 Found 43 hits with Last Name = 'bolton' and Initial = 'r'
Found 43 hits with Last Name = 'bolton' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine protease 1
(Homo sapiens (Human)) | BDBM50072292

((3S,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072285
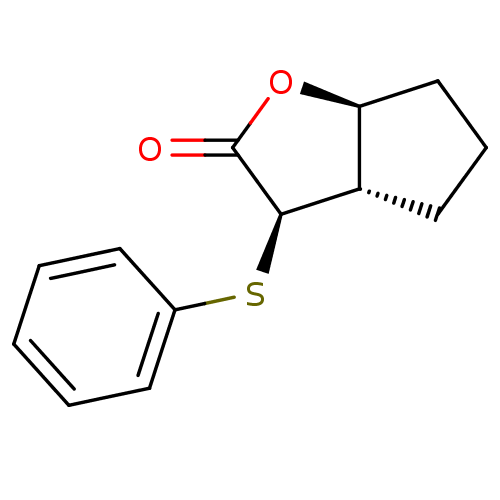
((3R,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072289

((3S,3aR,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10-,11+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072283
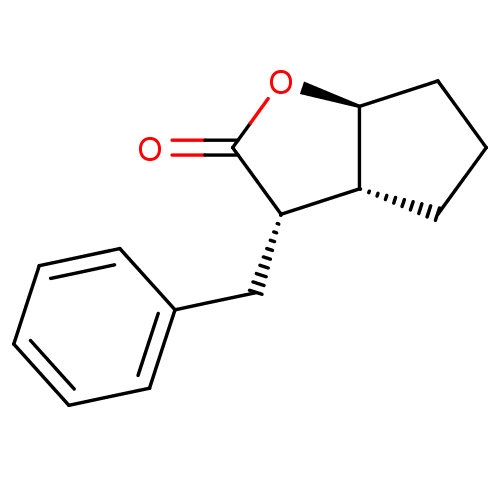
((3S,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072286
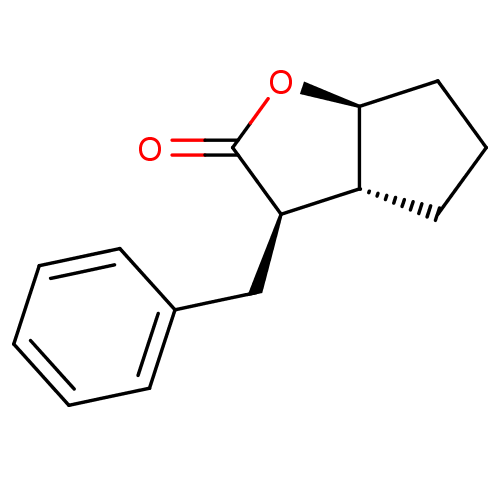
((3R,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072284

((1S,2R,4aR,6aS,6bS,7R,9aS,10aS)-2-Acetoxy-1,4a,6a,...)Show SMILES [H][C@@]12[#6]-[#6][C@]3([#6])[C@@]4([H])[#6]-[#6][C@@]5([#6])[C@]6([H])[#6@@H](-[#6]-[#6](=O)\[#6]=[#6](\[#6])-[#6])-[#6](=O)-[#8][C@@]6([H])[#6][C@]5([#6])[#6]4=[#6]-[#6][C@@]3([H])[C@]1([#6])[#6](=O)-[#8]2 |r,c:33| Show InChI InChI=1S/C30H40O5/c1-16(2)13-17(31)14-18-24-21(34-25(18)32)15-29(5)20-7-8-22-27(3,19(20)9-12-28(24,29)4)11-10-23-30(22,6)26(33)35-23/h7,13,18-19,21-24H,8-12,14-15H2,1-6H3/t18-,19+,21+,22-,23-,24-,27-,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072285

