 Found 251 hits with Last Name = 'xie' and Initial = 'p'
Found 251 hits with Last Name = 'xie' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(MOUSE) | BDBM50408679

(CHEMBL5287792)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C26H38N6O4/c1-30(2)17-18-11-12-21(36-18)25(33)31(3)13-9-7-8-10-14-32(4)26-28-20-16-23(35-6)22(34-5)15-19(20)24(27)29-26/h11-12,15-16H,7-10,13-14,17H2,1-6H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408509

(CHEMBL5277326)Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor in rat aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25391
(CHEMBL200622 | SB-590885 | SB590885 | [2-(4-{4-[(1...)Show SMILES CN(C)CCOc1ccc(cc1)-c1nc(c([nH]1)-c1ccc2C(CCc2c1)N=O)-c1ccncc1 Show InChI InChI=1S/C27H27N5O2/c1-32(2)15-16-34-22-7-3-19(4-8-22)27-29-25(18-11-13-28-14-12-18)26(30-27)21-5-9-23-20(17-21)6-10-24(23)31-33/h3-5,7-9,11-14,17,24H,6,10,15-16H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Pennsylvania
| Assay Description
BRAF kinase activity was quantified using an ELISA-based MEK phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curve f... |
J Med Chem 51: 6121-7 (2008)
Article DOI: 10.1021/jm800539g
BindingDB Entry DOI: 10.7270/Q2BC3WVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(MOUSE) | BDBM50408675
(CHEMBL5270915)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C24H32ClN5O4/c1-29(23(31)19-10-9-16(15-25)34-19)11-7-5-6-8-12-30(2)24-27-18-14-21(33-4)20(32-3)13-17(18)22(26)28-24/h9-10,13-14H,5-8,11-12,15H2,1-4H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408677

(CHEMBL5274117)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1CNCCCCCCN Show InChI InChI=1S/C32H49N7O3/c1-38(31(40)25-16-10-9-15-24(25)23-35-18-12-6-5-11-17-33)19-13-7-8-14-20-39(2)32-36-27-22-29(42-4)28(41-3)21-26(27)30(34)37-32/h9-10,15-16,21-22,35H,5-8,11-14,17-20,23,33H2,1-4H3,(H2,34,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21130
(N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)19-10-12-22(11-7-15-26-22)17-20(19)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at oxytocin receptor in Wistar rat uterus assessed as inhibition of oxytocin-induced uterotonic activity treated 1 min prior to o... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408676
(CHEMBL5272258)Show SMILES CNCCCCCCN(C)Cc1ccc(cc1)C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1 Show InChI InChI=1S/C34H53N7O3/c1-36-19-11-7-8-12-20-39(2)25-26-15-17-27(18-16-26)33(42)40(3)21-13-9-10-14-22-41(4)34-37-29-24-31(44-6)30(43-5)23-28(29)32(35)38-34/h15-18,23-24,36H,7-14,19-22,25H2,1-6H3,(H2,35,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408673

(CHEMBL5275873)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCSSCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C22H28ClN5O4S2/c1-27(21(29)17-6-5-14(13-23)32-17)7-9-33-34-10-8-28(2)22-25-16-12-19(31-4)18(30-3)11-15(16)20(24)26-22/h5-6,11-12H,7-10,13H2,1-4H3,(H2,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408816

