 Found 64 hits with Last Name = 'thanigaimalai' and Initial = 'p'
Found 64 hits with Last Name = 'thanigaimalai' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356166
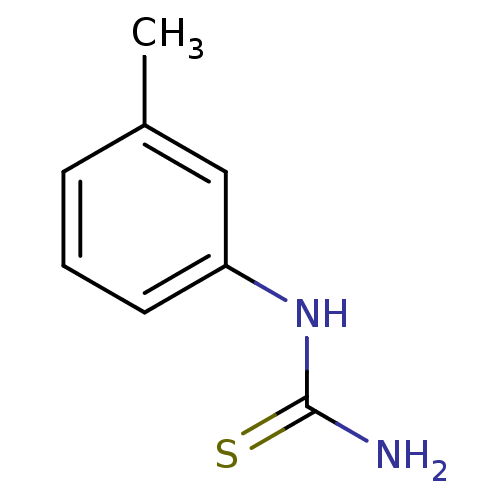
(CHEMBL1088657)Show InChI InChI=1S/C8H10N2S/c1-6-3-2-4-7(5-6)10-8(9)11/h2-5H,1H3,(H3,9,10,11) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356164
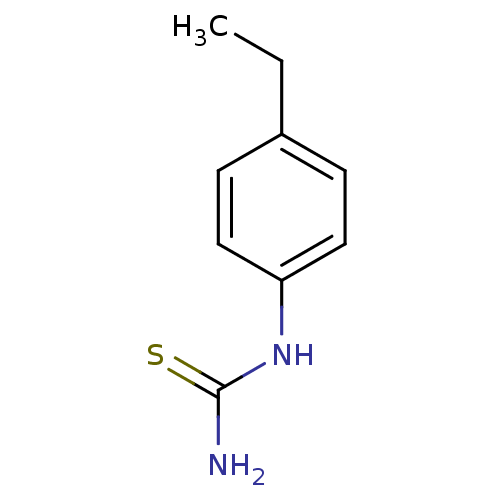
(CHEMBL1088361)Show InChI InChI=1S/C9H12N2S/c1-2-7-3-5-8(6-4-7)11-9(10)12/h3-6H,2H2,1H3,(H3,10,11,12) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356167
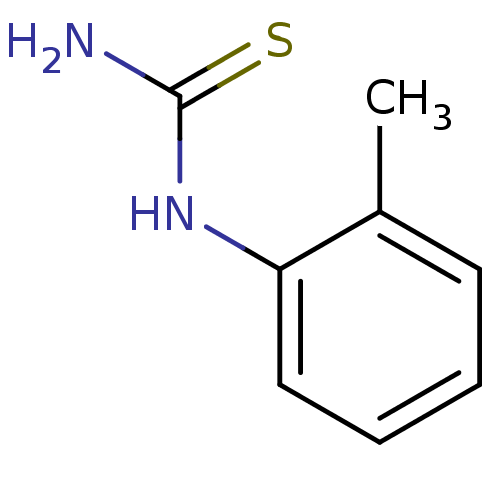
(CHEMBL1088658)Show InChI InChI=1S/C8H10N2S/c1-6-4-2-3-5-7(6)10-8(9)11/h2-5H,1H3,(H3,9,10,11) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50242276
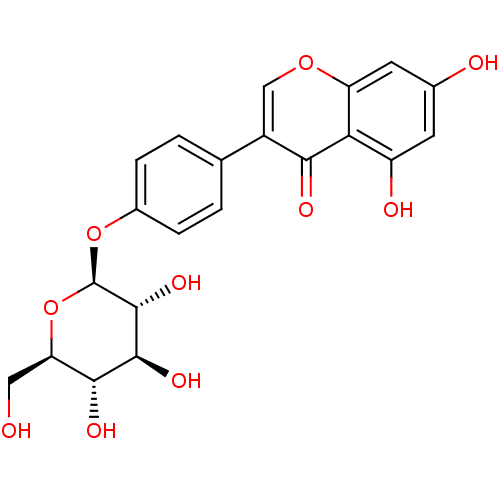
(CHEMBL486626 | Sophoricoside)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(cc2)-c2coc3cc(O)cc(O)c3c2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O10/c22-7-15-18(26)19(27)20(28)21(31-15)30-11-3-1-9(2-4-11)12-8-29-14-6-10(23)5-13(24)16(14)17(12)25/h1-6,8,15,18-24,26-28H,7H2/t15-,18-,19+,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50242276

(CHEMBL486626 | Sophoricoside)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(cc2)-c2coc3cc(O)cc(O)c3c2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O10/c22-7-15-18(26)19(27)20(28)21(31-15)30-11-3-1-9(2-4-11)12-8-29-14-6-10(23)5-13(24)16(14)17(12)25/h1-6,8,15,18-24,26-28H,7H2/t15-,18-,19+,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356163
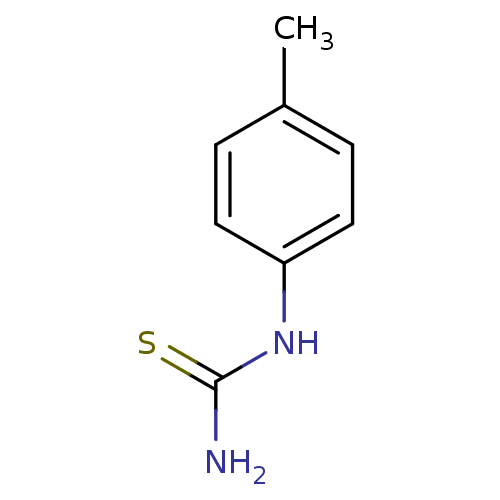
(CHEMBL1088360)Show InChI InChI=1S/C8H10N2S/c1-6-2-4-7(5-3-6)10-8(9)11/h2-5H,1H3,(H3,9,10,11) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50242276

(CHEMBL486626 | Sophoricoside)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(cc2)-c2coc3cc(O)cc(O)c3c2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O10/c22-7-15-18(26)19(27)20(28)21(31-15)30-11-3-1-9(2-4-11)12-8-29-14-6-10(23)5-13(24)16(14)17(12)25/h1-6,8,15,18-24,26-28H,7H2/t15-,18-,19+,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50240041