((3R,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072290

((3S,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...)Show InChI InChI=1S/C10H14O2/c1-2-4-8-7-5-3-6-9(7)12-10(8)11/h2,7-9H,1,3-6H2/t7-,8+,9+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075121
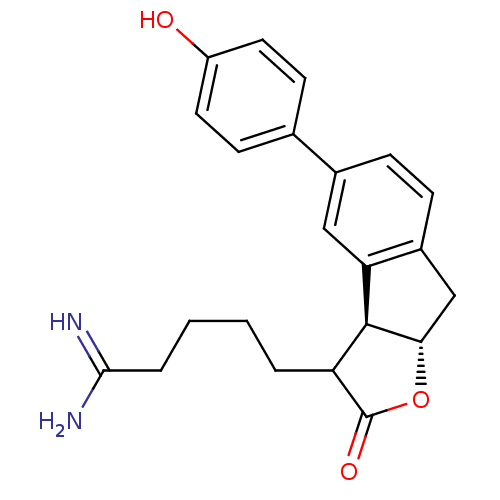
(5-[(3aS,8aS)-5-(4-Hydroxy-phenyl)-2-oxo-3,3a,8,8a-...)Show SMILES NC(=N)CCCCC1[C@@H]2[C@H](Cc3ccc(cc23)-c2ccc(O)cc2)OC1=O Show InChI InChI=1S/C22H24N2O3/c23-20(24)4-2-1-3-17-21-18-11-14(13-7-9-16(25)10-8-13)5-6-15(18)12-19(21)27-22(17)26/h5-11,17,19,21,25H,1-4,12H2,(H3,23,24)/t17?,19-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin was determined |
Bioorg Med Chem Lett 9: 431-6 (1999)
BindingDB Entry DOI: 10.7270/Q26T0KS3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072292

((3S,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072287

((3S,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahydro-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10?,11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072289

((3S,3aR,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10-,11+,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072290

((3S,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...)Show InChI InChI=1S/C10H14O2/c1-2-4-8-7-5-3-6-9(7)12-10(8)11/h2,7-9H,1,3-6H2/t7-,8+,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072287

((3S,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahydro-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10?,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072282

((3R,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...)Show InChI InChI=1S/C10H14O2/c1-2-4-8-7-5-3-6-9(7)12-10(8)11/h2,7-9H,1,3-6H2/t7-,8-,9+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072293
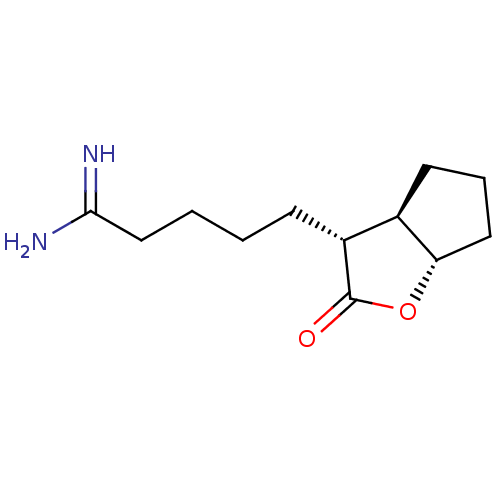
(5-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C12H20N2O2/c13-11(14)7-2-1-4-9-8-5-3-6-10(8)16-12(9)15/h8-10H,1-7H2,(H3,13,14)/t8-,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072282

((3R,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...)Show InChI InChI=1S/C10H14O2/c1-2-4-8-7-5-3-6-9(7)12-10(8)11/h2,7-9H,1,3-6H2/t7-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072282

((3R,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...)Show InChI InChI=1S/C10H14O2/c1-2-4-8-7-5-3-6-9(7)12-10(8)11/h2,7-9H,1,3-6H2/t7-,8-,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072284

((1S,2R,4aR,6aS,6bS,7R,9aS,10aS)-2-Acetoxy-1,4a,6a,...)Show SMILES [H][C@@]12[#6]-[#6][C@]3([#6])[C@@]4([H])[#6]-[#6][C@@]5([#6])[C@]6([H])[#6@@H](-[#6]-[#6](=O)\[#6]=[#6](\[#6])-[#6])-[#6](=O)-[#8][C@@]6([H])[#6][C@]5([#6])[#6]4=[#6]-[#6][C@@]3([H])[C@]1([#6])[#6](=O)-[#8]2 |r,c:33| Show InChI InChI=1S/C30H40O5/c1-16(2)13-17(31)14-18-24-21(34-25(18)32)15-29(5)20-7-8-22-27(3,19(20)9-12-28(24,29)4)11-10-23-30(22,6)26(33)35-23/h7,13,18-19,21-24H,8-12,14-15H2,1-6H3/t18-,19+,21+,22-,23-,24-,27-,28+,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072284