(CHEMBL5279890)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCCCCC1)C(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C43H68N12O8/c44-29(15-10-20-49-41(45)46)35(57)51-30(16-11-21-50-42(47)48)36(58)53-43(18-8-2-1-3-9-19-43)40(63)52-31(25-56)37(59)54-24-28-14-5-4-12-26(28)22-33(54)38(60)55-32-17-7-6-13-27(32)23-34(55)39(61)62/h4-5,12,14,27,29-34,56H,1-3,6-11,13,15-25,44H2,(H,51,57)(H,52,63)(H,53,58)(H,61,62)(H4,45,46,49)(H4,47,48,50)/t27-,29+,30-,31-,32-,33+,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H1 receptor in guinea pig ileum assessed as histamine-induced contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408813
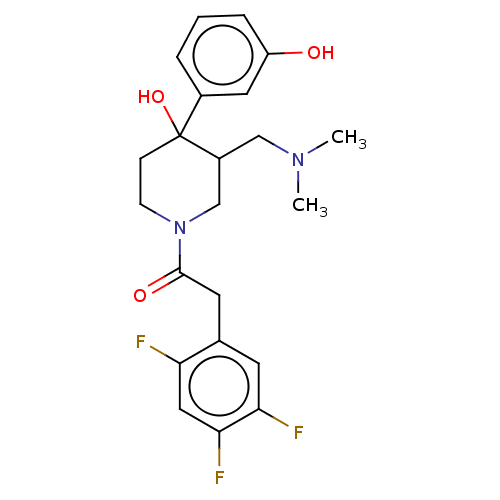
(CHEMBL5273356)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C46H76N12O8/c47-33(19-14-24-53-45(48)49)40(61)56-34(20-15-25-54-46(50)51)41(62)52-23-13-7-5-3-1-2-4-6-8-22-39(60)55-35(29-59)42(63)57-28-32-18-10-9-16-30(32)26-37(57)43(64)58-36-21-12-11-17-31(36)27-38(58)44(65)66/h9-10,16,18,31,33-38,59H,1-8,11-15,17,19-29,47H2,(H,52,62)(H,55,60)(H,56,61)(H,65,66)(H4,48,49,53)(H4,50,51,54)/t31-,33+,34-,35-,36-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at MTL receptor in rabbit duodenum homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408514
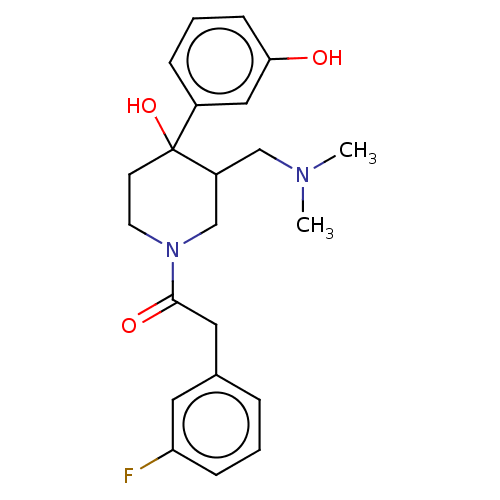
(CHEMBL5281561)Show InChI InChI=1S/C18H22N2OS/c1-19(2)10-5-11-20-15-6-3-4-7-17(15)22-18-9-8-14(13-21)12-16(18)20/h3-4,6-9,12,21H,5,10-11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21008
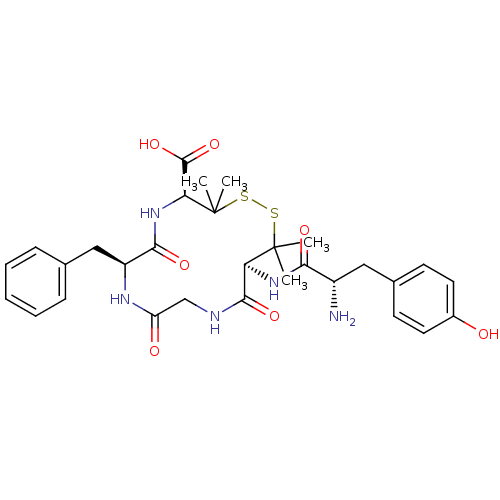
((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as luciferase activity by Schild assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408669
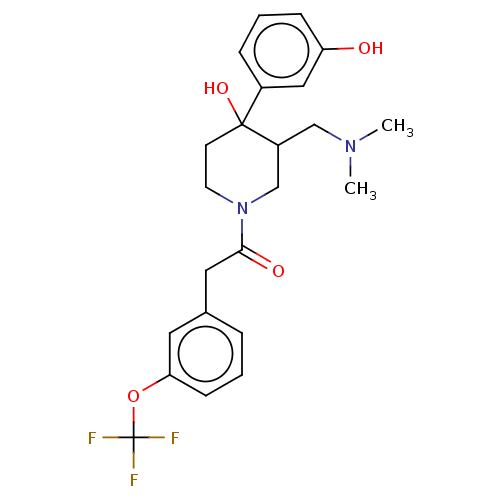
(CHEMBL5272171)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1CN(C)C Show InChI InChI=1S/C28H40N6O3/c1-32(2)19-20-13-9-10-14-21(20)27(35)33(3)15-11-7-8-12-16-34(4)28-30-23-18-25(37-6)24(36-5)17-22(23)26(29)31-28/h9-10,13-14,17-18H,7-8,11-12,15-16,19H2,1-6H3,(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408678
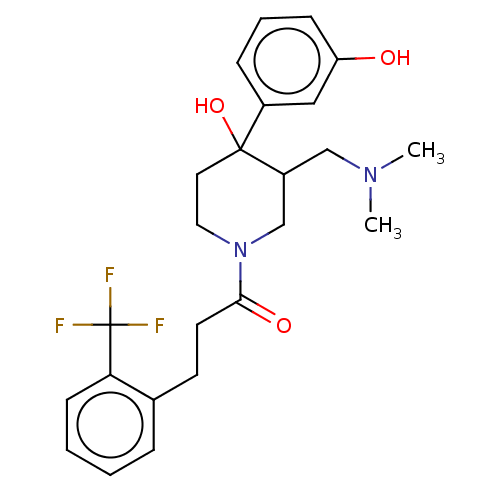
(CHEMBL5281054)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C28H40N6O3/c1-32(2)19-20-11-13-21(14-12-20)27(35)33(3)15-9-7-8-10-16-34(4)28-30-23-18-25(37-6)24(36-5)17-22(23)26(29)31-28/h11-14,17-18H,7-10,15-16,19H2,1-6H3,(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408513
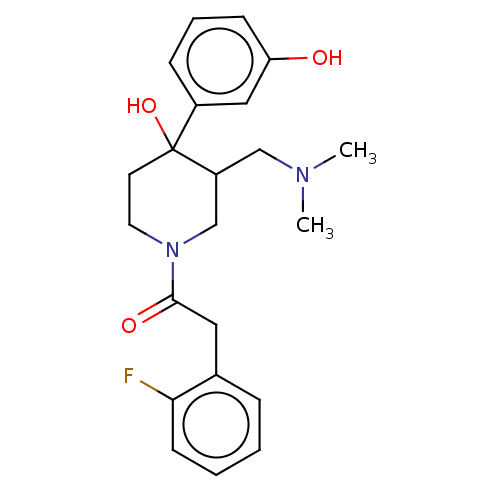
(CHEMBL5272744)Show InChI InChI=1S/C14H9BrClNOS/c15-8-14(18)17-10-3-1-2-4-12(10)19-13-6-5-9(16)7-11(13)17/h1-7H,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408511
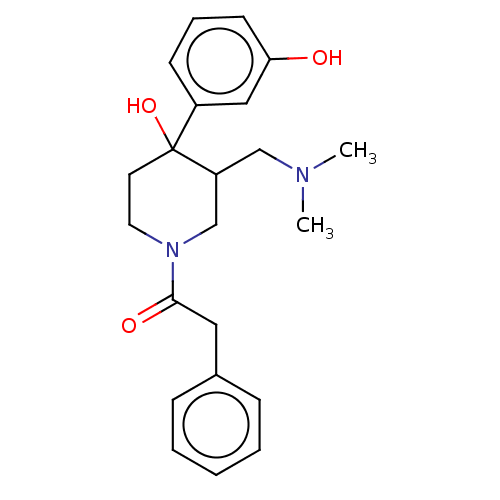
(CHEMBL5274208)Show InChI InChI=1S/C23H30N2OS/c1-4-5-6-11-21(26)18-13-14-23-20(17-18)25(16-9-15-24(2)3)19-10-7-8-12-22(19)27-23/h7-8,10,12-14,17H,4-6,9,11,15-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant CXCR3 receptor assessed as human IP10-induced calcium mobilization by FLIPR assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408509

