

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097869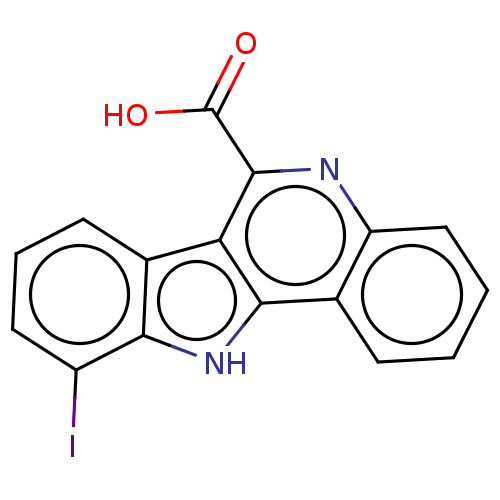 (CHEMBL3589662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397977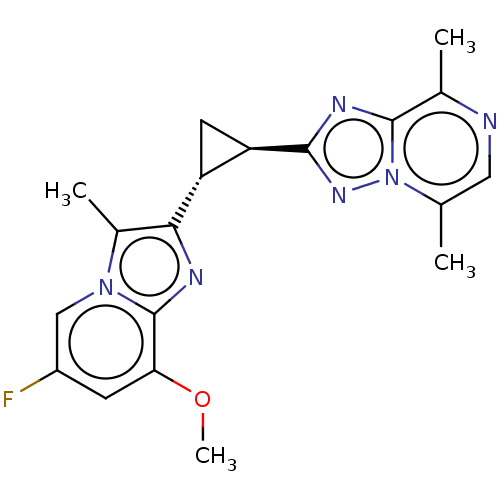 (US10604536, Compound 50 | US9994590, Compound 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397957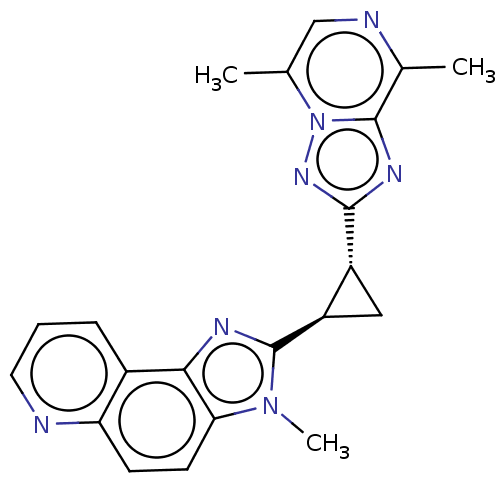 (US10604536, Compound 29 | US9994590, Compound 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397792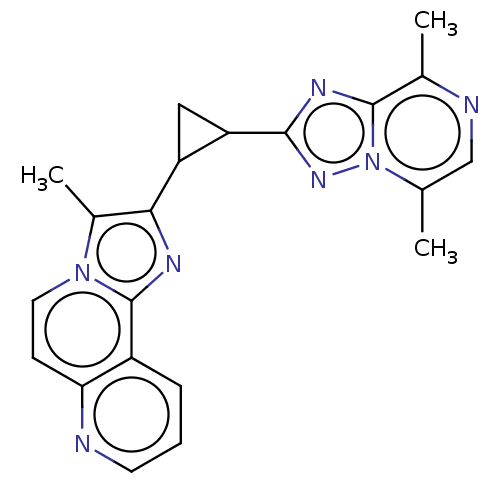 (US10604536, Compound 1 | US9994590, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397931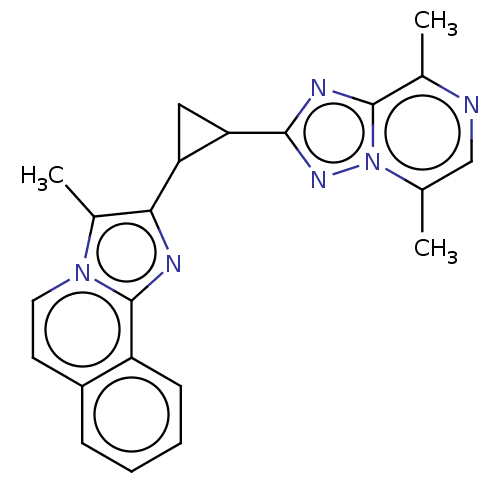 (US10604536, Compound 3 | US9994590, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397955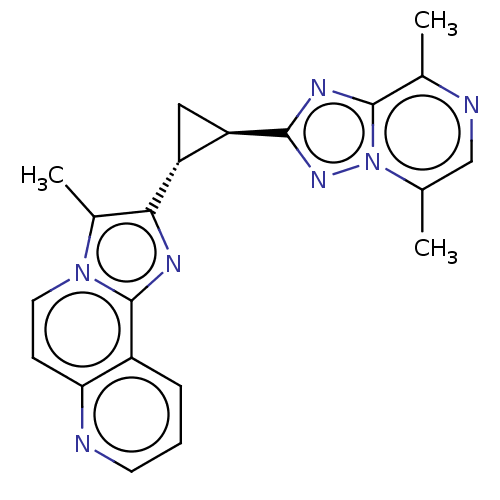 (US10604536, Compound 27 | US9994590, Compound 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397957 (US10604536, Compound 29 | US9994590, Compound 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397977 (US10604536, Compound 50 | US9994590, Compound 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397792 (US10604536, Compound 1 | US9994590, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397931 (US10604536, Compound 3 | US9994590, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397955 (US10604536, Compound 27 | US9994590, Compound 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 2 (Homo sapiens (Human)) | BDBM50097868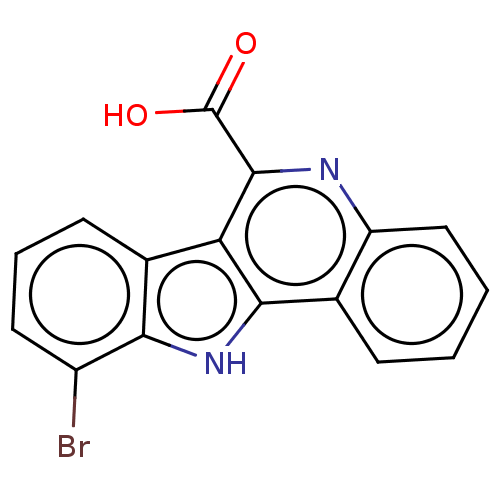 (CHEMBL3589661) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK2 expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097855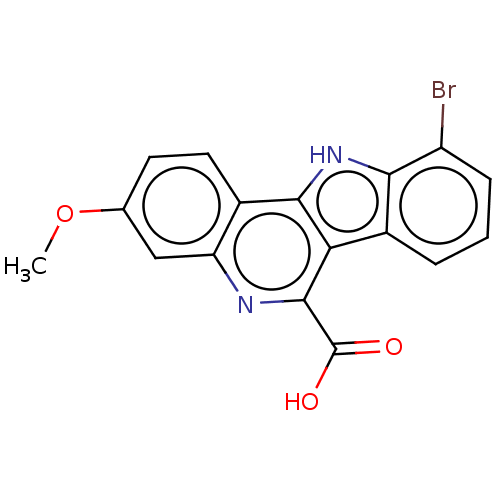 (CHEMBL3589666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097868 (CHEMBL3589661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Mus musculus) | BDBM50097855 (CHEMBL3589666) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CLK1 expressed in Escherichia coli using RS peptide as substrate after 30 mins by scintillation counting analysis | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097856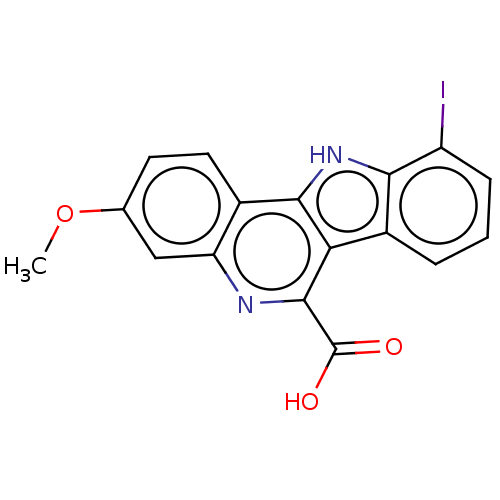 (CHEMBL3589667) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of recombinant VEGFR2 using poly(Glu,Tyr)4 as substrate assessed as inhibition of [33P]Phosphate incorporation into substrate after 80 min... | Eur J Med Chem 53: 254-63 (2012) Article DOI: 10.1016/j.ejmech.2012.04.007 BindingDB Entry DOI: 10.7270/Q2W66MT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097854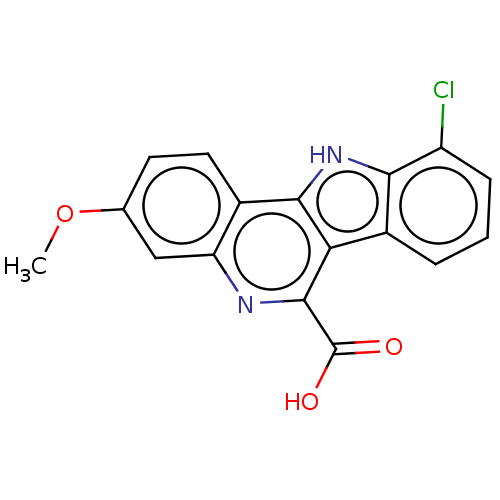 (CHEMBL3589665) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 