

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... | J Med Chem 63: 3935-3955 (2020) Article DOI: 10.1021/acs.jmedchem.9b01713 BindingDB Entry DOI: 10.7270/Q2G1648T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00525 BindingDB Entry DOI: 10.7270/Q29W0K29 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50245852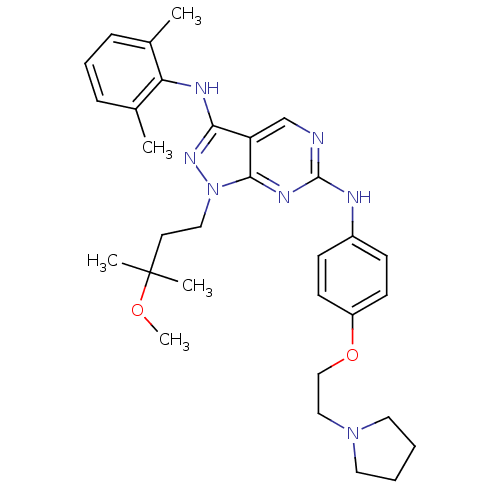 (CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of LCK (unknown origin) | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000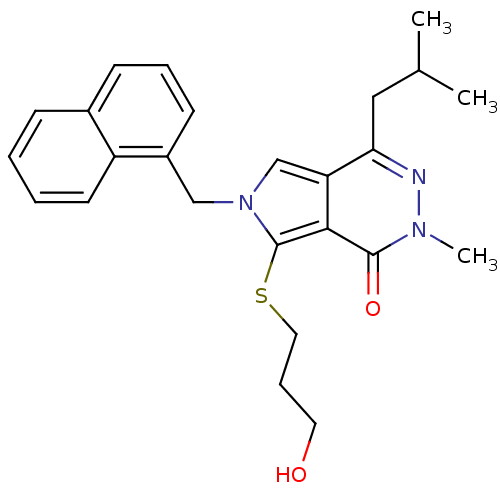 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50245852 (CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of BTK (unknown origin) | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001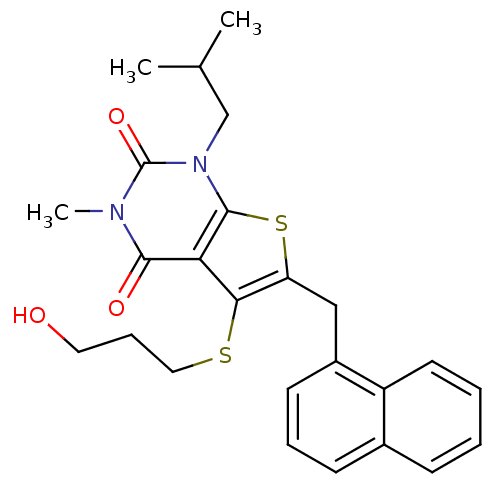 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499195 (CHEMBL3735504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002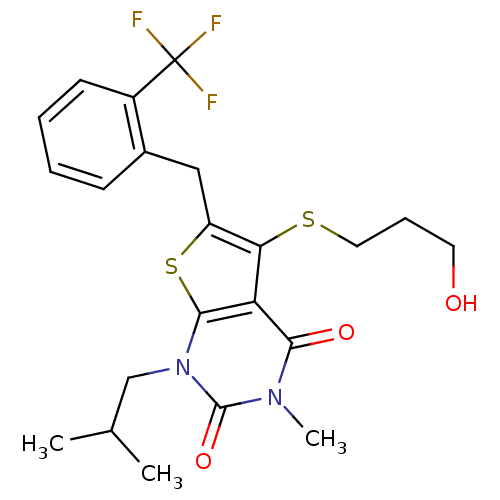 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499203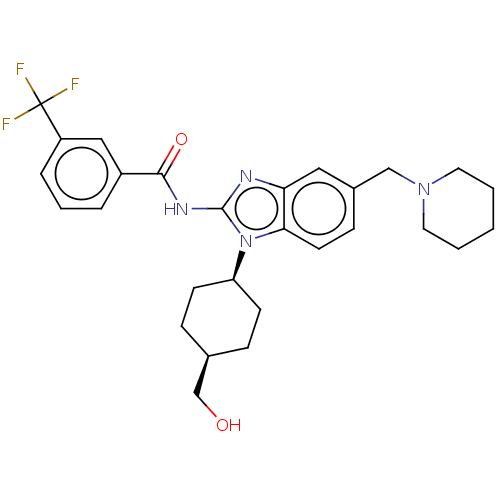 (CHEMBL3736036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499205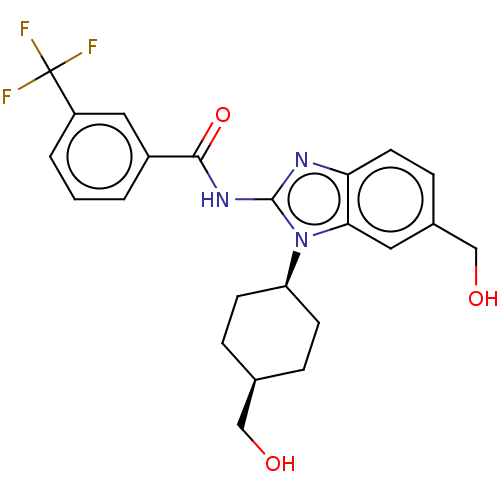 (CHEMBL3734814) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499208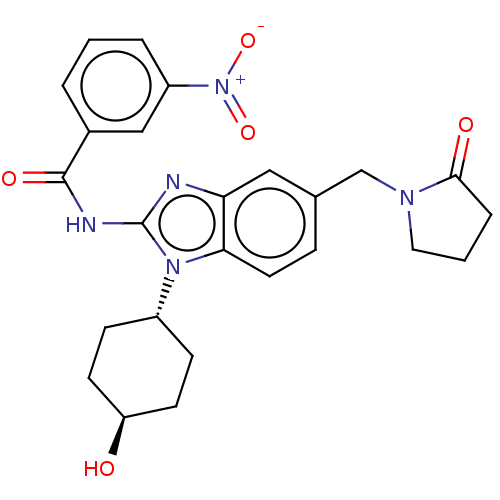 (CHEMBL3734872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM29882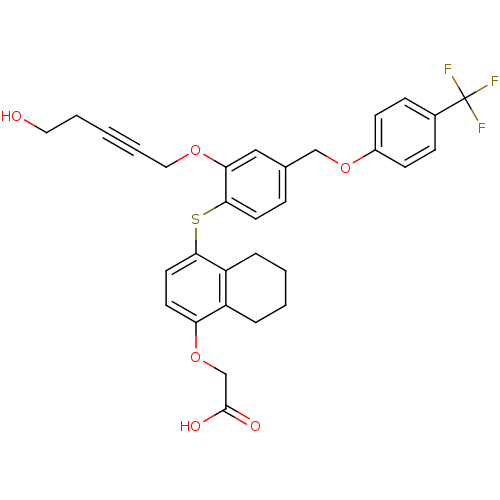 (alkynyl ether, 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Amgen | Assay Description The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... | Bioorg Med Chem Lett 19: 3550-4 (2009) Article DOI: 10.1016/j.bmcl.2009.04.151 BindingDB Entry DOI: 10.7270/Q2NK3CC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50602541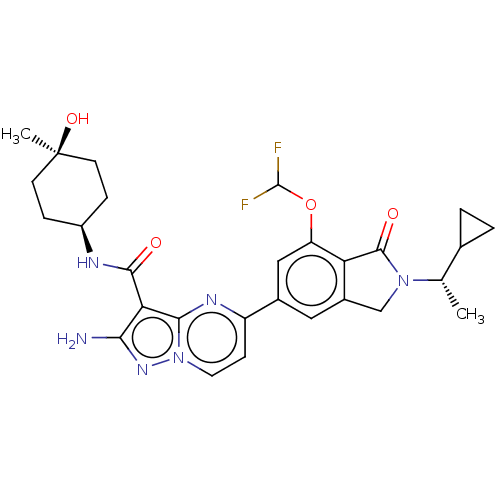 (CHEMBL5209268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01153 BindingDB Entry DOI: 10.7270/Q2KH0SCH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499194 (CHEMBL3734854) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448144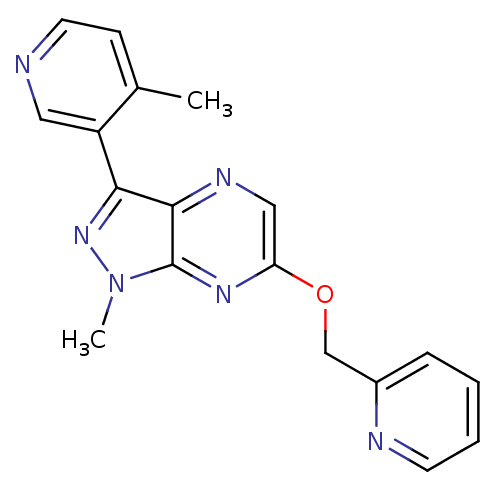 (CHEMBL3122212) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240887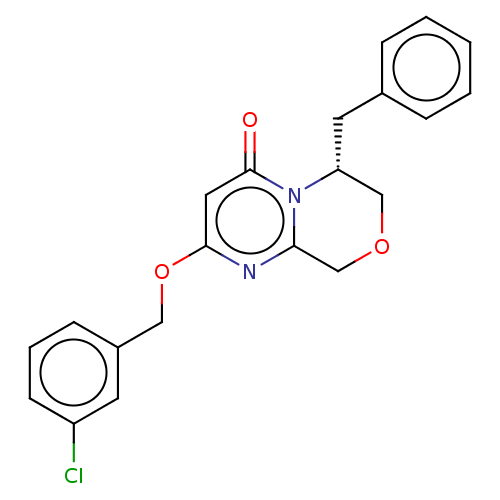 (CHEMBL4066731) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50248569 ((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 using variable cofactor NADPH concentration by Lineweaver burk plot | Bioorg Med Chem 16: 8922-31 (2008) Article DOI: 10.1016/j.bmc.2008.08.065 BindingDB Entry DOI: 10.7270/Q22F7N8T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499197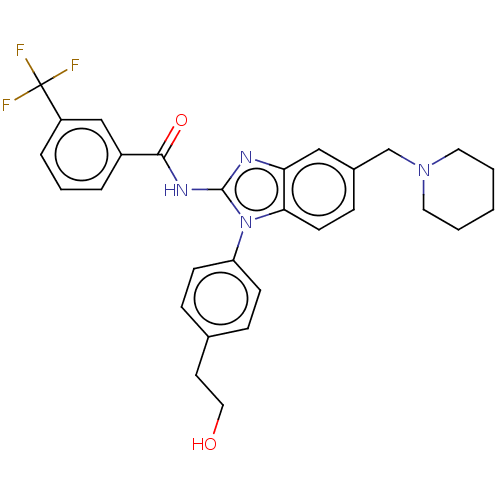 (CHEMBL3736465) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448157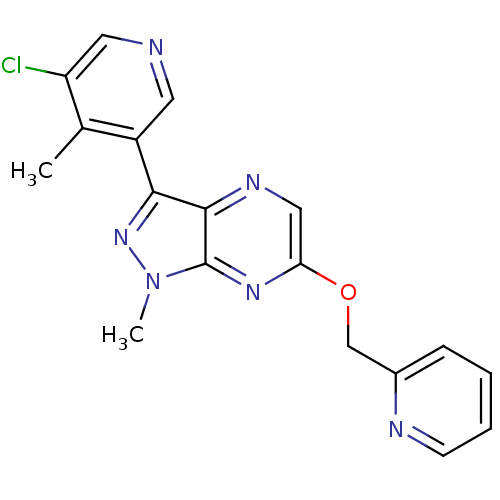 (CHEMBL3122215) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240900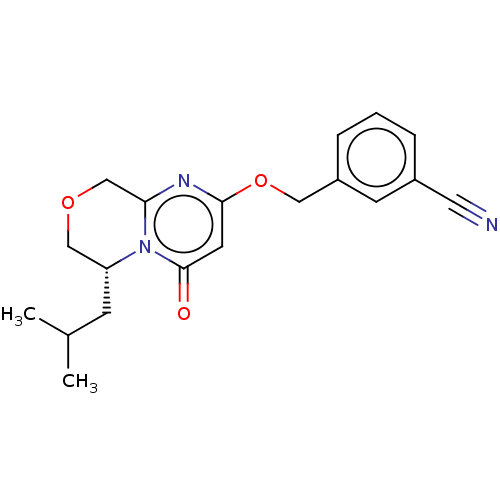 (CHEMBL4091620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499206 (CHEMBL3735949) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190788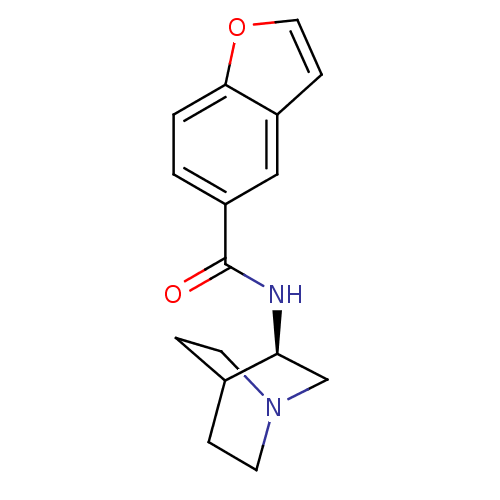 (CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates | J Med Chem 49: 4425-36 (2006) Article DOI: 10.1021/jm0602413 BindingDB Entry DOI: 10.7270/Q25B023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50246162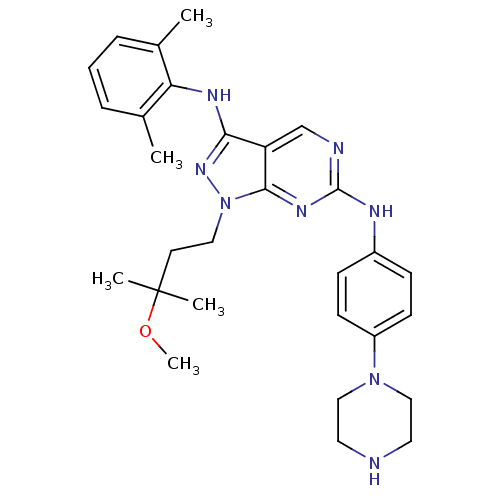 (CHEMBL472392 | N3-(2,6-dimethylphenyl)-1-(3-methox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50246214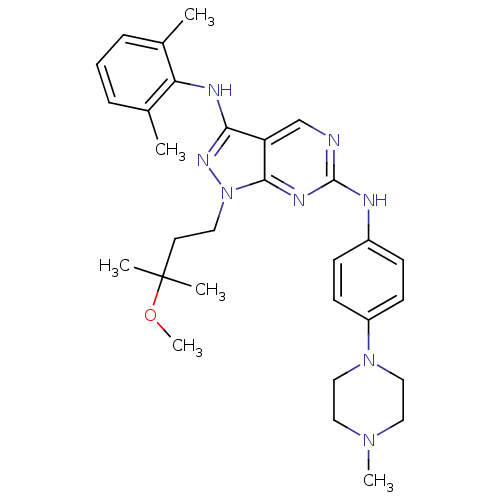 (CHEMBL487897 | N3-(2,6-dimethylphenyl)-1-(3-methox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50245854 (CHEMBL504331 | N3-(2,6-dichlorophenyl)-N6-(3-fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM29881 (alkynyl ether, 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Amgen | Assay Description The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... | Bioorg Med Chem Lett 19: 3550-4 (2009) Article DOI: 10.1016/j.bmcl.2009.04.151 BindingDB Entry DOI: 10.7270/Q2NK3CC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190785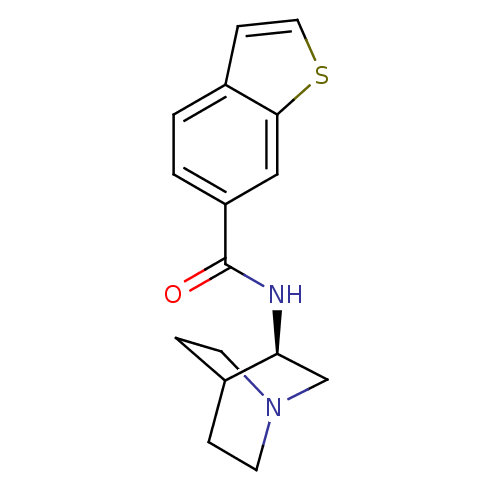 (CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates | J Med Chem 49: 4425-36 (2006) Article DOI: 10.1021/jm0602413 BindingDB Entry DOI: 10.7270/Q25B023S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448161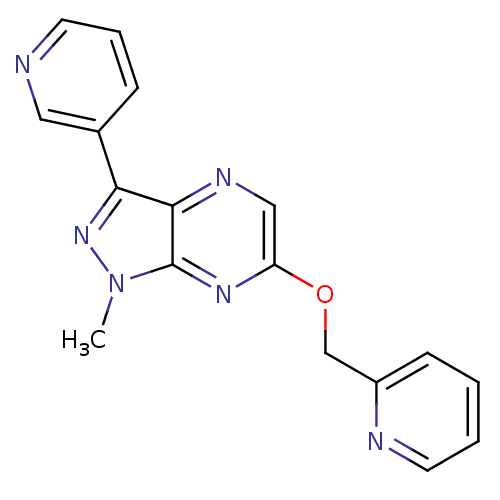 (CHEMBL3122210) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50248569 ((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 using variable substrate cortisol concentration by Lineweaver burk plot | Bioorg Med Chem 16: 8922-31 (2008) Article DOI: 10.1016/j.bmc.2008.08.065 BindingDB Entry DOI: 10.7270/Q22F7N8T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499199 (CHEMBL3735673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50448159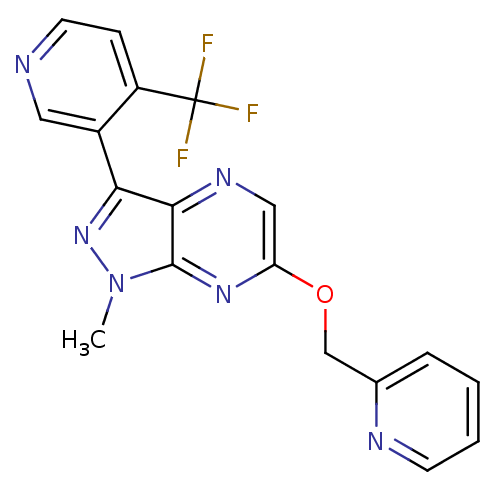 (CHEMBL3122213) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-MPEPy from human mGluR5 expressed in HEK293FT cells after 1 hr by liquid scintillation counting analysis | J Med Chem 57: 861-77 (2014) Article DOI: 10.1021/jm401622k BindingDB Entry DOI: 10.7270/Q27P90WR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499198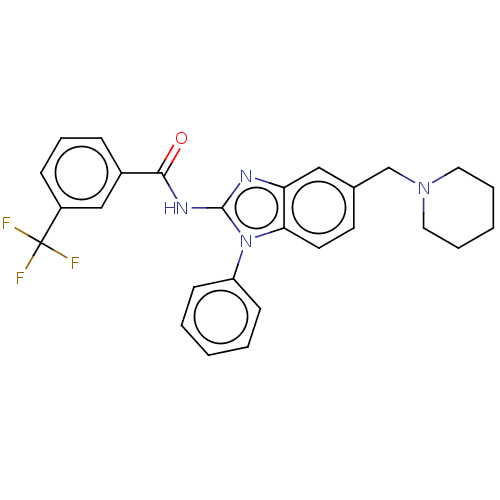 (CHEMBL3736278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50377050 (CHEMBL403858 | PH-709829) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MLA from alpha-7 nACh receptor in rat brain | Bioorg Med