

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50464028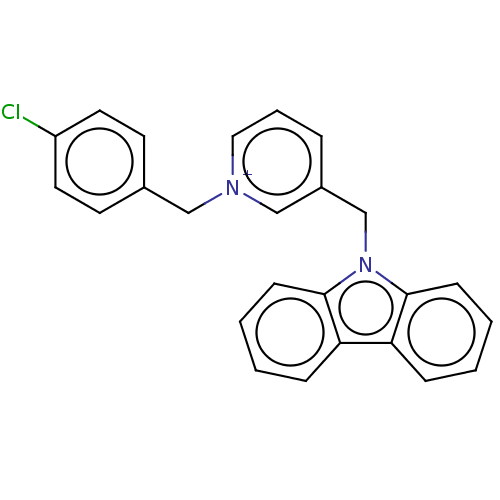 (CHEMBL4241164) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BuChE using varying levels of butyrylthiocholine iodide as substrate preincubated for 10 mins followed by subst... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50422387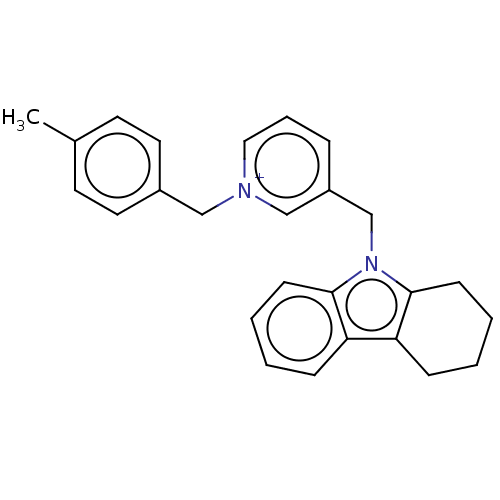 (CHEMBL4159171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate by Lineweaver-burk plot analysis | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50422394 (CHEMBL3558149) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 6 mi... