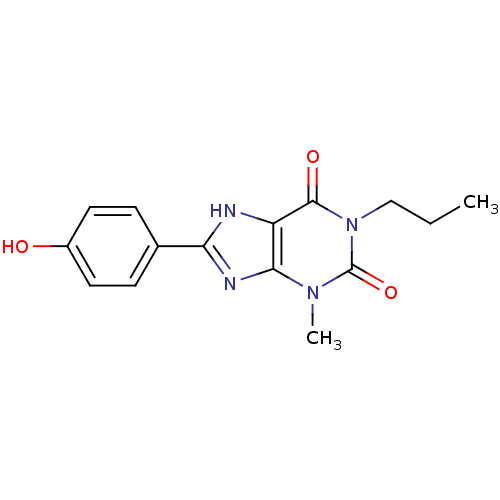 (8-(4-Hydroxy-phenyl)-3-methyl-1-propyl-3,7-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50446130 (AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115819 BindingDB Entry DOI: 10.7270/Q21N84R1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585913 (CHEMBL5093295) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011835 (8-Cyclohexyl-1-methyl-3-propyl-3,7-dihydro-purine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011825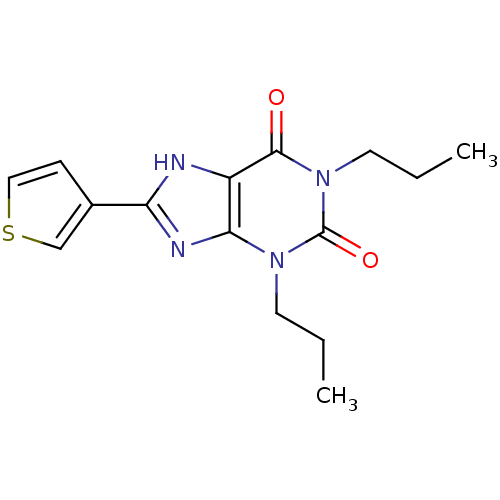 (1,3-Dipropyl-8-thiophen-3-yl-3,7-dihydro-purine-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585914 (CHEMBL5079273) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 1 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011836 (8-(4-Hydroxy-phenyl)-1-methyl-3-propyl-3,7-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423452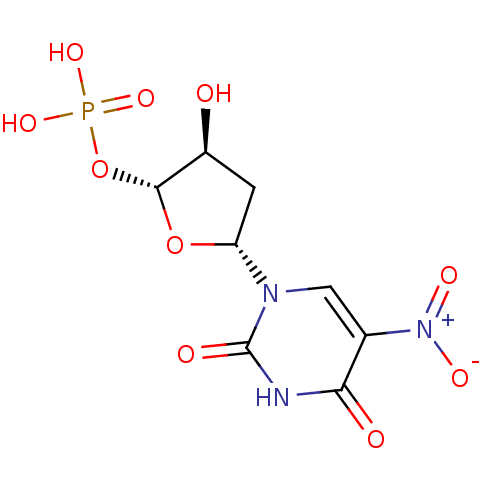 (CHEMBL401166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM82025 (1,3-Dipropyl-8-phenylxanthine | 8-Phenyl-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50084626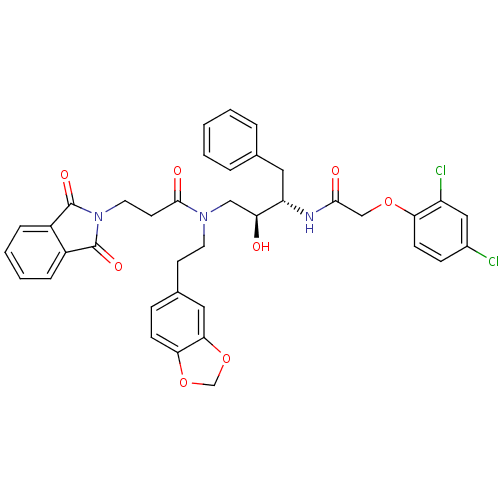 (CHEMBL284440 | N-(2-Benzo[1,3]dioxol-5-yl-ethyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585912 (CHEMBL5075486) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011841 (1,3-Dipropyl-8-pyridin-4-yl-3,7-dihydro-purine-2,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50005366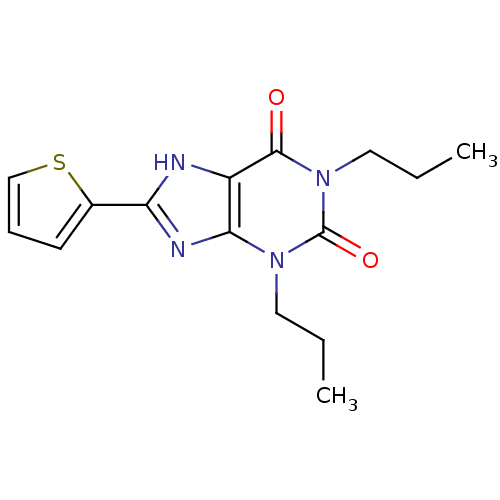 (1,3-Dipropyl-8-thiophen-2-yl-3,7-dihydro-purine-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor in rat cortex by displacement of [3H]-cyclohexyladenosine (CHA) | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585909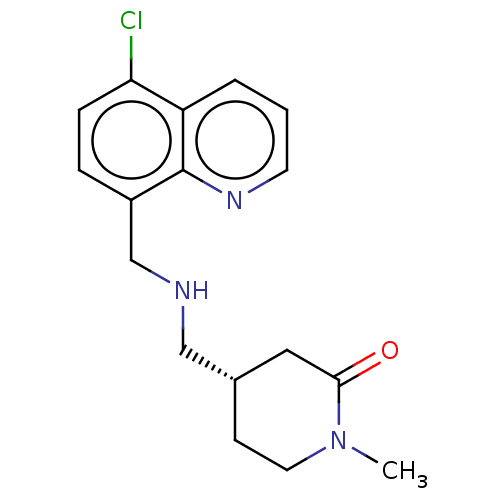 (CHEMBL5089996) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011833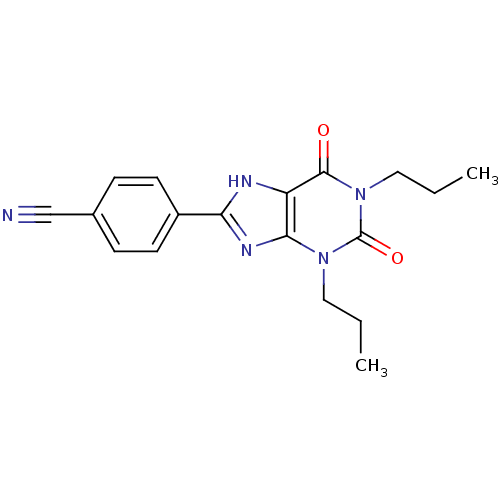 (4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-pu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011846 (1-Methyl-3-propyl-8-thiophen-3-yl-3,7-dihydro-puri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor in rat cortex by displacement of [3H]-cyclohexyladenosine (CHA) | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011839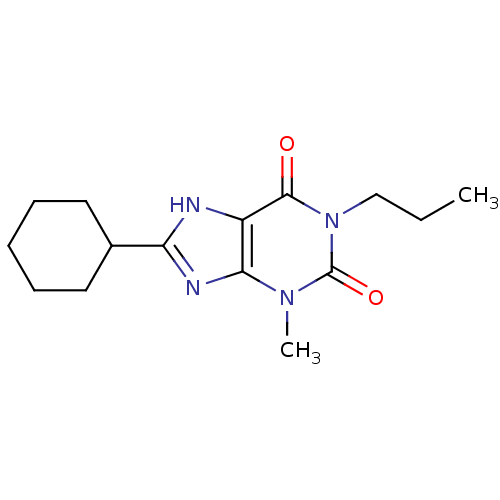 (8-Cyclohexyl-3-methyl-1-propyl-3,7-dihydro-purine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50110934 (CHEMBL30483 | N-((1S,3S)-1-Benzyl-3-{[2-(2,4-dichl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of compound against human Cathepsin D | J Med Chem 45: 1412-9 (2002) BindingDB Entry DOI: 10.7270/Q25T3JSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011845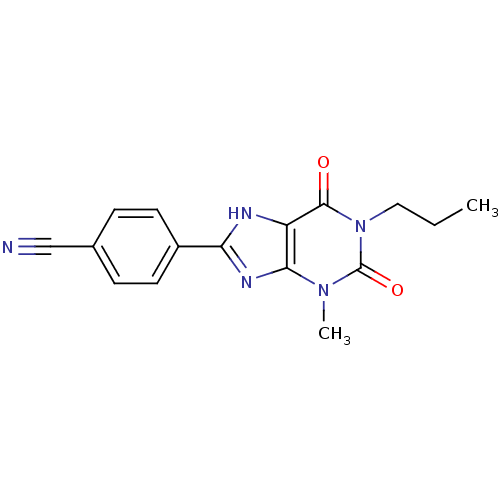 (4-(3-Methyl-2,6-dioxo-1-propyl-2,3,6,7-tetrahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50540611 (CHEMBL4643466) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from MOR in rat brain membranes incubated for 45 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011840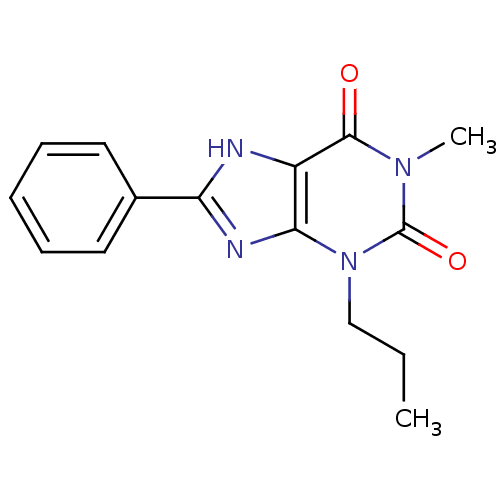 (1-Methyl-8-phenyl-3-propyl-3,7-dihydro-purine-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011843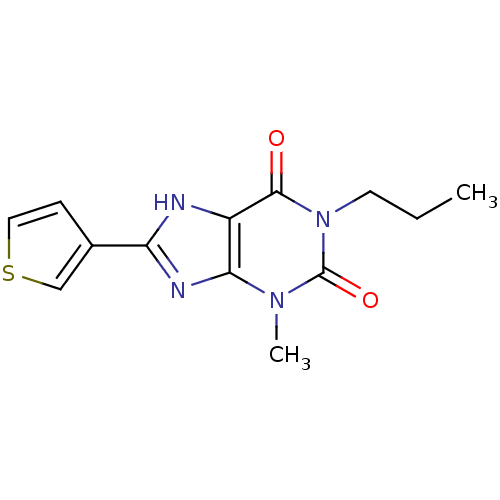 (3-Methyl-1-propyl-8-thiophen-3-yl-3,7-dihydro-puri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor in rat cortex by displacement of [3H]-cyclohexyladenosine (CHA) | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585911 (CHEMBL5093969) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50585910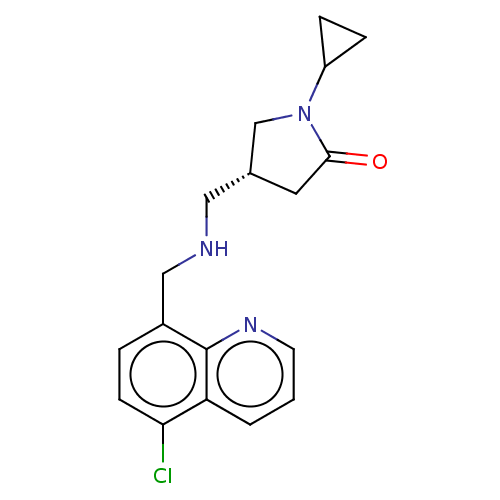 (CHEMBL5094012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-5-CT