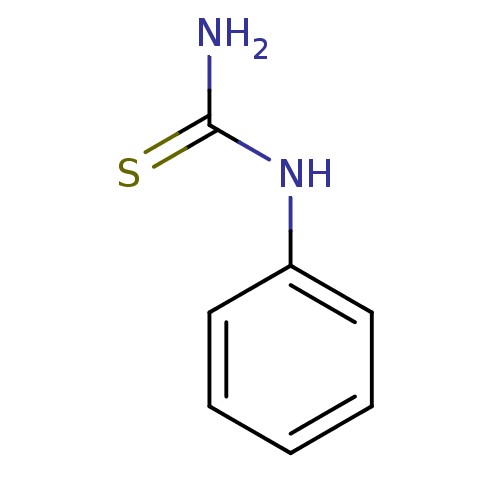
(1-phenyl-2-thiourea | 1-phenylthiourea | CHEMBL263...)Show InChI InChI=1S/C7H8N2S/c8-7(10)9-6-4-2-1-3-5-6/h1-5H,(H3,8,9,10) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356165
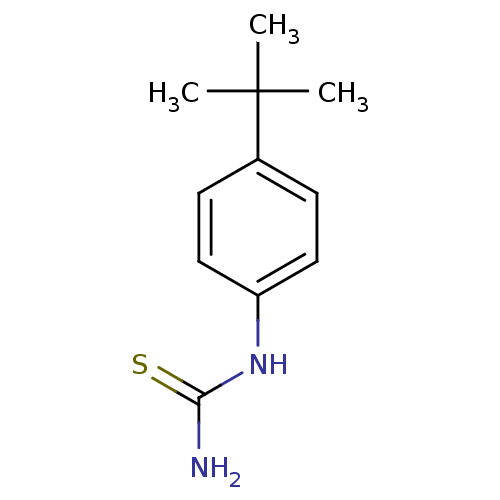
(CHEMBL1088498)Show InChI InChI=1S/C11H16N2S/c1-11(2,3)8-4-6-9(7-5-8)13-10(12)14/h4-7H,1-3H3,(H3,12,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50240041

(1-phenyl-2-thiourea | 1-phenylthiourea | CHEMBL263...)Show InChI InChI=1S/C7H8N2S/c8-7(10)9-6-4-2-1-3-5-6/h1-5H,(H3,8,9,10) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase |
Bioorg Med Chem 18: 1555-62 (2010)
Article DOI: 10.1016/j.bmc.2010.01.005
BindingDB Entry DOI: 10.7270/Q23F4PQ7 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50240041

(1-phenyl-2-thiourea | 1-phenylthiourea | CHEMBL263...)Show InChI InChI=1S/C7H8N2S/c8-7(10)9-6-4-2-1-3-5-6/h1-5H,(H3,8,9,10) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in alpha-MSH-induced mouse B16 cells |
Bioorg Med Chem Lett 20: 4771-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.123
BindingDB Entry DOI: 10.7270/Q27M0BR0 |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320916
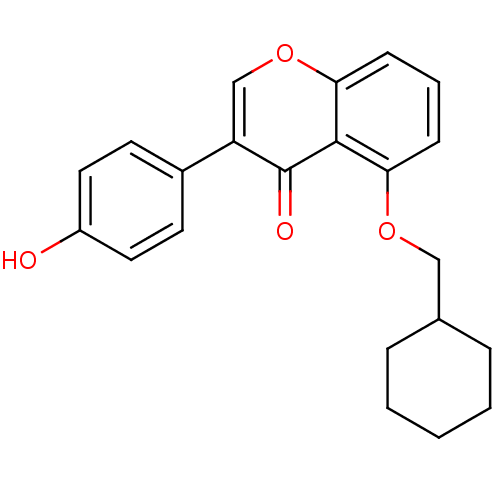
(5-(cyclohexylmethoxy)-3-(4-hydroxyphenyl)-4H-chrom...)Show InChI InChI=1S/C22H22O4/c23-17-11-9-16(10-12-17)18-14-26-20-8-4-7-19(21(20)22(18)24)25-13-15-5-2-1-3-6-15/h4,7-12,14-15,23H,1-3,5-6,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320923
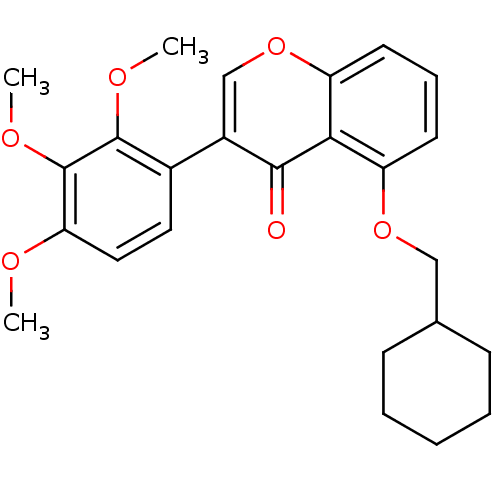
(5-(Cyclohexylmethoxy)-3-(3,4,5-trimethoxyphenyl)-4...)Show SMILES COc1ccc(c(OC)c1OC)-c1coc2cccc(OCC3CCCCC3)c2c1=O Show InChI InChI=1S/C25H28O6/c1-27-21-13-12-17(24(28-2)25(21)29-3)18-15-31-20-11-7-10-19(22(20)23(18)26)30-14-16-8-5-4-6-9-16/h7,10-13,15-16H,4-6,8-9,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321929