2 (Homo sapiens (Human)) | BDBM50097855 (CHEMBL3589666) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK2 expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Mus musculus) | BDBM50097854 (CHEMBL3589665) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CLK1 expressed in Escherichia coli using RS peptide as substrate after 30 mins by scintillation counting analysis | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 3 (Homo sapiens (Human)) | BDBM50097863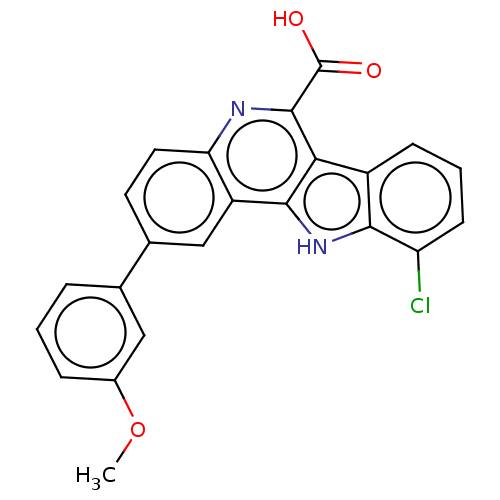 (CHEMBL3589674) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK3 expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097867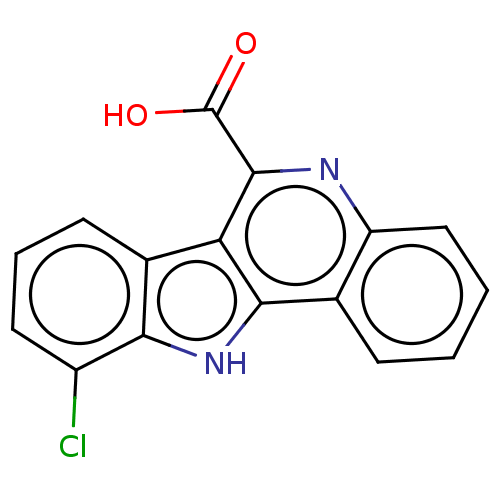 (CHEMBL3589660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Mus musculus) | BDBM50097868 (CHEMBL3589661) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CLK1 expressed in Escherichia coli using RS peptide as substrate after 30 mins by scintillation counting analysis | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097872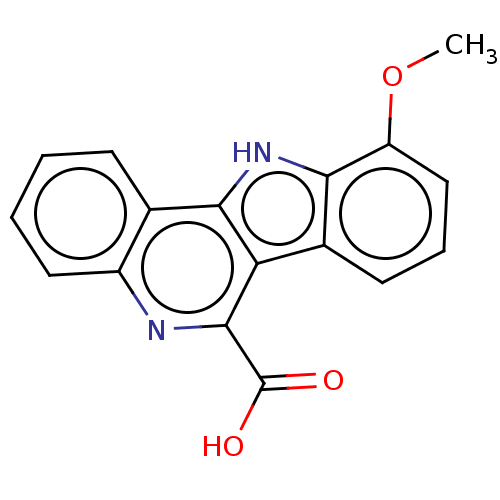 (CHEMBL3589663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 2 (Homo sapiens (Human)) | BDBM50097854 (CHEMBL3589665) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK2 expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK4 (Mus musculus) | BDBM50097868 (CHEMBL3589661) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CLK4 expressed in Escherichia coli using RS peptide as substrate after 30 mins by scintillation counting analysis | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097862 (CHEMBL3589673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 2 (Homo sapiens (Human)) | BDBM50097867 (CHEMBL3589660) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK2 expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM50097863 (CHEMBL3589674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of human recombinant DYRK1A expressed in Escherichia coli using RS peptide as substrate | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK4 (Mus musculus) | BDBM50097855 (CHEMBL3589666) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CLK4 expressed in Escherichia coli using RS peptide as substrate after 30 mins by scintillation counting analysis | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK4 (Mus musculus) | BDBM50097867 (CHEMBL3589660) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CLK4 expressed in Escherichia coli using RS peptide as substrate after 30 mins by scintillation counting analysis | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK1 (Mus musculus) | BDBM50097865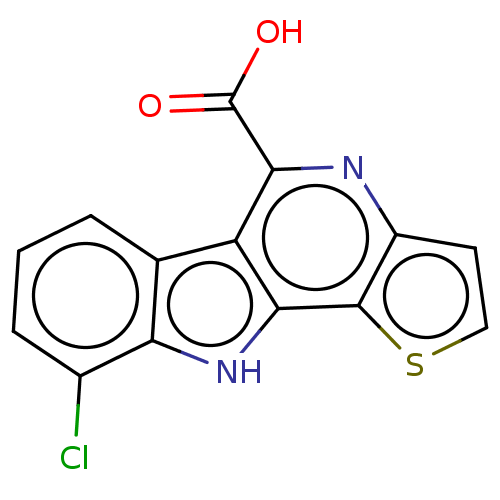 (CHEMBL3589774) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CLK1 expressed in Escherichia coli using RS peptide as substrate after 30 mins by scintillation counting analysis | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397960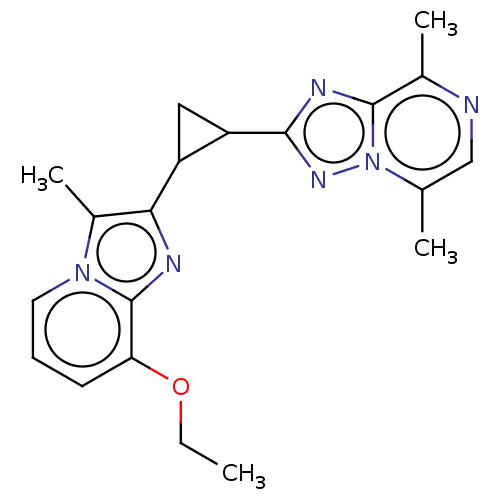 (US10604536, Compound 32 | US9994590, Compound 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397962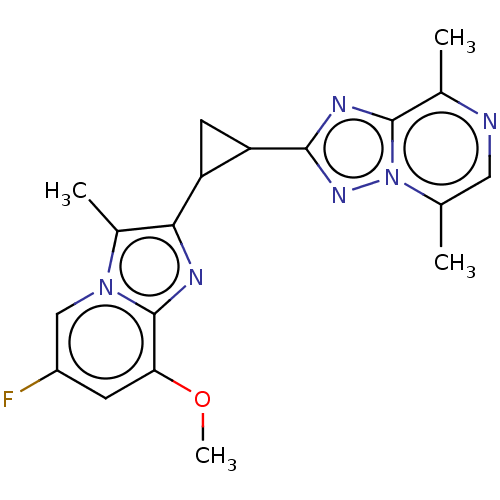 (US10604536, Compound 34 | US9994590, Compound 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397963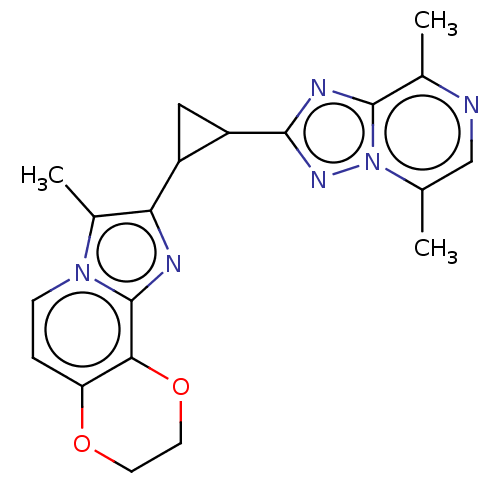 (US10604536, Compound 35 | US9994590, Compound 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397964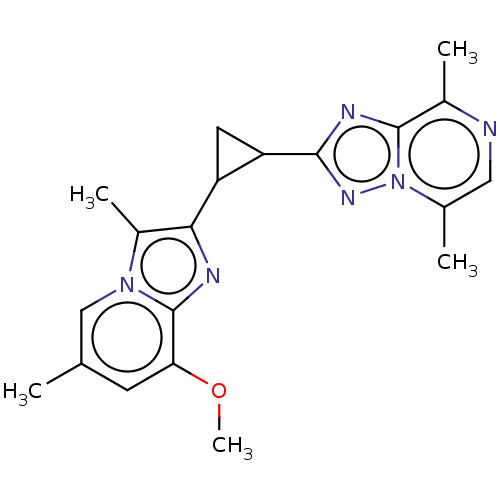 (US10604536, Compound 36 | US9994590, Compound 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397965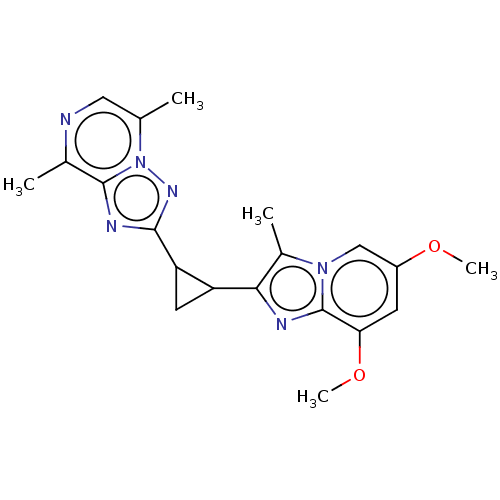 (US10604536, Compound 37 | US9994590, Compound 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397967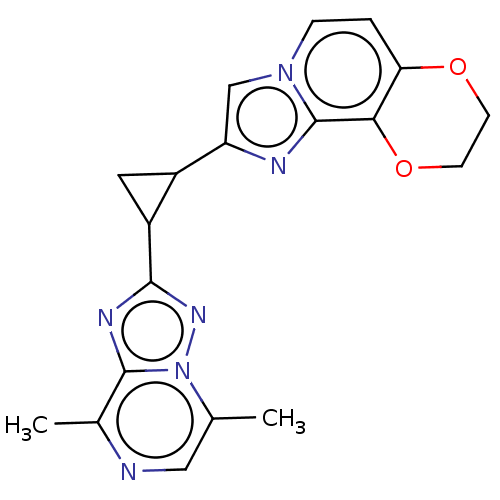 (US10604536, Compound 39 | US9994590, Compound 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397972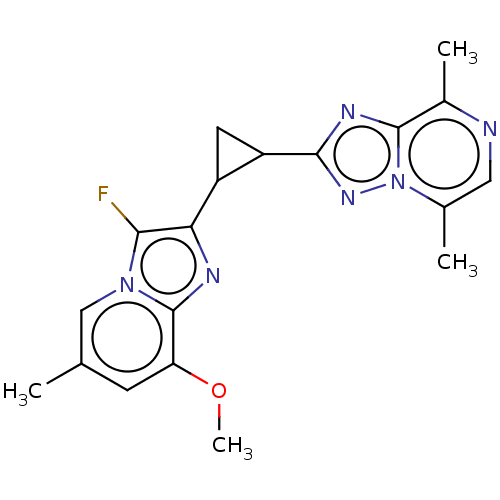 (US10604536, Compound 44 | US9994590, Compound 