(CHEMBL5277326)Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as inhibition of 2-[3-(imidazol-4-yl)-propyl]-1-(4-iodo-... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50408679

(CHEMBL5287792)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C26H38N6O4/c1-30(2)17-18-11-12-21(36-18)25(33)31(3)13-9-7-8-10-14-32(4)26-28-20-16-23(35-6)22(34-5)15-19(20)24(27)29-26/h11-12,15-16H,7-10,13-14,17H2,1-6H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant PAC1 receptor expressed in CHO cells assessed as reduction in PACAP38-induced calcium mobilization by FLIPR ... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408674
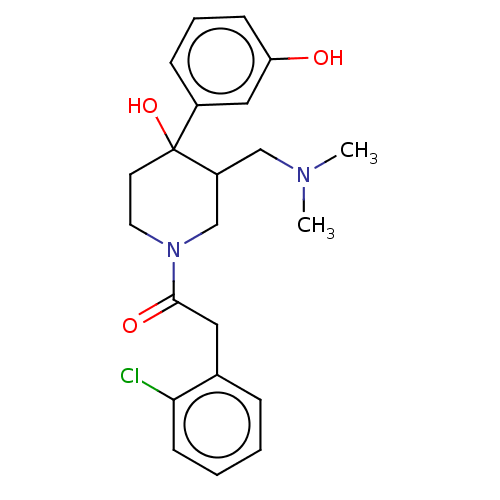
(CHEMBL5282901)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)cc1 Show InChI InChI=1S/C26H34ClN5O3/c1-31(25(33)19-11-9-18(17-27)10-12-19)13-7-5-6-8-14-32(2)26-29-21-16-23(35-4)22(34-3)15-20(21)24(28)30-26/h9-12,15-16H,5-8,13-14,17H2,1-4H3,(H2,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408672
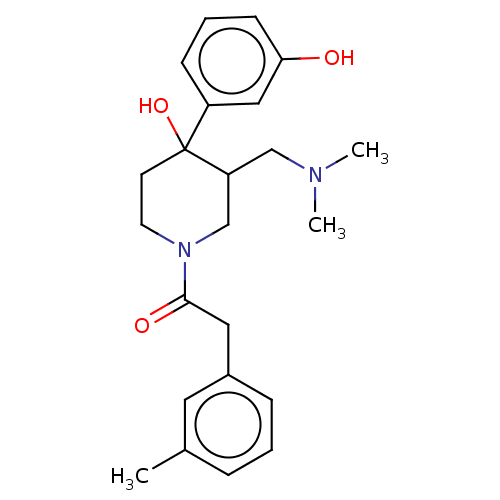
(CHEMBL5274664)Show SMILES CNCCCCCCN(C)Cc1cccc(c1)C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1 Show InChI InChI=1S/C34H53N7O3/c1-36-18-11-7-8-12-19-39(2)25-26-16-15-17-27(22-26)33(42)40(3)20-13-9-10-14-21-41(4)34-37-29-24-31(44-6)30(43-5)23-28(29)32(35)38-34/h15-17,22-24,36H,7-14,18-21,25H2,1-6H3,(H2,35,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50176263
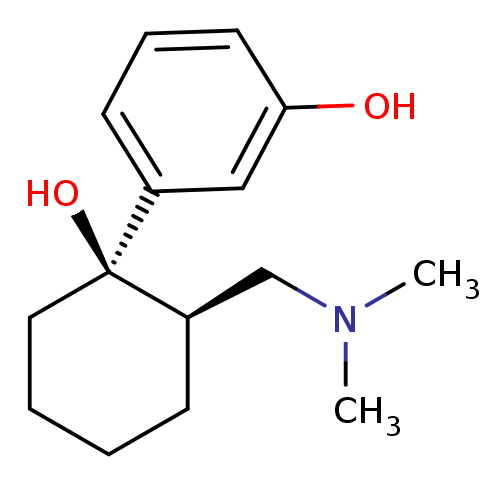
(3-((1R,2R)-2-((dimethylamino)methyl)-1-hydroxycycl...)Show InChI InChI=1S/C15H23NO2/c1-16(2)11-13-6-3-4-9-15(13,18)12-7-5-8-14(17)10-12/h5,7-8,10,13,17-18H,3-4,6,9,11H2,1-2H3/t13-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50408668