(3-((1-Cyclohexyl-3-hydroxypropan-2-ylamino)methyl)...)Show SMILES OCC(CC1CCCCC1)NCc1coc2cccc(OCC3CCCCC3)c2c1=O Show InChI InChI=1S/C26H37NO4/c28-16-22(14-19-8-3-1-4-9-19)27-15-21-18-31-24-13-7-12-23(25(24)26(21)29)30-17-20-10-5-2-6-11-20/h7,12-13,18-20,22,27-28H,1-6,8-11,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320921
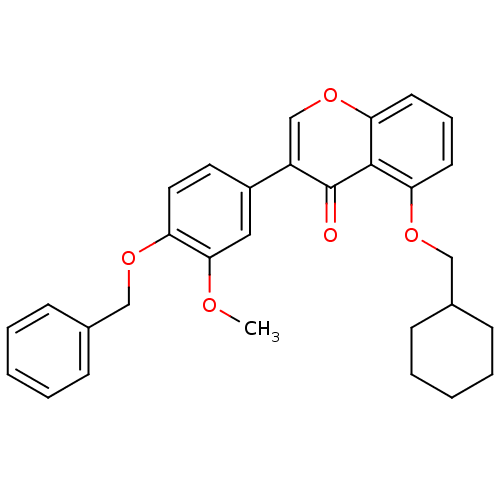
(3-(4-(Benzyloxy)-3-methoxyphenyl)-5-(cyclohexylmet...)Show SMILES COc1cc(ccc1OCc1ccccc1)-c1coc2cccc(OCC3CCCCC3)c2c1=O Show InChI InChI=1S/C30H30O5/c1-32-28-17-23(15-16-25(28)33-18-21-9-4-2-5-10-21)24-20-35-27-14-8-13-26(29(27)30(24)31)34-19-22-11-6-3-7-12-22/h2,4-5,8-10,13-17,20,22H,3,6-7,11-12,18-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321926
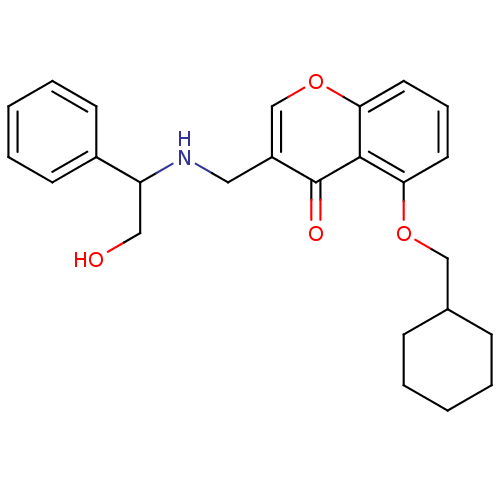
(5-(Cyclohexylmethoxy)-3-((2-hydroxy-1-phenylethyla...)Show SMILES OCC(NCc1coc2cccc(OCC3CCCCC3)c2c1=O)c1ccccc1 Show InChI InChI=1S/C25H29NO4/c27-15-21(19-10-5-2-6-11-19)26-14-20-17-30-23-13-7-12-22(24(23)25(20)28)29-16-18-8-3-1-4-9-18/h2,5-7,10-13,17-18,21,26-27H,1,3-4,8-9,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356170
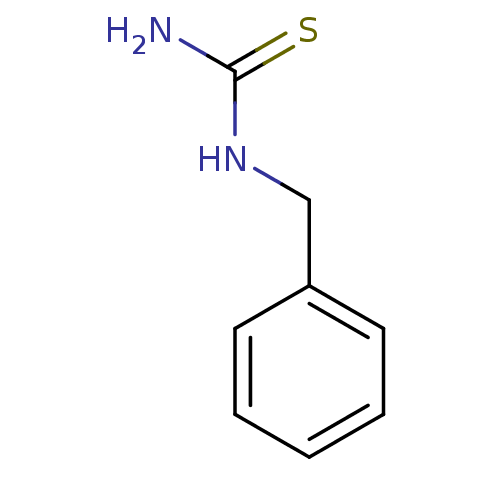
(CHEMBL1087841)Show InChI InChI=1S/C8H10N2S/c9-8(11)10-6-7-4-2-1-3-5-7/h1-5H,6H2,(H3,9,10,11) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321928
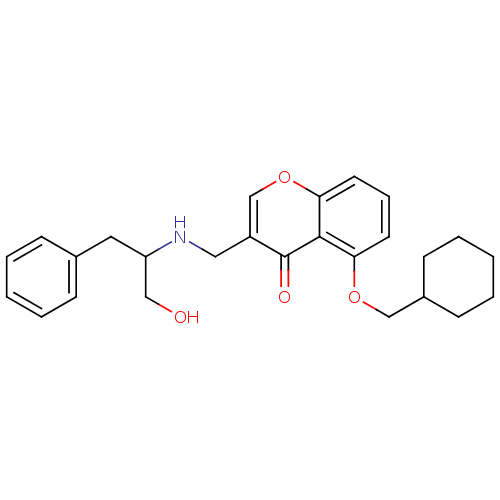
(5-(Cyclohexylmethoxy)-3-((1-hydroxy-3-phenylpropan...)Show SMILES OCC(Cc1ccccc1)NCc1coc2cccc(OCC3CCCCC3)c2c1=O Show InChI InChI=1S/C26H31NO4/c28-16-22(14-19-8-3-1-4-9-19)27-15-21-18-31-24-13-7-12-23(25(24)26(21)29)30-17-20-10-5-2-6-11-20/h1,3-4,7-9,12-13,18,20,22,27-28H,2,5-6,10-11,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50354850

(BUDESONIDE | US10869929, Compound Budesonide | US1...)Show SMILES CCCC1O[C@@H]2C[C@H]3[C@@H]4CCC5=CC(=O)C=C[C@]5(C)[C@H]4[C@@H](O)C[C@]3(C)[C@@]2(O1)C(=O)CO |r,c:15,t:11| Show InChI InChI=1S/C25H34O6/c1-4-5-21-30-20-11-17-16-7-6-14-10-15(27)8-9-23(14,2)22(16)18(28)12-24(17,3)25(20,31-21)19(29)13-26/h8-10,16-18,20-22,26,28H,4-7,11-13H2,1-3H3/t16-,17-,18-,20+,21?,22+,23-,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50354850