((1S,2R,4aR,6aS,6bS,7R,9aS,10aS)-2-Acetoxy-1,4a,6a,...)Show SMILES [H][C@@]12[#6]-[#6][C@]3([#6])[C@@]4([H])[#6]-[#6][C@@]5([#6])[C@]6([H])[#6@@H](-[#6]-[#6](=O)\[#6]=[#6](\[#6])-[#6])-[#6](=O)-[#8][C@@]6([H])[#6][C@]5([#6])[#6]4=[#6]-[#6][C@@]3([H])[C@]1([#6])[#6](=O)-[#8]2 |r,c:33| Show InChI InChI=1S/C30H40O5/c1-16(2)13-17(31)14-18-24-21(34-25(18)32)15-29(5)20-7-8-22-27(3,19(20)9-12-28(24,29)4)11-10-23-30(22,6)26(33)35-23/h7,13,18-19,21-24H,8-12,14-15H2,1-6H3/t18-,19+,21+,22-,23-,24-,27-,28+,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Coagulation factor X |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072283

((3S,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12+,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075122
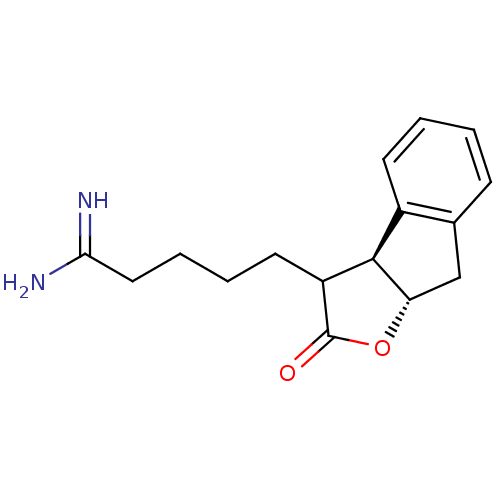
(5-((3aS,8aS)-2-Oxo-3,3a,8,8a-tetrahydro-2H-indeno[...)Show InChI InChI=1S/C16H20N2O2/c17-14(18)8-4-3-7-12-15-11-6-2-1-5-10(11)9-13(15)20-16(12)19/h1-2,5-6,12-13,15H,3-4,7-9H2,(H3,17,18)/t12?,13-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin was determined |
Bioorg Med Chem Lett 9: 431-6 (1999)
BindingDB Entry DOI: 10.7270/Q26T0KS3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072286

((3R,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072290

((3S,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...)Show InChI InChI=1S/C10H14O2/c1-2-4-8-7-5-3-6-9(7)12-10(8)11/h2,7-9H,1,3-6H2/t7-,8+,9+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072283

((3S,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12+,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072286

((3R,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072285

((3R,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072289

((3S,3aR,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10-,11+,12+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072292

((3S,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072293

(5-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C12H20N2O2/c13-11(14)7-2-1-4-9-8-5-3-6-10(8)16-12(9)15/h8-10H,1-7H2,(H3,13,14)/t8-,9-,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin was determined |
Bioorg Med Chem Lett 9: 431-6 (1999)
BindingDB Entry DOI: 10.7270/Q26T0KS3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072293