(CHEMBL5277164)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1cccc(CN)c1 Show InChI InChI=1S/C26H36N6O3/c1-31(25(33)19-11-9-10-18(14-19)17-27)12-7-5-6-8-13-32(2)26-29-21-16-23(35-4)22(34-3)15-20(21)24(28)30-26/h9-11,14-16H,5-8,12-13,17,27H2,1-4H3,(H2,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H3 receptor in guinea pig jejunum assessed as electrically evoked contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408671
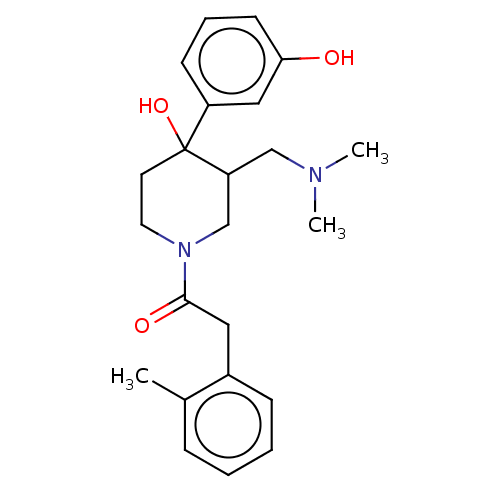
(CHEMBL5281864)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1 Show InChI InChI=1S/C25H33N5O3/c1-29(24(31)18-12-8-7-9-13-18)14-10-5-6-11-15-30(2)25-27-20-17-22(33-4)21(32-3)16-19(20)23(26)28-25/h7-9,12-13,16-17H,5-6,10-11,14-15H2,1-4H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408675