(BUDESONIDE | US10869929, Compound Budesonide | US1...)Show SMILES CCCC1O[C@@H]2C[C@H]3[C@@H]4CCC5=CC(=O)C=C[C@]5(C)[C@H]4[C@@H](O)C[C@]3(C)[C@@]2(O1)C(=O)CO |r,c:15,t:11| Show InChI InChI=1S/C25H34O6/c1-4-5-21-30-20-11-17-16-7-6-14-10-15(27)8-9-23(14,2)22(16)18(28)12-24(17,3)25(20,31-21)19(29)13-26/h8-10,16-18,20-22,26,28H,4-7,11-13H2,1-3H3/t16-,17-,18-,20+,21?,22+,23-,24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321941
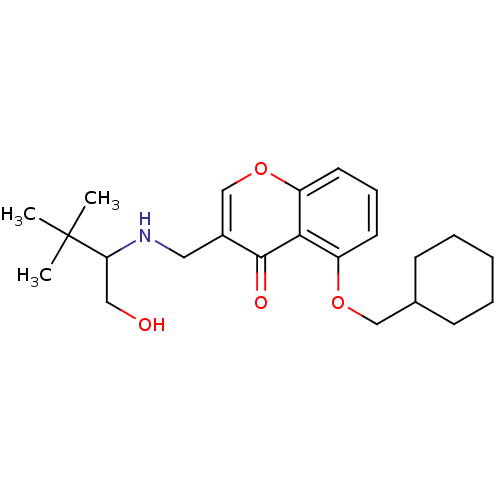
(5-(Cyclohexylmethoxy)-3-((1-hydroxy-3,3-dimethylbu...)Show SMILES CC(C)(C)C(CO)NCc1coc2cccc(OCC3CCCCC3)c2c1=O Show InChI InChI=1S/C23H33NO4/c1-23(2,3)20(13-25)24-12-17-15-28-19-11-7-10-18(21(19)22(17)26)27-14-16-8-5-4-6-9-16/h7,10-11,15-16,20,24-25H,4-6,8-9,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321940
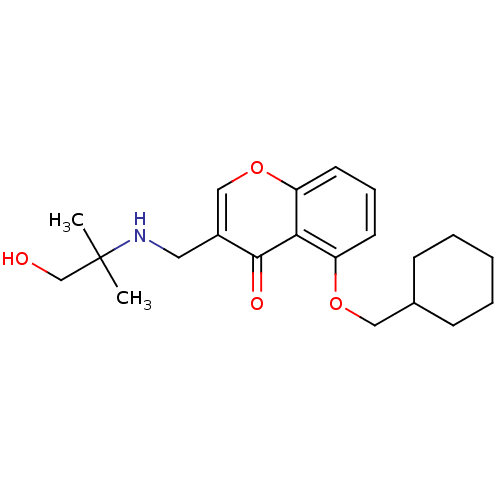
(5-(Cyclohexylmethoxy)-3-((1-hydroxy-2-methylpropan...)Show InChI InChI=1S/C21H29NO4/c1-21(2,14-23)22-11-16-13-26-18-10-6-9-17(19(18)20(16)24)25-12-15-7-4-3-5-8-15/h6,9-10,13,15,22-23H,3-5,7-8,11-12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Homo sapiens (Human)) | BDBM50354850

(BUDESONIDE | US10869929, Compound Budesonide | US1...)Show SMILES CCCC1O[C@@H]2C[C@H]3[C@@H]4CCC5=CC(=O)C=C[C@]5(C)[C@H]4[C@@H](O)C[C@]3(C)[C@@]2(O1)C(=O)CO |r,c:15,t:11| Show InChI InChI=1S/C25H34O6/c1-4-5-21-30-20-11-17-16-7-6-14-10-15(27)8-9-23(14,2)22(16)18(28)12-24(17,3)25(20,31-21)19(29)13-26/h8-10,16-18,20-22,26,28H,4-7,11-13H2,1-3H3/t16-,17-,18-,20+,21?,22+,23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356168
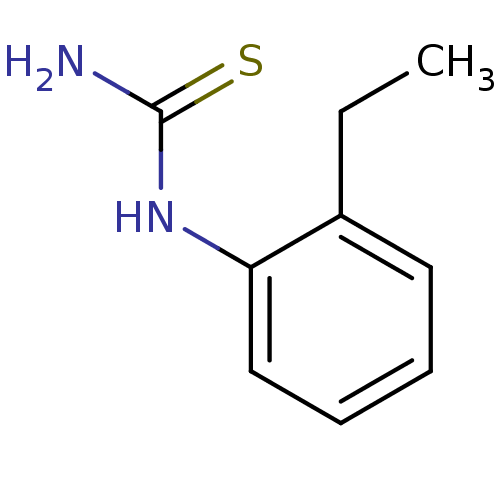
(CHEMBL1087472)Show InChI InChI=1S/C9H12N2S/c1-2-7-5-3-4-6-8(7)11-9(10)12/h3-6H,2H2,1H3,(H3,10,11,12) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321935
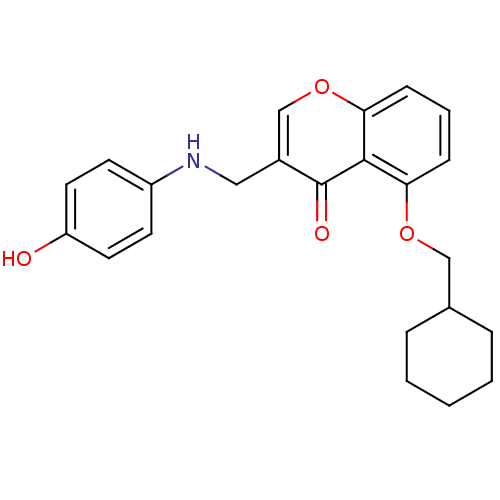
(5-(Cyclohexylmethoxy)-3-((4-hydroxyphenylamino)-me...)Show InChI InChI=1S/C23H25NO4/c25-19-11-9-18(10-12-19)24-13-17-15-28-21-8-4-7-20(22(21)23(17)26)27-14-16-5-2-1-3-6-16/h4,7-12,15-16,24-25H,1-3,5-6,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321932