(5-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C12H20N2O2/c13-11(14)7-2-1-4-9-8-5-3-6-10(8)16-12(9)15/h8-10H,1-7H2,(H3,13,14)/t8-,9-,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072291
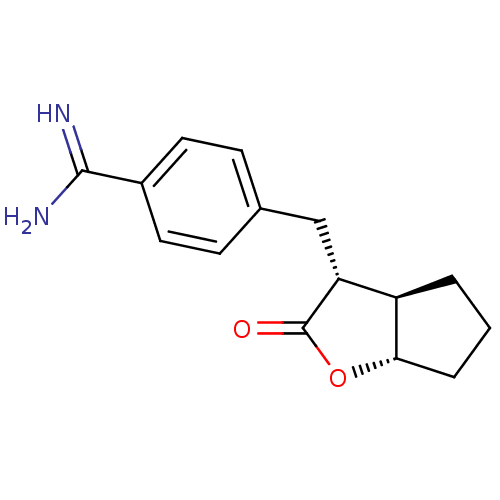
(4-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show SMILES NC(=N)c1ccc(C[C@@H]2[C@H]3CCC[C@@H]3OC2=O)cc1 Show InChI InChI=1S/C15H18N2O2/c16-14(17)10-6-4-9(5-7-10)8-12-11-2-1-3-13(11)19-15(12)18/h4-7,11-13H,1-3,8H2,(H3,16,17)/t11-,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075123
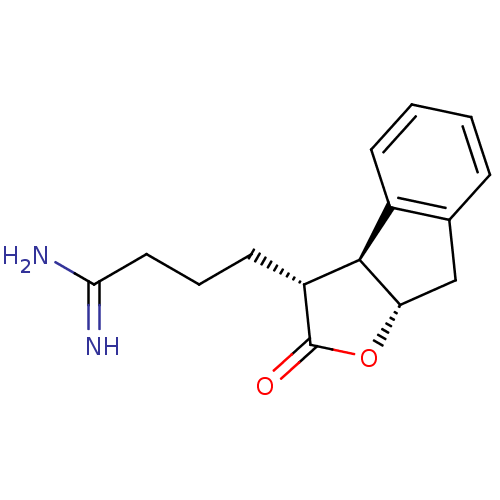
(4-((3R,3aS,8aS)-2-Oxo-3,3a,8,8a-tetrahydro-2H-inde...)Show SMILES NC(=N)CCC[C@@H]1[C@@H]2[C@H](Cc3ccccc23)OC1=O Show InChI InChI=1S/C15H18N2O2/c16-13(17)7-3-6-11-14-10-5-2-1-4-9(10)8-12(14)19-15(11)18/h1-2,4-5,11-12,14H,3,6-8H2,(H3,16,17)/t11-,12+,14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin was determined |
Bioorg Med Chem Lett 9: 431-6 (1999)
BindingDB Entry DOI: 10.7270/Q26T0KS3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075120
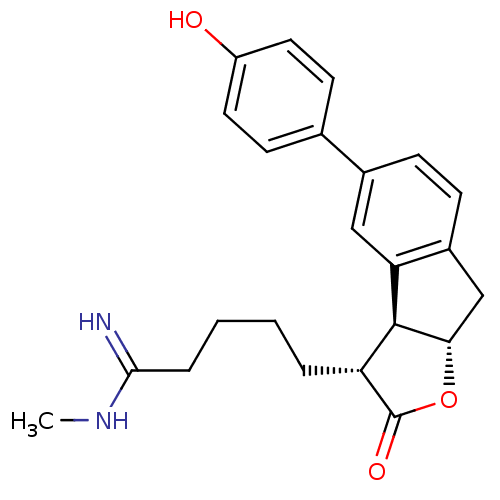
(5-[(3R,3aS,8aS)-5-(4-Hydroxy-phenyl)-2-oxo-3,3a,8,...)Show SMILES CNC(=N)CCCC[C@@H]1[C@@H]2[C@H](Cc3ccc(cc23)-c2ccc(O)cc2)OC1=O Show InChI InChI=1S/C23H26N2O3/c1-25-21(24)5-3-2-4-18-22-19-12-15(14-8-10-17(26)11-9-14)6-7-16(19)13-20(22)28-23(18)27/h6-12,18,20,22,26H,2-5,13H2,1H3,(H2,24,25)/t18-,20+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin was determined |
Bioorg Med Chem Lett 9: 431-6 (1999)
BindingDB Entry DOI: 10.7270/Q26T0KS3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075119
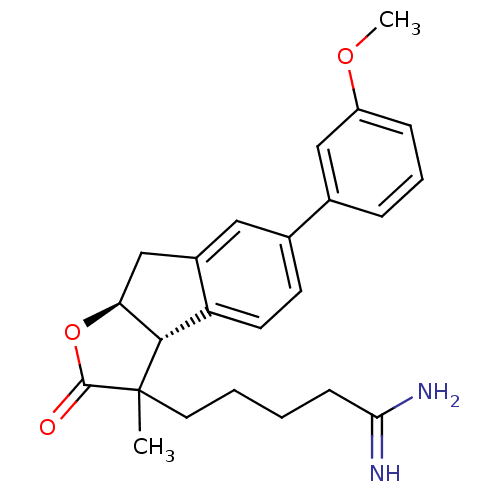
(5-[(3aR,8aS)-6-(3-Methoxy-phenyl)-3-methyl-2-oxo-3...)Show SMILES COc1cccc(c1)-c1ccc2[C@H]3[C@H](Cc2c1)OC(=O)C3(C)CCCCC(N)=N Show InChI InChI=1S/C24H28N2O3/c1-24(11-4-3-8-21(25)26)22-19-10-9-16(15-6-5-7-18(13-15)28-2)12-17(19)14-20(22)29-23(24)27/h5-7,9-10,12-13,20,22H,3-4,8,11,14H2,1-2H3,(H3,25,26)/t20-,22-,24?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human thrombin was determined |
Bioorg Med Chem Lett 9: 431-6 (1999)
BindingDB Entry DOI: 10.7270/Q26T0KS3 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072288