(CHEMBL5270915)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C24H32ClN5O4/c1-29(23(31)19-10-9-16(15-25)34-19)11-7-5-6-8-12-30(2)24-27-18-14-21(33-4)20(32-3)13-17(18)22(26)28-24/h9-10,13-14H,5-8,11-12,15H2,1-4H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at oxytocin receptor in Wistar rat uterus assessed as inhibition of oxytocin-induced uterotonic activity treated 1 min prior to o... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408721
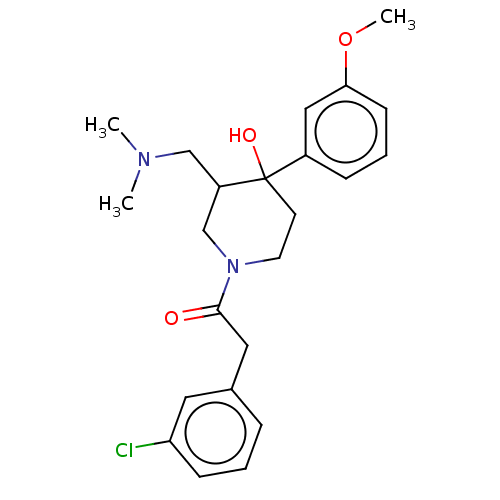
(CHEMBL5281114)Show InChI InChI=1S/C22H34N2O2/c1-2-16-26-21-13-7-5-11-19(21)22(25)23-20-12-6-4-10-18(20)17-24-14-8-3-9-15-24/h5,7,11,13,18,20H,2-4,6,8-10,12,14-17H2,1H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity rat recombinant histamine H3 receptor expressed in human SK-N-MC cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408804
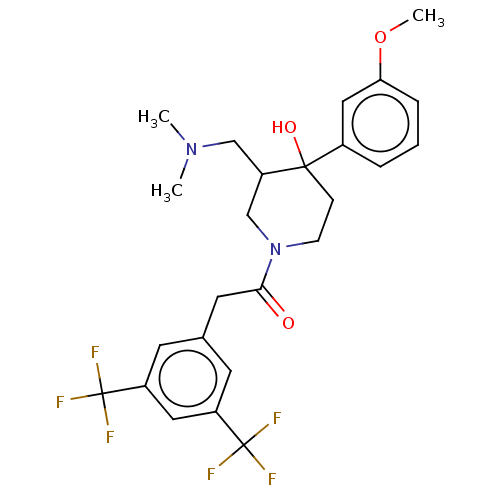
(CHEMBL5290025)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(C)=O)C(O)=O Show InChI InChI=1S/C57H81N13O12/c1-4-34(2)48(56(81)82)68-51(76)43(31-37-23-24-38-17-8-9-18-39(38)29-37)66-52(77)44(33-71)67-50(75)42(30-36-15-6-5-7-16-36)64-47(73)32-62-53(78)45-21-13-27-69(45)55(80)46-22-14-28-70(46)54(79)41(20-12-26-61-57(59)60)65-49(74)40(63-35(3)72)19-10-11-25-58/h5-9,15-18,23-24,29,34,40-46,48,71H,4,10-14,19-22,25-28,30-33,58H2,1-3H3,(H,62,78)(H,63,72)(H,64,73)(H,65,74)(H,66,77)(H,67,75)(H,68,76)(H,81,82)(H4,59,60,61)/t34-,40-,41-,42-,43+,44-,45-,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at MTL receptor in rabbit duodenum homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408801
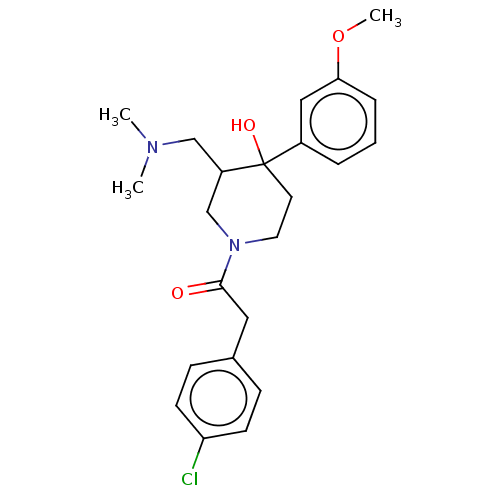
(CHEMBL5289463)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N[C@H](C1Cc2ccccc2C1)C(=O)N1[C@H](C[C@H]2CCCC[C@@H]12)C(O)=O Show InChI InChI=1S/C46H76N12O8/c47-33(18-13-23-53-45(48)49)40(61)56-34(19-14-24-54-46(50)51)41(62)52-22-12-6-4-2-1-3-5-7-21-38(60)55-35(28-59)42(63)57-39(32-25-29-15-8-9-16-30(29)26-32)43(64)58-36-20-11-10-17-31(36)27-37(58)44(65)66/h8-9,15-16,31-37,39,59H,1-7,10-14,17-28,47H2,(H,52,62)(H,55,60)(H,56,61)(H,57,63)(H,65,66)(H4,48,49,53)(H4,50,51,54)/t31-,33-,34+,35+,36-,37-,39-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity rat recombinant histamine H3 receptor expressed in human SK-N-MC cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408512
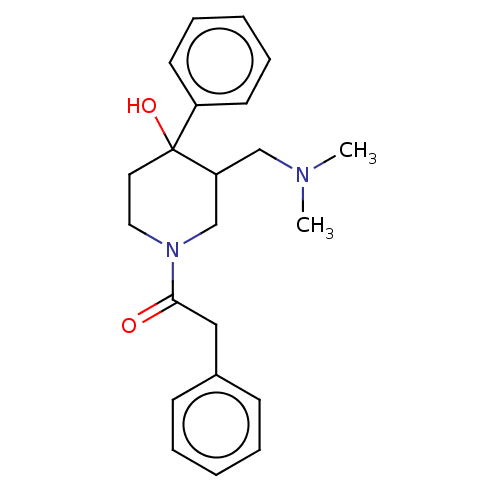
(CHEMBL5270488)Show InChI InChI=1S/C19H22N2O2S/c1-20(2)11-6-12-21-15-7-4-5-8-17(15)24-18-10-9-14(13-16(18)21)19(22)23-3/h4-5,7-10,13H,6,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant CXCR3 receptor assessed as human IP10-induced calcium mobilization by FLIPR assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408668