(5-(Cyclohexylmethoxy)-3-((4-hydroxybenzylamino)met...)Show InChI InChI=1S/C24H27NO4/c26-20-11-9-17(10-12-20)13-25-14-19-16-29-22-8-4-7-21(23(22)24(19)27)28-15-18-5-2-1-3-6-18/h4,7-12,16,18,25-26H,1-3,5-6,13-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320925
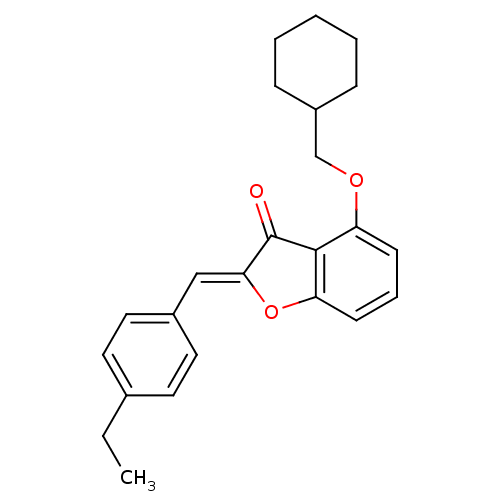
((Z)-4-(cyclohexylmethoxy)-2-(4-ethylbenzylidene)be...)Show SMILES CCc1ccc(\C=C2/Oc3cccc(OCC4CCCCC4)c3C2=O)cc1 Show InChI InChI=1S/C24H26O3/c1-2-17-11-13-18(14-12-17)15-22-24(25)23-20(9-6-10-21(23)27-22)26-16-19-7-4-3-5-8-19/h6,9-15,19H,2-5,7-8,16H2,1H3/b22-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320924

(5-(Cyclohexylmethoxy)-3-(4-ethylphenyl)-4H-chromen...)Show InChI InChI=1S/C24H26O3/c1-2-17-11-13-19(14-12-17)20-16-27-22-10-6-9-21(23(22)24(20)25)26-15-18-7-4-3-5-8-18/h6,9-14,16,18H,2-5,7-8,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320922
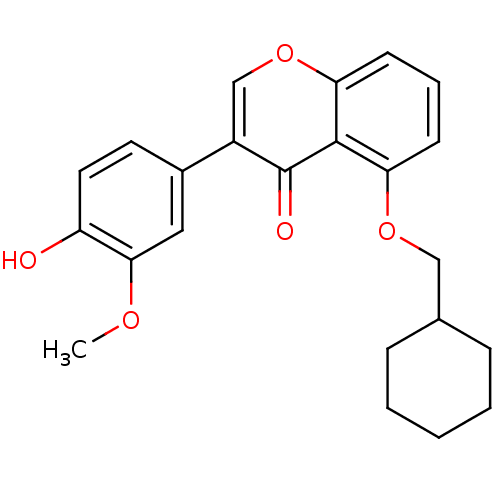
(5-(Cyclohexylmethoxy)-3-(4-hydroxy-3-methoxyphenyl...)Show InChI InChI=1S/C23H24O5/c1-26-21-12-16(10-11-18(21)24)17-14-28-20-9-5-8-19(22(20)23(17)25)27-13-15-6-3-2-4-7-15/h5,8-12,14-15,24H,2-4,6-7,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320917
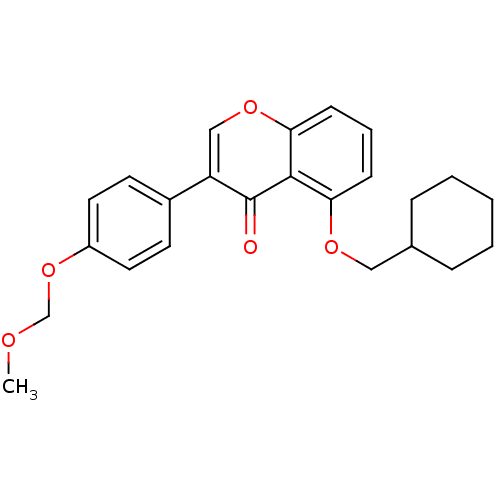
(5-(Cyclohexylmethoxy)-3-[4-(methoxymethoxy)phenyl]...)Show SMILES COCOc1ccc(cc1)-c1coc2cccc(OCC3CCCCC3)c2c1=O Show InChI InChI=1S/C24H26O5/c1-26-16-29-19-12-10-18(11-13-19)20-15-28-22-9-5-8-21(23(22)24(20)25)27-14-17-6-3-2-4-7-17/h5,8-13,15,17H,2-4,6-7,14,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320930
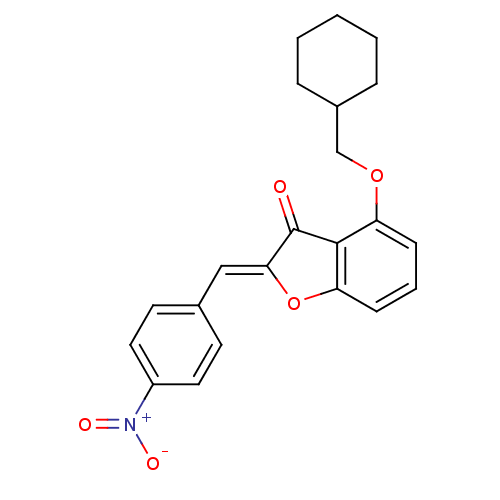
((Z)-4-(Cyclohexylmethoxy)-2-(4-nitrobenzylidene)be...)Show SMILES [O-][N+](=O)c1ccc(\C=C2/Oc3cccc(OCC4CCCCC4)c3C2=O)cc1 Show InChI InChI=1S/C22H21NO5/c24-22-20(13-15-9-11-17(12-10-15)23(25)26)28-19-8-4-7-18(21(19)22)27-14-16-5-2-1-3-6-16/h4,7-13,16H,1-3,5-6,14H2/b20-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320929
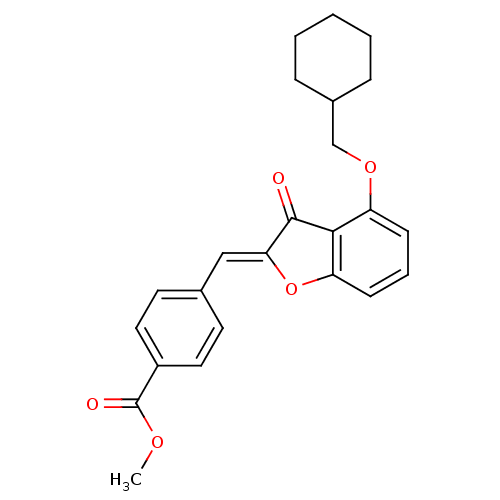
(CHEMBL1165763 | Methyl(Z)-4-[4-(cyclohexylmethoxy)...)Show SMILES COC(=O)c1ccc(\C=C2/Oc3cccc(OCC4CCCCC4)c3C2=O)cc1 Show InChI InChI=1S/C24H24O5/c1-27-24(26)18-12-10-16(11-13-18)14-21-23(25)22-19(8-5-9-20(22)29-21)28-15-17-6-3-2-4-7-17/h5,8-14,17H,2-4,6-7,15H2,1H3/b21-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320928