(3-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show SMILES NC(=N)c1cccc(C[C@@H]2[C@H]3CCC[C@@H]3OC2=O)c1 Show InChI InChI=1S/C15H18N2O2/c16-14(17)10-4-1-3-9(7-10)8-12-11-5-2-6-13(11)19-15(12)18/h1,3-4,7,11-13H,2,5-6,8H2,(H3,16,17)/t11-,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50072284

((1S,2R,4aR,6aS,6bS,7R,9aS,10aS)-2-Acetoxy-1,4a,6a,...)Show SMILES [H][C@@]12[#6]-[#6][C@]3([#6])[C@@]4([H])[#6]-[#6][C@@]5([#6])[C@]6([H])[#6@@H](-[#6]-[#6](=O)\[#6]=[#6](\[#6])-[#6])-[#6](=O)-[#8][C@@]6([H])[#6][C@]5([#6])[#6]4=[#6]-[#6][C@@]3([H])[C@]1([#6])[#6](=O)-[#8]2 |r,c:33| Show InChI InChI=1S/C30H40O5/c1-16(2)13-17(31)14-18-24-21(34-25(18)32)15-29(5)20-7-8-22-27(3,19(20)9-12-28(24,29)4)11-10-23-30(22,6)26(33)35-23/h7,13,18-19,21-24H,8-12,14-15H2,1-6H3/t18-,19+,21+,22-,23-,24-,27-,28+,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of human leukocyte elastase(HLE). |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072291

(4-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show SMILES NC(=N)c1ccc(C[C@@H]2[C@H]3CCC[C@@H]3OC2=O)cc1 Show InChI InChI=1S/C15H18N2O2/c16-14(17)10-6-4-9(5-7-10)8-12-11-2-1-3-13(11)19-15(12)18/h4-7,11-13H,1-3,8H2,(H3,16,17)/t11-,12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50072284

((1S,2R,4aR,6aS,6bS,7R,9aS,10aS)-2-Acetoxy-1,4a,6a,...)Show SMILES [H][C@@]12[#6]-[#6][C@]3([#6])[C@@]4([H])[#6]-[#6][C@@]5([#6])[C@]6([H])[#6@@H](-[#6]-[#6](=O)\[#6]=[#6](\[#6])-[#6])-[#6](=O)-[#8][C@@]6([H])[#6][C@]5([#6])[#6]4=[#6]-[#6][C@@]3([H])[C@]1([#6])[#6](=O)-[#8]2 |r,c:33| Show InChI InChI=1S/C30H40O5/c1-16(2)13-17(31)14-18-24-21(34-25(18)32)15-29(5)20-7-8-22-27(3,19(20)9-12-28(24,29)4)11-10-23-30(22,6)26(33)35-23/h7,13,18-19,21-24H,8-12,14-15H2,1-6H3/t18-,19+,21+,22-,23-,24-,27-,28+,29-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072291

(4-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show SMILES NC(=N)c1ccc(C[C@@H]2[C@H]3CCC[C@@H]3OC2=O)cc1 Show InChI InChI=1S/C15H18N2O2/c16-14(17)10-6-4-9(5-7-10)8-12-11-2-1-3-13(11)19-15(12)18/h4-7,11-13H,1-3,8H2,(H3,16,17)/t11-,12-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072293

(5-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C12H20N2O2/c13-11(14)7-2-1-4-9-8-5-3-6-10(8)16-12(9)15/h8-10H,1-7H2,(H3,13,14)/t8-,9-,10+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072288

(3-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show SMILES NC(=N)c1cccc(C[C@@H]2[C@H]3CCC[C@@H]3OC2=O)c1 Show InChI InChI=1S/C15H18N2O2/c16-14(17)10-4-1-3-9(7-10)8-12-11-5-2-6-13(11)19-15(12)18/h1,3-4,7,11-13H,2,5-6,8H2,(H3,16,17)/t11-,12-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072288

(3-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...)Show SMILES NC(=N)c1cccc(C[C@@H]2[C@H]3CCC[C@@H]3OC2=O)c1 Show InChI InChI=1S/C15H18N2O2/c16-14(17)10-4-1-3-9(7-10)8-12-11-5-2-6-13(11)19-15(12)18/h1,3-4,7,11-13H,2,5-6,8H2,(H3,16,17)/t11-,12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data