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397976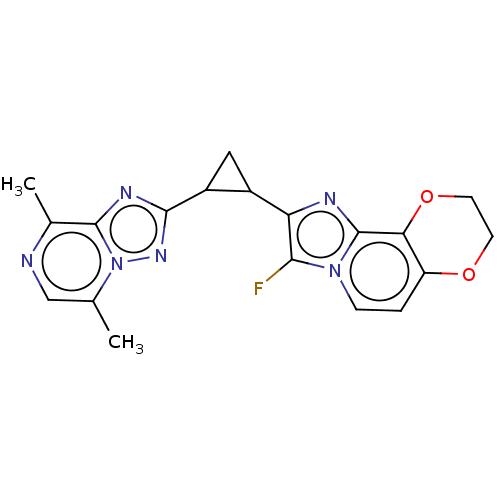 (US10604536, Compound 48 | US9994590, Compound 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397979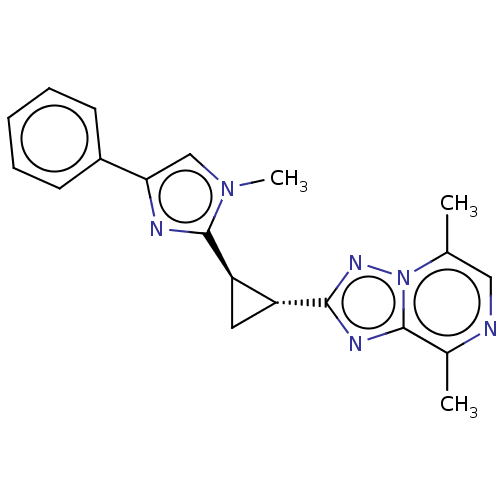 (US10604536, Compound 52 | US9994590, Compound 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397981 (US10604536, Compound 54 | US9994590, Compound 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397984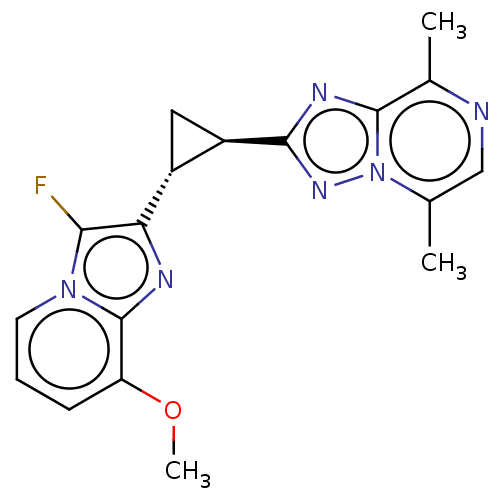 (US10604536, Compound 57 | US9994590, Compound 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397983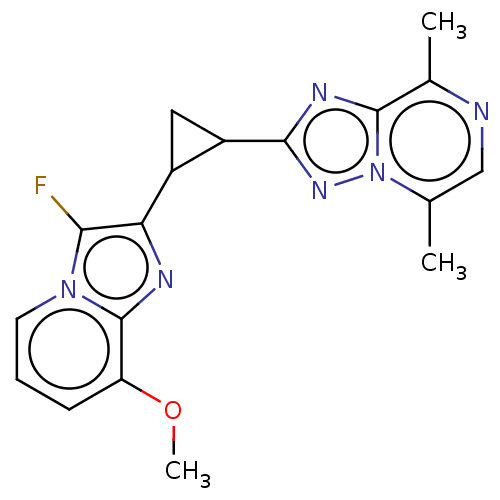 (US10604536, Compound 55 | US9994590, Compound 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharamaceuticals Inc. US Patent | Assay Description The compounds were determined using the Molecular Devices IMAP PDE Fluorescence Polarization assay using recombinant human PDE-10 enzyme expressed in... | US Patent US10604536 (2020) BindingDB Entry DOI: 10.7270/Q2QN69TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein kinase CLK2 (Mus musculus) | BDBM50097868 (CHEMBL3589661) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Braunschweig Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CLK2 expressed in Escherichia