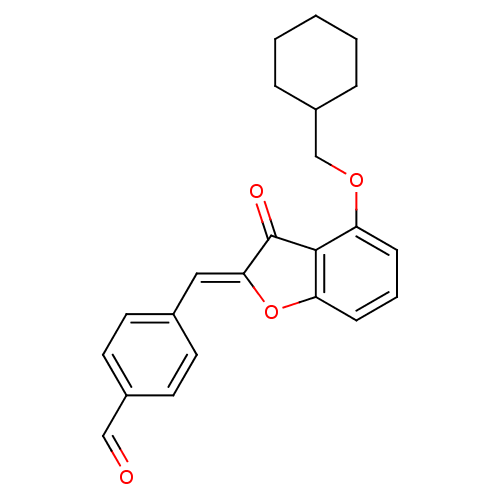
((Z)-4-{[4-(Cyclohexylmethoxy)-3-oxobenzofuran-2(3H...)Show SMILES O=Cc1ccc(\C=C2/Oc3cccc(OCC4CCCCC4)c3C2=O)cc1 Show InChI InChI=1S/C23H22O4/c24-14-17-11-9-16(10-12-17)13-21-23(25)22-19(7-4-8-20(22)27-21)26-15-18-5-2-1-3-6-18/h4,7-14,18H,1-3,5-6,15H2/b21-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320927
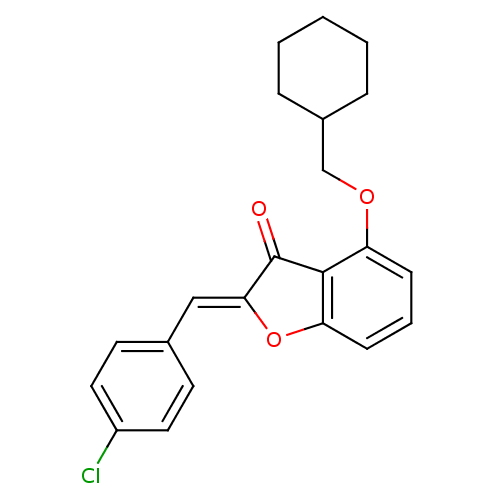
((Z)-2-(4-Chlorobenzylidene)-4-(cyclohexylmethoxy)b...)Show SMILES Clc1ccc(\C=C2/Oc3cccc(OCC4CCCCC4)c3C2=O)cc1 Show InChI InChI=1S/C22H21ClO3/c23-17-11-9-15(10-12-17)13-20-22(24)21-18(7-4-8-19(21)26-20)25-14-16-5-2-1-3-6-16/h4,7-13,16H,1-3,5-6,14H2/b20-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320926
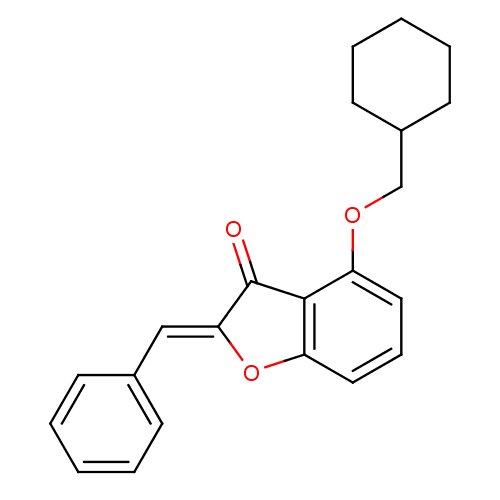
((Z)-2-(Benzylidene)-4-(cyclohexylmethoxy)benzofura...)Show InChI InChI=1S/C22H22O3/c23-22-20(14-16-8-3-1-4-9-16)25-19-13-7-12-18(21(19)22)24-15-17-10-5-2-6-11-17/h1,3-4,7-9,12-14,17H,2,5-6,10-11,15H2/b20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320918
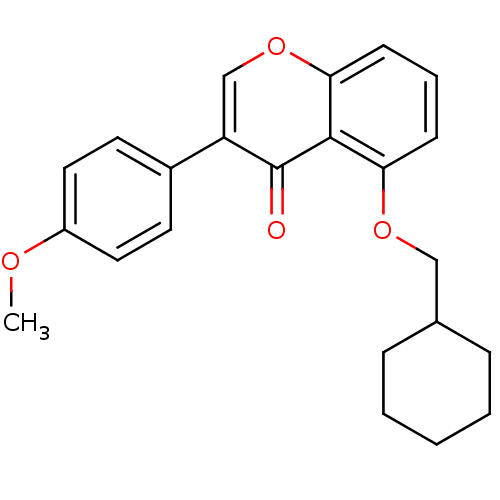
(5-(Cyclohexylmethoxy)-3-(4-methoxyphenyl)-4H-chrom...)Show InChI InChI=1S/C23H24O4/c1-25-18-12-10-17(11-13-18)19-15-27-21-9-5-8-20(22(21)23(19)24)26-14-16-6-3-2-4-7-16/h5,8-13,15-16H,2-4,6-7,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321931
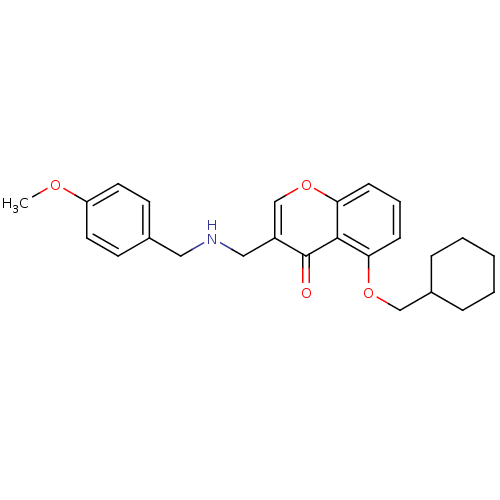
(5-(Cyclohexylmethoxy)-3-((4-methoxybenzylamino)-me...)Show SMILES COc1ccc(CNCc2coc3cccc(OCC4CCCCC4)c3c2=O)cc1 Show InChI InChI=1S/C25H29NO4/c1-28-21-12-10-18(11-13-21)14-26-15-20-17-30-23-9-5-8-22(24(23)25(20)27)29-16-19-6-3-2-4-7-19/h5,8-13,17,19,26H,2-4,6-7,14-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320919
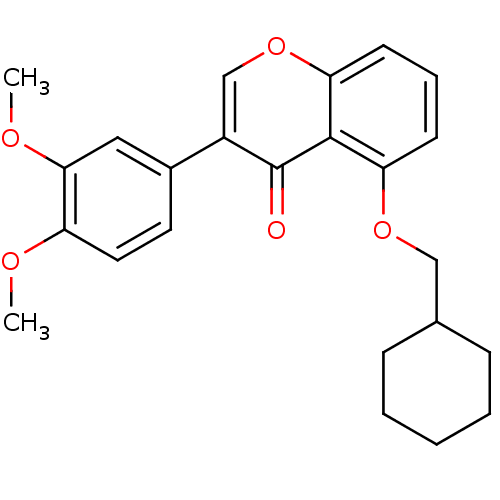
(5-(Cyclohexylmethoxy)-3-(3,4-dimethoxyphenyl)-4Hch...)Show SMILES COc1ccc(cc1OC)-c1coc2cccc(OCC3CCCCC3)c2c1=O Show InChI InChI=1S/C24H26O5/c1-26-19-12-11-17(13-22(19)27-2)18-15-29-21-10-6-9-20(23(21)24(18)25)28-14-16-7-4-3-5-8-16/h6,9-13,15-16H,3-5,7-8,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50320920