(CHEMBL5277164)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1cccc(CN)c1 Show InChI InChI=1S/C26H36N6O3/c1-31(25(33)19-11-9-10-18(14-19)17-27)12-7-5-6-8-13-32(2)26-29-21-16-23(35-4)22(34-3)15-20(21)24(28)30-26/h9-11,14-16H,5-8,12-13,17,27H2,1-4H3,(H2,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408808
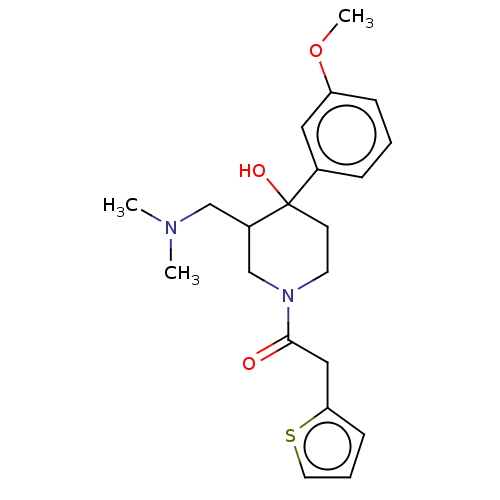
(CHEMBL5271351)Show SMILES NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N[C@H](C1Cc2ccccc2C1)C(=O)N1[C@H](C[C@@H]2CCCC[C@H]12)C(O)=O Show InChI InChI=1S/C52H88N12O9/c53-26-14-12-21-38(55)46(67)61-40(22-13-15-27-54)48(69)62-39(23-17-29-59-52(56)57)47(68)58-28-16-6-4-2-1-3-5-7-25-44(66)60-41(33-65)49(70)63-45(37-30-34-18-8-9-19-35(34)31-37)50(71)64-42-24-11-10-20-36(42)32-43(64)51(72)73/h8-9,18-19,36-43,45,65H,1-7,10-17,20-33,53-55H2,(H,58,68)(H,60,66)(H,61,67)(H,62,69)(H,63,70)(H,72,73)(H4,56,57,59)/t36-,38-,39-,40-,41-,42-,43+,45+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at MTL receptor in rabbit duodenum homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 38 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
University of Pennsylvania
| Assay Description
BRAF kinase activity was quantified using an ELISA-based MEK phosphorylation assay. IC50 values were derived from the sigmoidal dose-response curve f... |
J Med Chem 51: 6121-7 (2008)
Article DOI: 10.1021/jm800539g
BindingDB Entry DOI: 10.7270/Q2BC3WVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408818
(CHEMBL5287134)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCC(=O)NC1(CCCCCC1)C(=O)NCCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C48H76N14O10/c49-32(14-9-21-56-46(50)51)40(66)59-33(15-10-22-57-47(52)53)41(67)54-23-18-39(65)60-48(19-7-1-2-8-20-48)45(72)55-24-17-38(64)58-34(28-63)42(68)61-27-31-13-4-3-11-29(31)25-36(61)43(69)62-35-16-6-5-12-30(35)26-37(62)44(70)71/h3-4,11,13,30,32-37,63H,1-2,5-10,12,14-28,49H2,(H,54,67)(H,55,72)(H,58,64)(H,59,66)(H,60,65)(H,70,71)(H4,50,51,56)(H4,52,53,57)/t30-,32+,33-,34-,35-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at wild type guinea pig NK3 receptor A1142.58T mutant expressed in HEK293 cells assessed as inhibition of [MePhe7]NKB-induced acc... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408510
(CHEMBL5277743)Show InChI InChI=1S/C16H12ClNO3S/c17-10-5-6-14-12(9-10)18(15(19)7-8-16(20)21)11-3-1-2-4-13(11)22-14/h1-6,9H,7-8H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant CXCR3 receptor assessed as human IP10-induced calcium mobilization by FLIPR assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408667

(CHEMBL5272486)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCSSCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C24H34N6O4S2/c1-28(2)15-16-7-8-19(34-16)23(31)29(3)9-11-35-36-12-10-30(4)24-26-18-14-21(33-6)20(32-5)13-17(18)22(25)27-24/h7-8,13-14H,9-12,15H2,1-6H3,(H2,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat Alpha-1D adrenoceptor assessed as inhibition if norepenephrine-induced contraction of thoracic aorta |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50408509

(CHEMBL5277326)Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H3 receptor in guinea pig jejunum assessed as electrically evoked contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408676

(CHEMBL5272258)Show SMILES CNCCCCCCN(C)Cc1ccc(cc1)C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1 Show InChI InChI=1S/C34H53N7O3/c1-36-19-11-7-8-12-20-39(2)25-26-15-17-27(18-16-26)33(42)40(3)21-13-9-10-14-22-41(4)34-37-29-24-31(44-6)30(43-5)23-28(29)32(35)38-34/h15-18,23-24,36H,7-14,19-22,25H2,1-6H3,(H2,35,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at vasopressin V1a receptor in phenoxybenzamine-treated Wistar rat uterus assessed as inhibition of vasopressin-induced arteial v... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50408669