Chem Lett 18: 3611-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.070 BindingDB Entry DOI: 10.7270/Q2KD1ZS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240888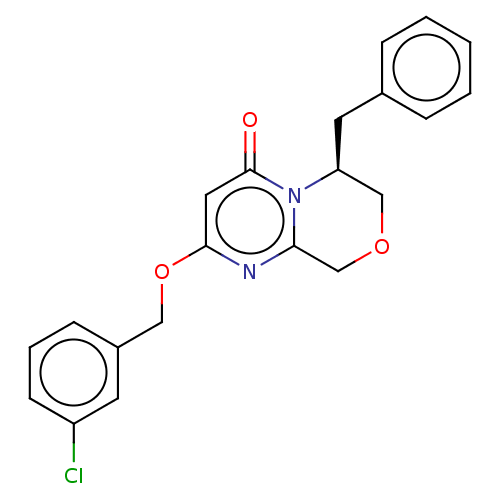 (CHEMBL4094256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50245855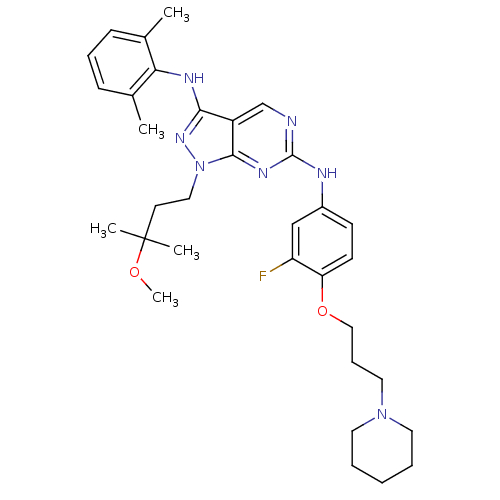 (CHEMBL450918 | N3-(2,6-dimethylphenyl)-N6-(3-fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50245852 (CHEMBL458333 | N3-(2,6-dimethylphenyl)-1-(3-methox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50246164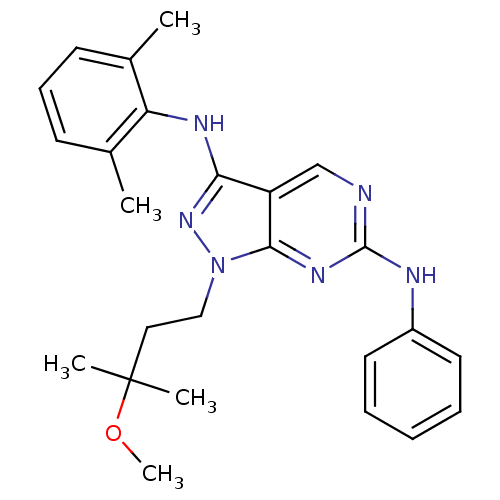 (CHEMBL487242 | N3-(2,6-dimethylphenyl)-1-(3-methox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50245853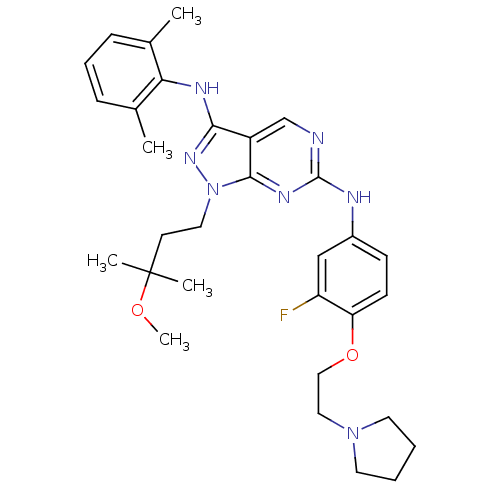 (CHEMBL502156 | N3-(2,6-dimethylphenyl)-N6-(3-fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of histidine-tagged ACK1 (amino acids 117 to 489) (unknown origin) expressed in SF9 cells | Bioorg Med Chem Lett 18: 6352-6 (2008) Article DOI: 10.1016/j.bmcl.2008.10.092 BindingDB Entry DOI: 