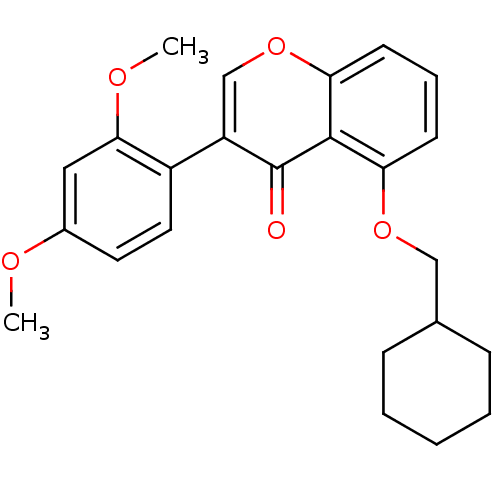
(5-(Cyclohexylmethoxy)-3-(2,5-dimethoxyphenyl)-4Hch...)Show SMILES COc1ccc(c(OC)c1)-c1coc2cccc(OCC3CCCCC3)c2c1=O Show InChI InChI=1S/C24H26O5/c1-26-17-11-12-18(22(13-17)27-2)19-15-29-21-10-6-9-20(23(21)24(19)25)28-14-16-7-4-3-5-8-16/h6,9-13,15-16H,3-5,7-8,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of IL5-mediated proliferation of mouse Y16 cells by WST1 assay |
Bioorg Med Chem 18: 4441-5 (2010)
Article DOI: 10.1016/j.bmc.2010.04.075
BindingDB Entry DOI: 10.7270/Q2H41SDX |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321937
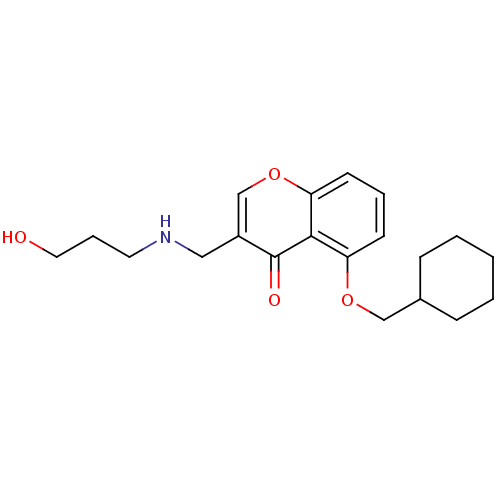
(5-(Cyclohexylmethoxy)-3-((3-hydroxypropylamino)-me...)Show InChI InChI=1S/C20H27NO4/c22-11-5-10-21-12-16-14-25-18-9-4-8-17(19(18)20(16)23)24-13-15-6-2-1-3-7-15/h4,8-9,14-15,21-22H,1-3,5-7,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321936
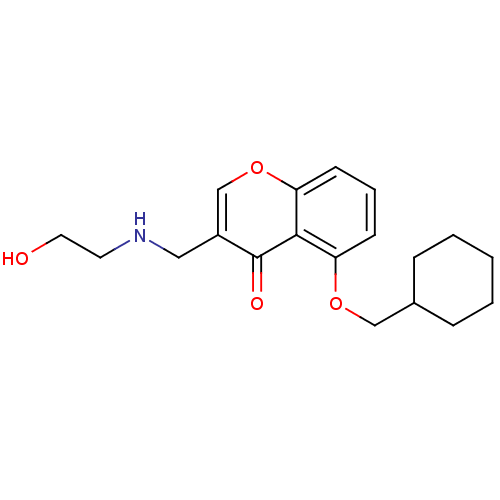
(5-(Cyclohexylmethoxy)-3-((2-hydroxyethylamino)-met...)Show InChI InChI=1S/C19H25NO4/c21-10-9-20-11-15-13-24-17-8-4-7-16(18(17)19(15)22)23-12-14-5-2-1-3-6-14/h4,7-8,13-14,20-21H,1-3,5-6,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321927
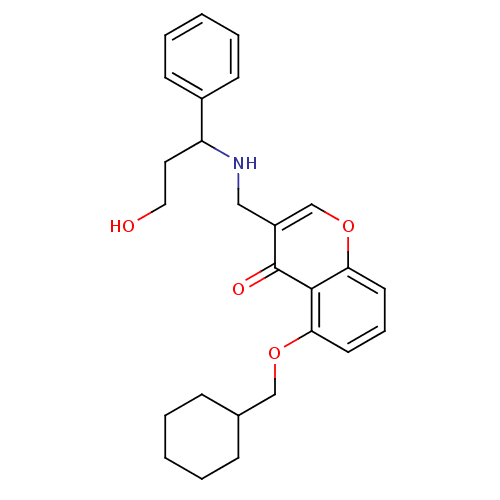
(5-(Cyclohexylmethoxy)-3-((3-hydroxy-1-phenylpropyl...)Show SMILES OCCC(NCc1coc2cccc(OCC3CCCCC3)c2c1=O)c1ccccc1 Show InChI InChI=1S/C26H31NO4/c28-15-14-22(20-10-5-2-6-11-20)27-16-21-18-31-24-13-7-12-23(25(24)26(21)29)30-17-19-8-3-1-4-9-19/h2,5-7,10-13,18-19,22,27-28H,1,3-4,8-9,14-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321934
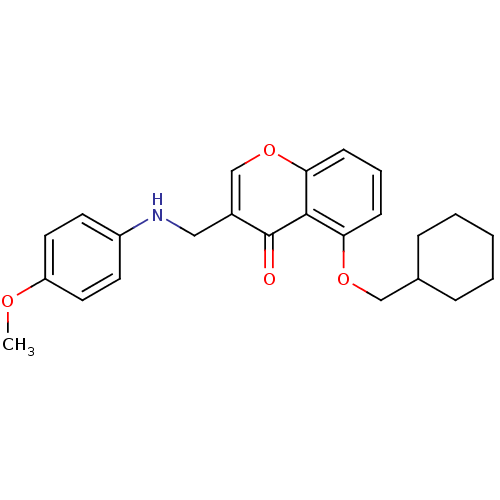
(5-(Cyclohexylmethoxy)-3-((4-methoxyphenylamino)-me...)Show InChI InChI=1S/C24H27NO4/c1-27-20-12-10-19(11-13-20)25-14-18-16-29-22-9-5-8-21(23(22)24(18)26)28-15-17-6-3-2-4-7-17/h5,8-13,16-17,25H,2-4,6-7,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321933
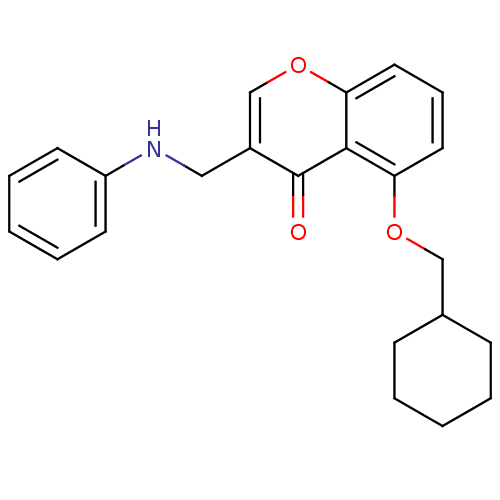