(CHEMBL5272171)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1CN(C)C Show InChI InChI=1S/C28H40N6O3/c1-32(2)19-20-13-9-10-14-21(20)27(35)33(3)15-11-7-8-12-16-34(4)28-30-23-18-25(37-6)24(36-5)17-22(23)26(29)31-28/h9-10,13-14,17-18H,7-8,11-12,15-16,19H2,1-6H3,(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H3 receptor in guinea pig jejunum assessed as electrically evoked contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408670
(CHEMBL5269558)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN2CCSC2)cc1 Show InChI InChI=1S/C29H40N6O3S/c1-33(28(36)22-11-9-21(10-12-22)19-35-15-16-39-20-35)13-7-5-6-8-14-34(2)29-31-24-18-26(38-4)25(37-3)17-23(24)27(30)32-29/h9-12,17-18H,5-8,13-16,19-20H2,1-4H3,(H2,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50408674

(CHEMBL5282901)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)cc1 Show InChI InChI=1S/C26H34ClN5O3/c1-31(25(33)19-11-9-18(17-27)10-12-19)13-7-5-6-8-14-32(2)26-29-21-16-23(35-4)22(34-3)15-20(21)24(28)30-26/h9-12,15-16H,5-8,13-14,17H2,1-4H3,(H2,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H1 receptor in guinea pig ileum assessed as histamine-induced contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50408675

(CHEMBL5270915)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C24H32ClN5O4/c1-29(23(31)19-10-9-16(15-25)34-19)11-7-5-6-8-12-30(2)24-27-18-14-21(33-4)20(32-3)13-17(18)22(26)28-24/h9-10,13-14H,5-8,11-12,15H2,1-4H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H1 receptor in guinea pig ileum assessed as histamine-induced contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408818

(CHEMBL5287134)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCC(=O)NC1(CCCCCC1)C(=O)NCCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C48H76N14O10/c49-32(14-9-21-56-46(50)51)40(66)59-33(15-10-22-57-47(52)53)41(67)54-23-18-39(65)60-48(19-7-1-2-8-20-48)45(72)55-24-17-38(64)58-34(28-63)42(68)61-27-31-13-4-3-11-29(31)25-36(61)43(69)62-35-16-6-5-12-30(35)26-37(62)44(70)71/h3-4,11,13,30,32-37,63H,1-2,5-10,12,14-28,49H2,(H,54,67)(H,55,72)(H,58,64)(H,59,66)(H,60,65)(H,70,71)(H4,50,51,56)(H4,52,53,57)/t30-,32+,33-,34-,35-,36+,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H3 receptor in guinea pig jejunum assessed as electrically evoked contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50408672

(CHEMBL5274664)Show SMILES CNCCCCCCN(C)Cc1cccc(c1)C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1 Show InChI InChI=1S/C34H53N7O3/c1-36-18-11-7-8-12-19-39(2)25-26-16-15-17-27(22-26)33(42)40(3)20-13-9-10-14-21-41(4)34-37-29-24-31(44-6)30(43-5)23-28(29)32(35)38-34/h15-17,22-24,36H,7-14,18-21,25H2,1-6H3,(H2,35,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H3 receptor in guinea pig jejunum assessed as electrically evoked contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408805

(CHEMBL5269806)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCCC(=O)NC1(CCCCCC1)C(=O)NCCCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C50H80N14O10/c51-34(16-9-25-58-48(52)53)42(68)61-35(17-10-26-59-49(54)55)43(69)56-23-12-20-41(67)62-50(21-7-1-2-8-22-50)47(74)57-24-11-19-40(66)60-36(30-65)44(70)63-29-33-15-4-3-13-31(33)27-38(63)45(71)64-37-18-6-5-14-32(37)28-39(64)46(72)73/h3-4,13,15,32,34-39,65H,1-2,5-12,14,16-30,51H2,(H,56,69)(H,57,74)(H,60,66)(H,61,68)(H,62,67)(H,72,73)(H4,52,53,58)(H4,54,55,59)/t32-,34+,35-,36-,37-,38+,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at MTL receptor in rabbit duodenum homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408673

(CHEMBL5275873)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCSSCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C22H28ClN5O4S2/c1-27(21(29)17-6-5-14(13-23)32-17)7-9-33-34-10-8-28(2)22-25-16-12-19(31-4)18(30-3)11-15(16)20(24)26-22/h5-6,11-12H,7-10,13H2,1-4H3,(H2,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at oxytocin receptor in Wistar rat uterus assessed as inhibition of oxytocin-induced uterotonic activity treated 1 min prior to o... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408813