from human 5-HT5A receptor at 32 uM incubated for 2 hr by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02031 BindingDB Entry DOI: 10.7270/Q2G73JM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011834 (3-Methyl-8-phenyl-1-propyl-3,7-dihydro-purine-2,6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50540598 (CHEMBL4633897) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from MOR in rat brain membranes incubated for 45 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50540602 (CHEMBL4642448) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from MOR in rat brain membranes incubated for 45 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Homo sapiens (Human)) | BDBM85807 (Beta-PEA | CAS_60-12-8 | NSC_6054) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by PDSP Ki Database | Proc Natl Acad Sci U S A 98: 8966-71 (2001) Article DOI: 10.1073/pnas.151105198 BindingDB Entry DOI: 10.7270/Q2G15ZD5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50423448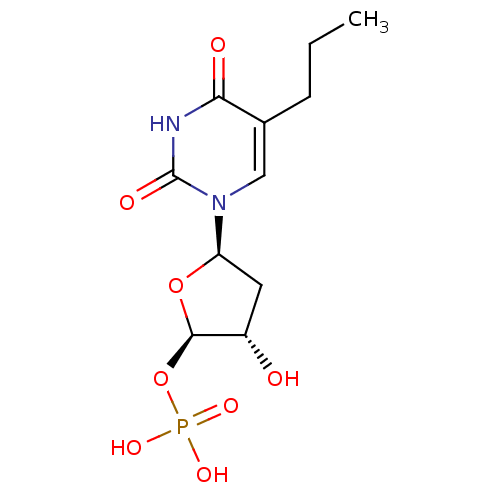 (CHEMBL254204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of mouse thymidylate synthase in mouse L1210 cells | Bioorg Med Chem 15: 2346-58 (2007) Article DOI: 10.1016/j.bmc.2007.01.021 BindingDB Entry DOI: 10.7270/Q21G0NK7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011828 (1-Methyl-3-propyl-8-thiophen-2-yl-3,7-dihydro-puri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011848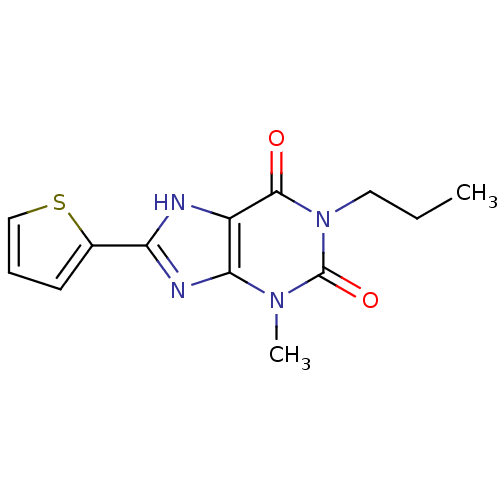 (3-Methyl-1-propyl-8-thiophen-2-yl-3,7-dihydro-puri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor in rat cortex by displacement of [3H]-cyclohexyladenosine (CHA) | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011849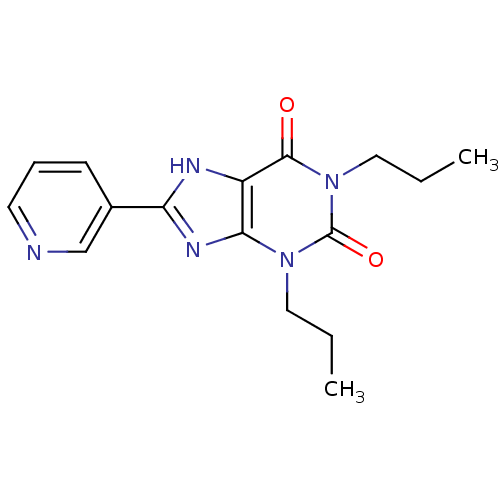 (1,3-Dipropyl-8-pyridin-3-yl-3,7-dihydro-purine-2,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor in rat cortex by displacement of [3H]-cyclohexyladenosine (CHA) | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011844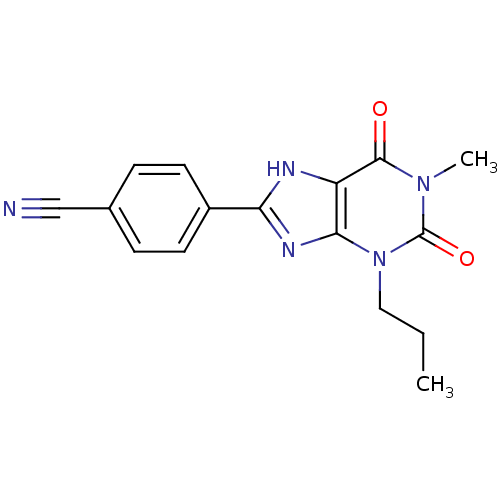 (4-(1-Methyl-2,6-dioxo-3-propyl-2,3,6,7-tetrahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50540601 (CHEMBL4641938) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from MOR in rat brain membranes incubated for 45 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50540600 (CHEMBL4638339) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from MOR in rat brain membranes incubated for 45 mins by liquid scintillation counting | ACS Med Chem Lett 11: 678-685 (2020) Article DOI: 10.1021/acsmedchemlett.9b00549 BindingDB Entry DOI: 10.7270/Q2Q81HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50006713 (1,3-Dipropyl-8-styryl-3,7-dihydro-purine-2,6-dione...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50011829 (1-Methyl-3-propyl-8-pyridin-4-yl-3,7-dihydro-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nova Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat cortex by the displacement of [3H]-cyclohexyladenosine (CHA). | J Med Chem 34: 1431-5 (1991) BindingDB Entry DOI: 10.7270/Q2G73CP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonuclease pancreatic (Homo sapiens (Human)) | BDBM50402338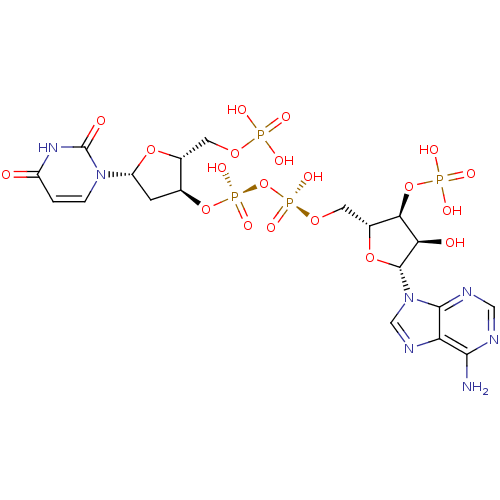 (CHEMBL401150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly Curated by ChEMBL | Assay Description Inhibition of RNase A | Bioorg Med Chem 20: 7184-93 (2012) Article DOI: 10.1016/j.bmc.2012.09.067 BindingDB Entry DOI: 10.7270/Q2M61MDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2094 total ) | Next | Last >> |