(5-(Cyclohexylmethoxy)-3-((phenylamino)methyl)-4Hch...)Show InChI InChI=1S/C23H25NO3/c25-23-18(14-24-19-10-5-2-6-11-19)16-27-21-13-7-12-20(22(21)23)26-15-17-8-3-1-4-9-17/h2,5-7,10-13,16-17,24H,1,3-4,8-9,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321938
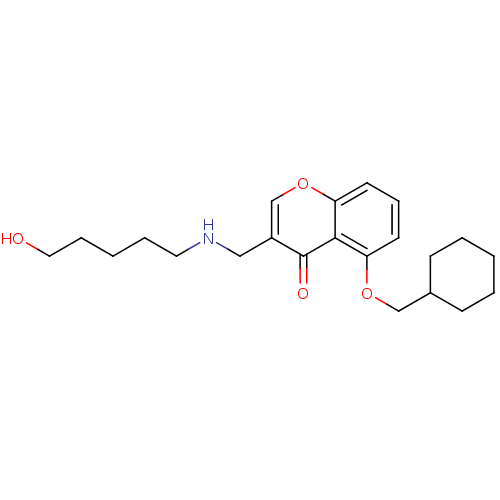
(5-(Cyclohexylmethoxy)-3-((5-hydroxypentylamino)-me...)Show InChI InChI=1S/C22H31NO4/c24-13-6-2-5-12-23-14-18-16-27-20-11-7-10-19(21(20)22(18)25)26-15-17-8-3-1-4-9-17/h7,10-11,16-17,23-24H,1-6,8-9,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321930
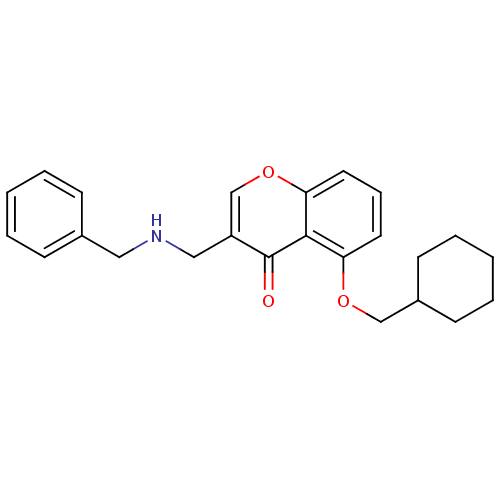
(3-((Benzylamino)methyl)-5-(cyclohexylmethoxy)-4Hch...)Show InChI InChI=1S/C24H27NO3/c26-24-20(15-25-14-18-8-3-1-4-9-18)17-28-22-13-7-12-21(23(22)24)27-16-19-10-5-2-6-11-19/h1,3-4,7-9,12-13,17,19,25H,2,5-6,10-11,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Interleukin-5
(Mus musculus) | BDBM50321939
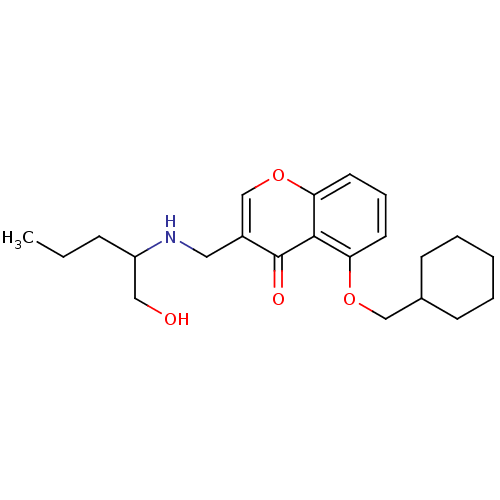
(5-(Cyclohexylmethoxy)-3-((1-hydroxypentan-2-ylamin...)Show InChI InChI=1S/C22H31NO4/c1-2-7-18(13-24)23-12-17-15-27-20-11-6-10-19(21(20)22(17)25)26-14-16-8-4-3-5-9-16/h6,10-11,15-16,18,23-24H,2-5,7-9,12-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of interleukin-5 in mouse Y16 cells |
Bioorg Med Chem 18: 4625-9 (2011)
Article DOI: 10.1016/j.bmc.2010.05.028
BindingDB Entry DOI: 10.7270/Q2B8593F |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356172
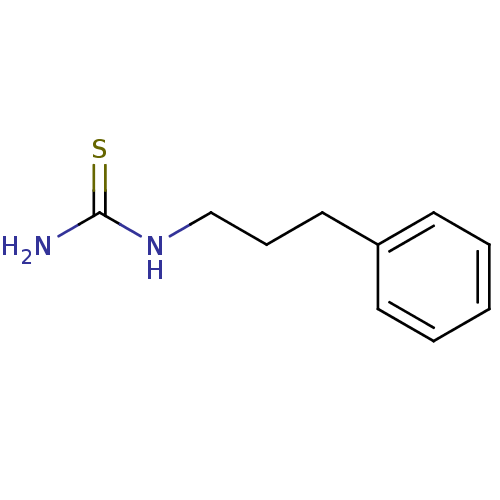
(CHEMBL1087843)Show InChI InChI=1S/C10H14N2S/c11-10(13)12-8-4-7-9-5-2-1-3-6-9/h1-3,5-6H,4,7-8H2,(H3,11,12,13) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50356169
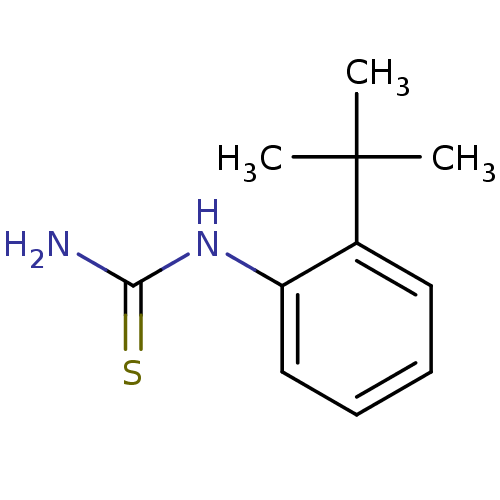
(CHEMBL1094725)Show InChI InChI=1S/C11H16N2S/c1-11(2,3)8-6-4-5-7-9(8)13-10(12)14/h4-7H,1-3H3,(H3,12,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase activity using L-tyrosine as substrate by spectrometric analysis |
Bioorg Med Chem Lett 21: 6824-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.024
BindingDB Entry DOI: 10.7270/Q2M9093C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data