(CHEMBL5273356)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C46H76N12O8/c47-33(19-14-24-53-45(48)49)40(61)56-34(20-15-25-54-46(50)51)41(62)52-23-13-7-5-3-1-2-4-6-8-22-39(60)55-35(29-59)42(63)57-28-32-18-10-9-16-30(32)26-37(57)43(64)58-36-21-12-11-17-31(36)27-38(58)44(65)66/h9-10,16,18,31,33-38,59H,1-8,11-15,17,19-29,47H2,(H,52,62)(H,55,60)(H,56,61)(H,65,66)(H4,48,49,53)(H4,50,51,54)/t31-,33+,34-,35-,36-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at wild type guinea pig NK3 receptor expressed in HEK293 cells assessed as inhibition of [MePhe7]NKB-induced accumulation of [3H]... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408802
(CHEMBL5275209)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)NC1(CCCCCC1)C(=O)NCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C46H72N14O10/c47-30(14-9-19-52-44(48)49)38(64)57-31(15-10-20-53-45(50)51)39(65)54-24-37(63)58-46(17-7-1-2-8-18-46)43(70)55-23-36(62)56-32(26-61)40(66)59-25-29-13-4-3-11-27(29)21-34(59)41(67)60-33-16-6-5-12-28(33)22-35(60)42(68)69/h3-4,11,13,28,30-35,61H,1-2,5-10,12,14-26,47H2,(H,54,65)(H,55,70)(H,56,62)(H,57,64)(H,58,63)(H,68,69)(H4,48,49,52)(H4,50,51,53)/t28-,30+,31-,32-,33-,34+,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity rat recombinant histamine H3 receptor expressed in human SK-N-MC cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408812
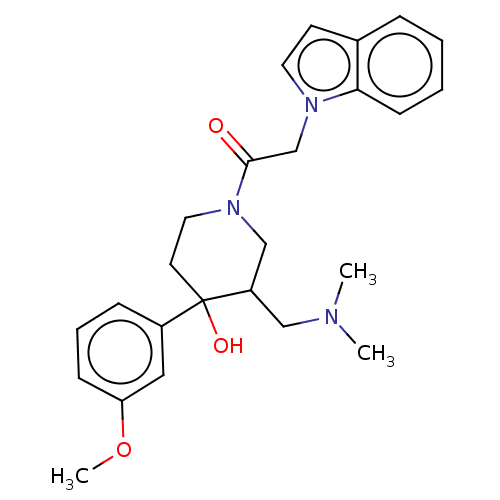
(CHEMBL5273683)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](CO)NC(=O)CCCCCCCCCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(C)=O)C(O)=O Show InChI InChI=1S/C47H76N10O9/c1-4-31(2)41(46(65)66)57-44(63)38(29-33-23-24-34-18-12-13-19-35(34)28-33)56-45(64)39(30-58)54-40(60)22-11-9-7-5-6-8-10-16-26-51-42(61)36(21-17-27-52-47(49)50)55-43(62)37(53-32(3)59)20-14-15-25-48/h12-13,18-19,23-24,28,31,36-39,41,58H,4-11,14-17,20-22,25-27,29-30,48H2,1-3H3,(H,51,61)(H,53,59)(H,54,60)(H,55,62)(H,56,64)(H,57,63)(H,65,66)(H4,49,50,52)/t31-,36-,37-,38+,39-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at MTL receptor in rabbit duodenum homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408672

(CHEMBL5274664)Show SMILES CNCCCCCCN(C)Cc1cccc(c1)C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1 Show InChI InChI=1S/C34H53N7O3/c1-36-18-11-7-8-12-19-39(2)25-26-16-15-17-27(22-26)33(42)40(3)20-13-9-10-14-21-41(4)34-37-29-24-31(44-6)30(43-5)23-28(29)32(35)38-34/h15-17,22-24,36H,7-14,18-21,25H2,1-6H3,(H2,35,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at oxytocin receptor in Wistar rat uterus assessed as inhibition of oxytocin-induced uterotonic activity treated 1 min prior to o... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408809
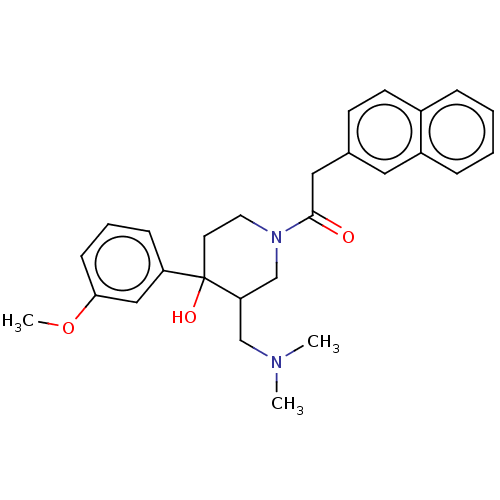
(CHEMBL5287093)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCCCCCC1)C(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C44H70N12O8/c45-30(16-11-21-50-42(46)47)36(58)52-31(17-12-22-51-43(48)49)37(59)54-44(19-9-3-1-2-4-10-20-44)41(64)53-32(26-57)38(60)55-25-29-15-6-5-13-27(29)23-34(55)39(61)56-33-18-8-7-14-28(33)24-35(56)40(62)63/h5-6,13,15,28,30-35,57H,1-4,7-12,14,16-26,45H2,(H,52,58)(H,53,64)(H,54,59)(H,62,63)(H4,46,47,50)(H4,48,49,51)/t28-,30+,31-,32-,33-,34+,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at MTL receptor in rabbit duodenum homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data