coli using RS peptide as substrate after 30 mins by scintillation counting analysis | J Med Chem 58: 3131-43 (2015) Article DOI: 10.1021/jm501994d BindingDB Entry DOI: 10.7270/Q2736SPK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397933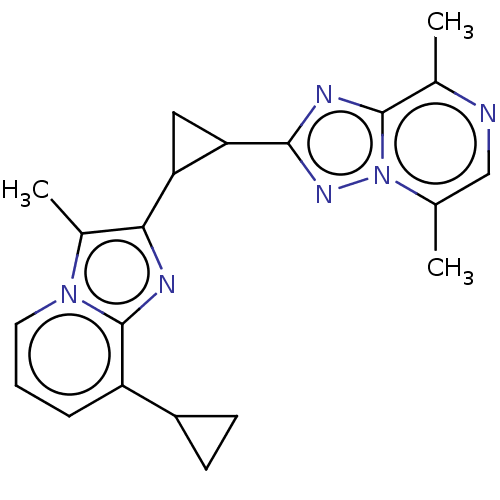 (US10604536, Compound 5 | US9994590, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397943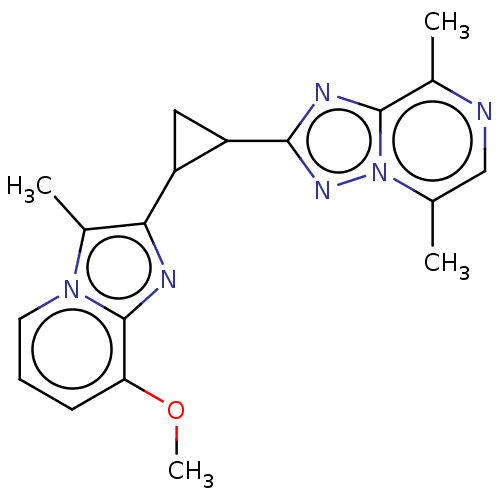 (US10604536, Compound 15 | US9994590, Compound 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397945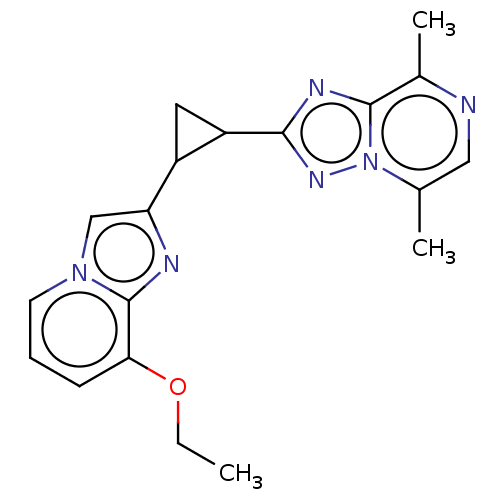 (US10604536, Compound 17 | US9994590, Compound 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397947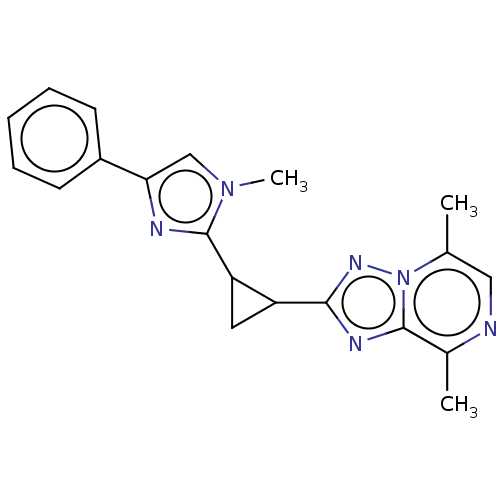 (US10604536, Compound 19 | US9994590, Compound 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM397949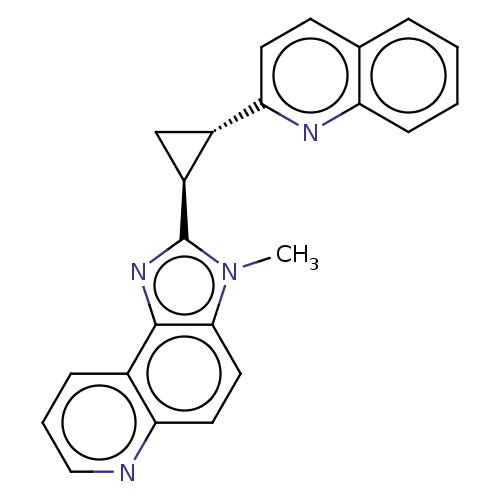 (US10604536, Compound 21 | US9994590, Compound 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona | Assay Description In one embodiment, the compounds provided herein were assayed for their ability to inhibit human PDE-10A. In one embodiment, the activities of the co... | J Med Chem 52: 5365-79 (2009) BindingDB Entry DOI: 10.7270/Q2VD71TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 435 total ) | Next | Last >> |