10.7270/Q2B56JKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22025 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240881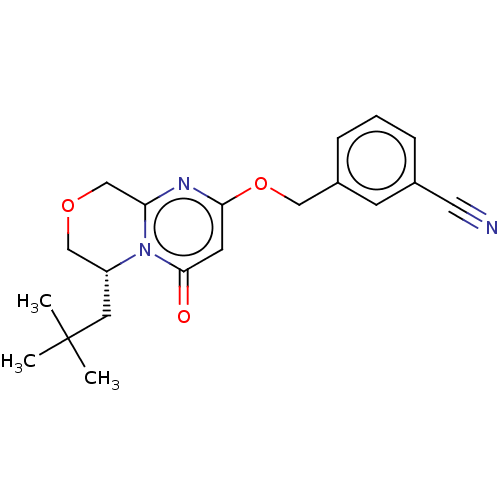 (CHEMBL4090712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50416571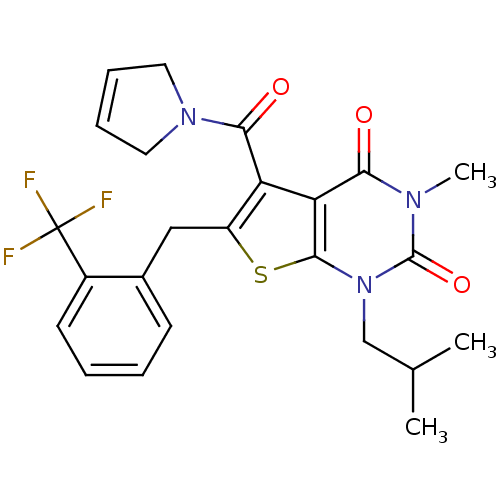 (CHEMBL1221551) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50416571 (CHEMBL1221551) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50240885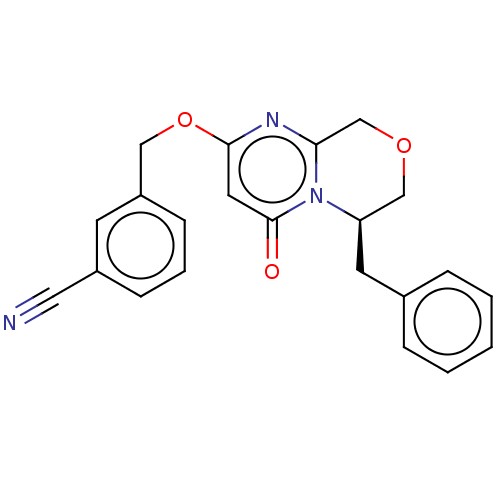 (CHEMBL4064010) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]MPEPy from human mGlu5 expressed in HEK293FT cell membranes after 1 hr by liquid scintillation counting | J Med Chem 60: 7764-7780 (2017) Article DOI: 10.1021/acs.jmedchem.7b00604 BindingDB Entry DOI: 10.7270/Q2DJ5HS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50499202 (CHEMBL3735523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA | Bioorg Med Chem Lett 25: 5546-50 (2015) Article DOI: 10.1016/j.bmcl.2015.10.060 BindingDB Entry DOI: 10.7270/Q29W0JG8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2105 total ) | Next | Last >> |