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 (1 to 460 residues) expressed in baculovirus-infected insect cells using Rh-EVNLDAEFK-quencher as substrate mea... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured f... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 mi... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10949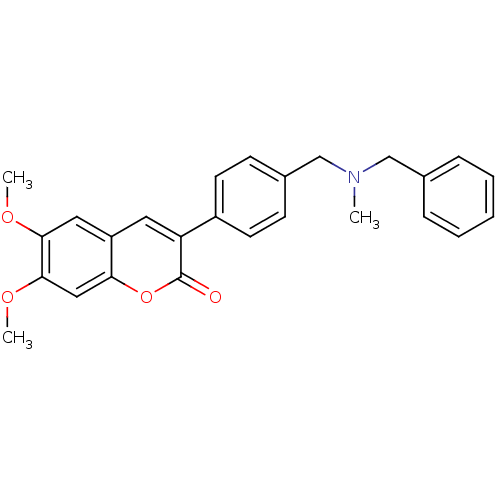 (3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human AChE | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464028 (CHEMBL4241164) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50422387 (CHEMBL4159171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50422386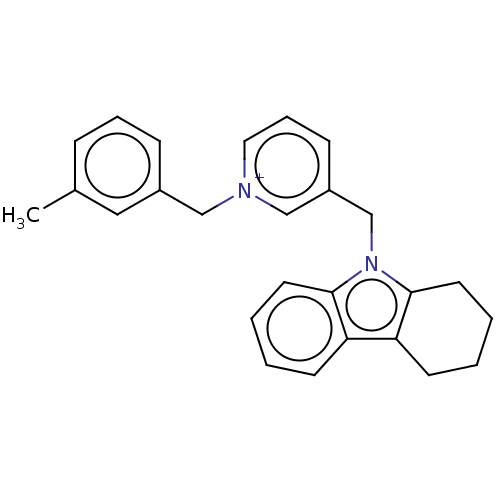 (CHEMBL4169820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464019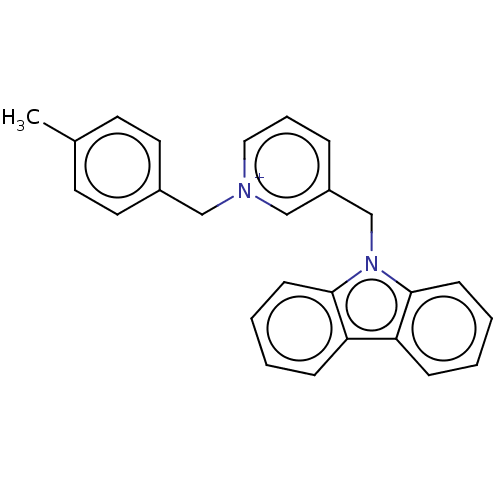 (CHEMBL4245106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464031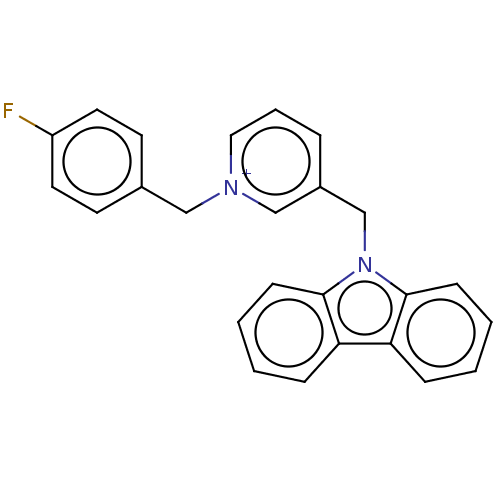 (CHEMBL4248896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464025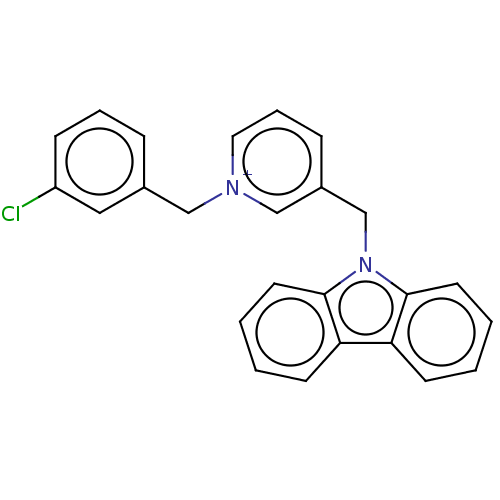 (CHEMBL4244170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421756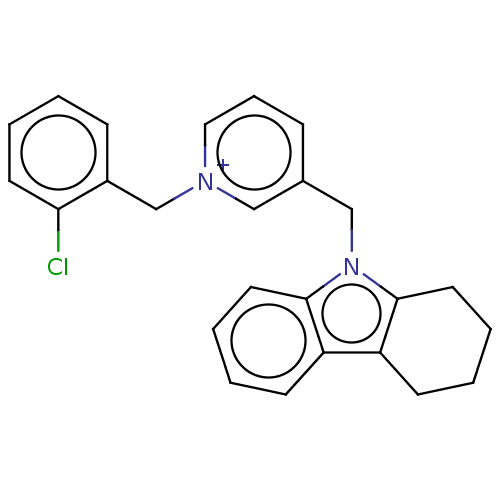 (CHEMBL4161385) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421747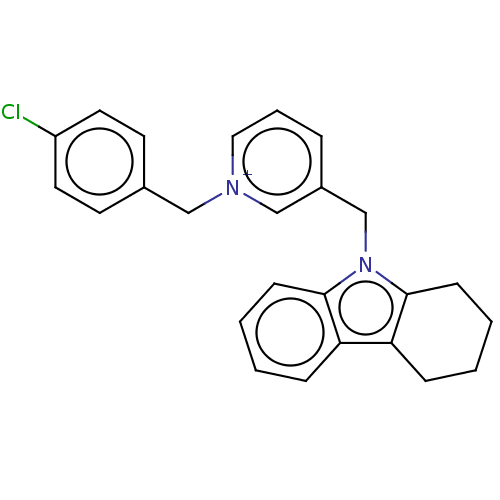 (CHEMBL4171576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464021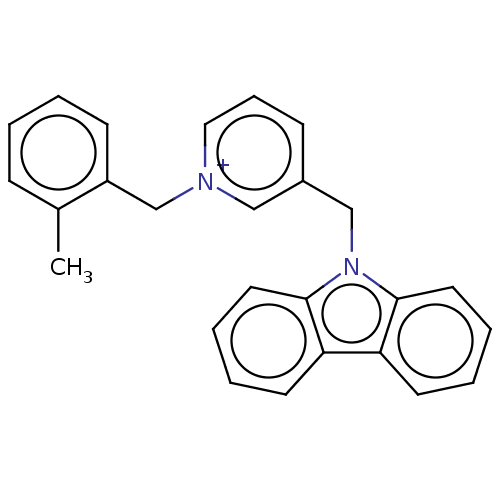 (CHEMBL4241338) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421752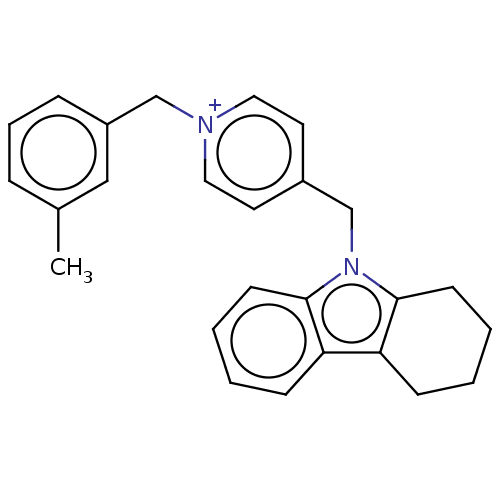 (CHEMBL4166704) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421757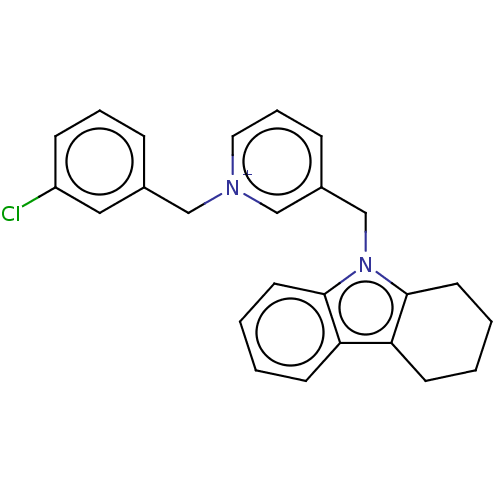 (CHEMBL4160961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421744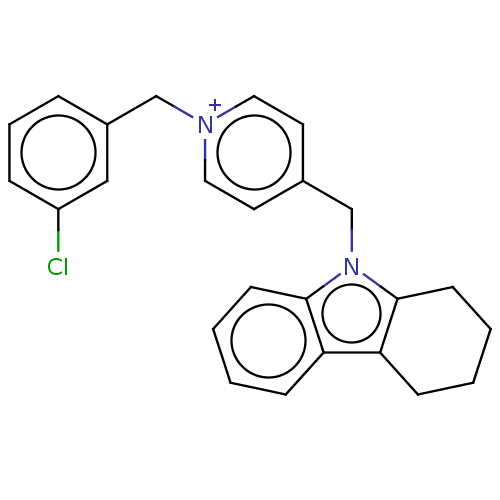 (CHEMBL4161962) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464029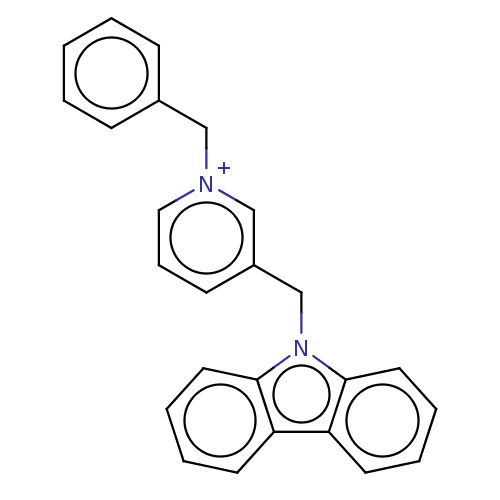 (CHEMBL4242583) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464030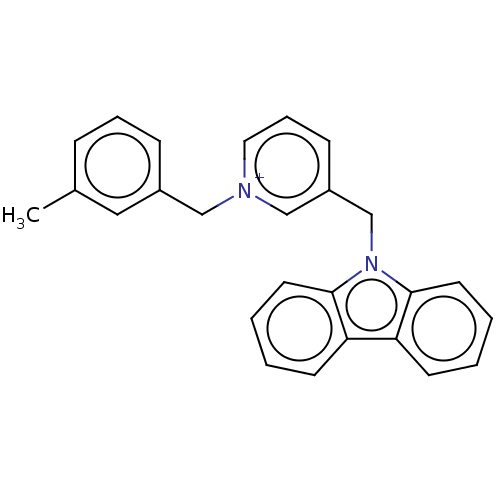 (CHEMBL4244620) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50422396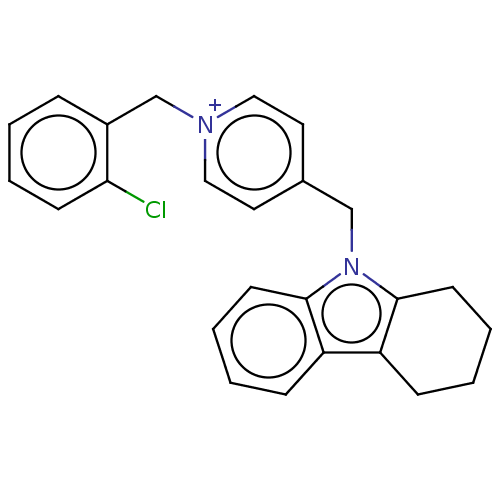 (CHEMBL4159647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464026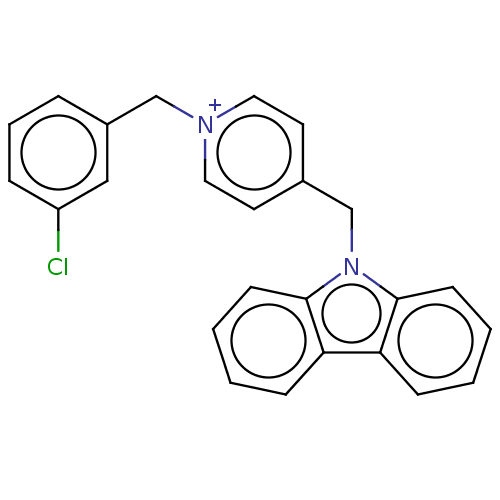 (CHEMBL4240662) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464027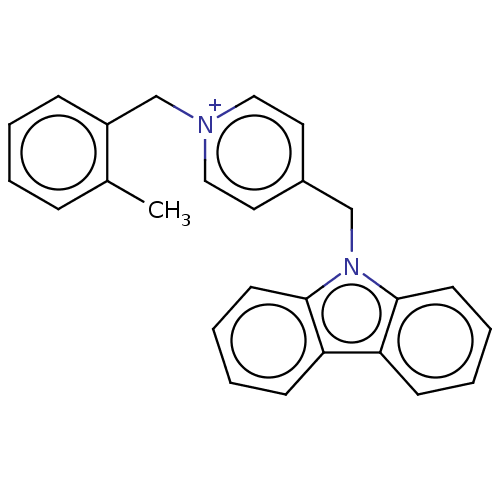 (CHEMBL4243615) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464017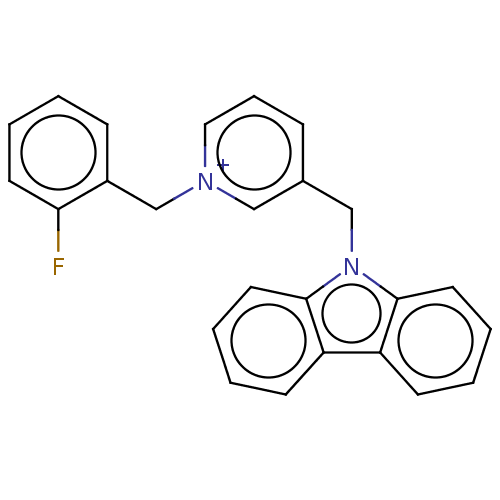 (CHEMBL4247756) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421749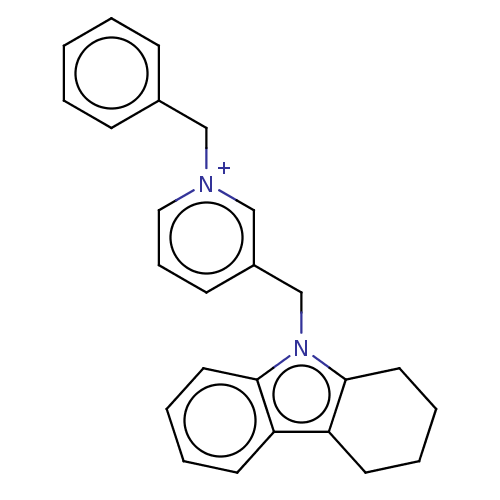 (CHEMBL4173247) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421748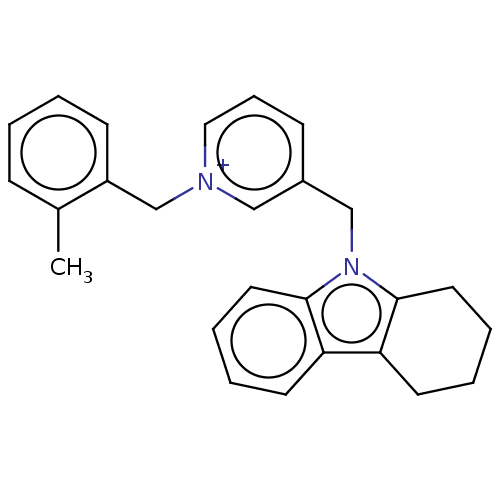 (CHEMBL4159886) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464022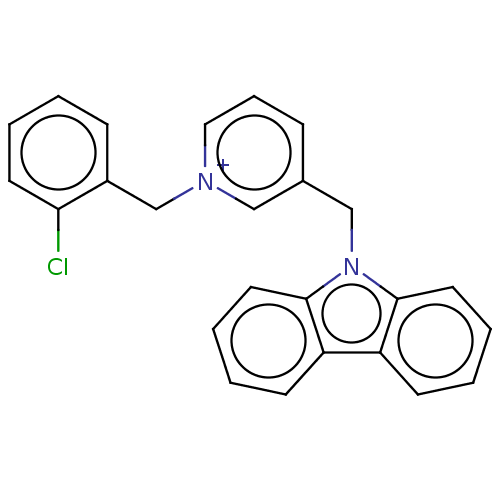 (CHEMBL4244767) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421751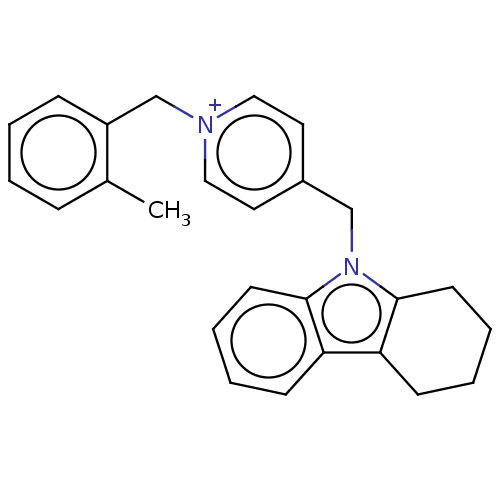 (CHEMBL4174929) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421755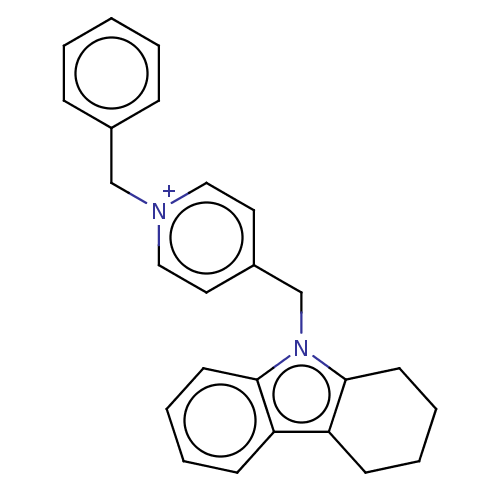 (CHEMBL4169308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421759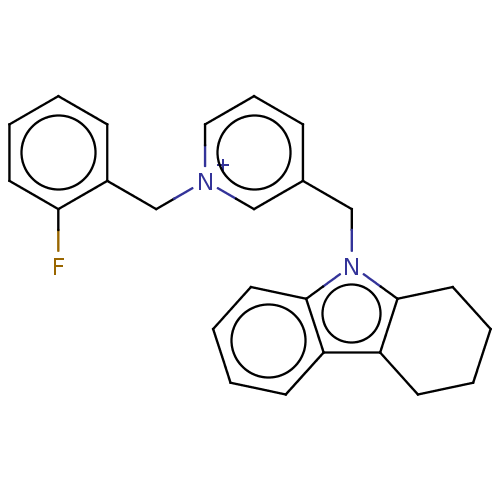 (CHEMBL4176078) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421970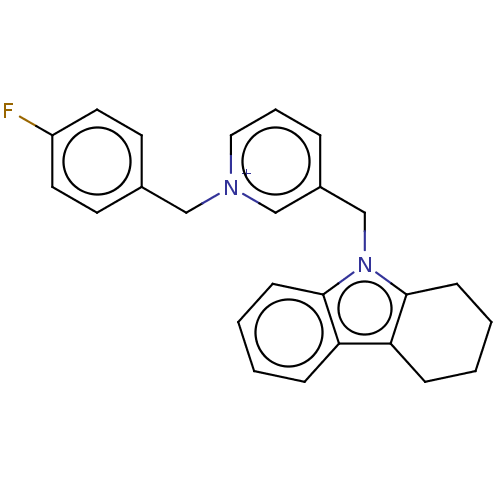 (CHEMBL4167804) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421880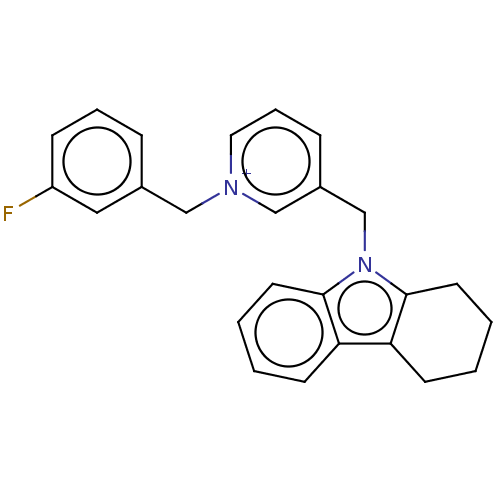 (CHEMBL4168234) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464034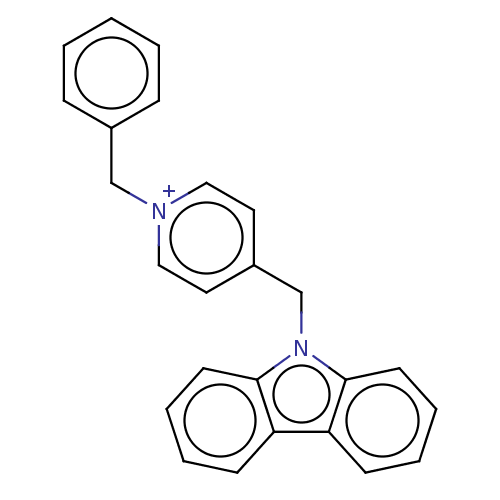 (CHEMBL4251230) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464033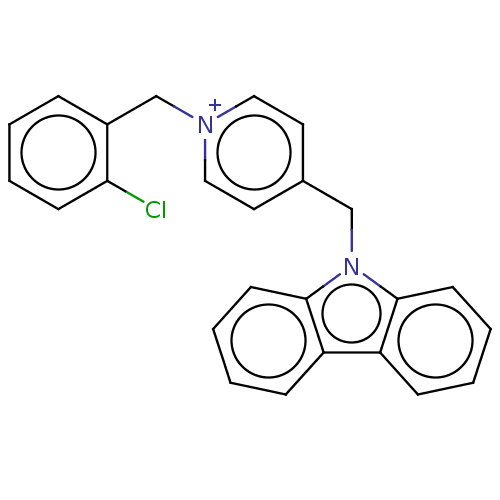 (CHEMBL4250760) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421753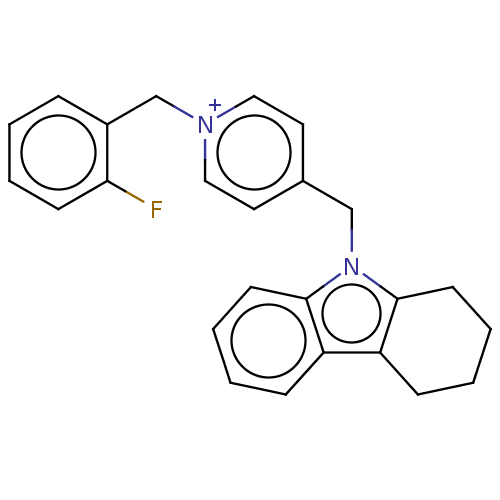 (CHEMBL4175629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464018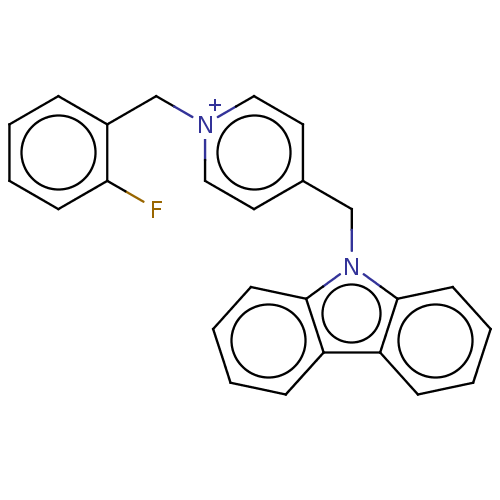 (CHEMBL4239674) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421745 (CHEMBL4165332) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464020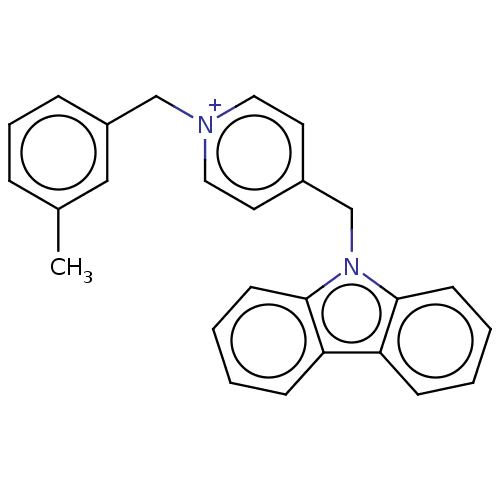 (CHEMBL4243151) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421746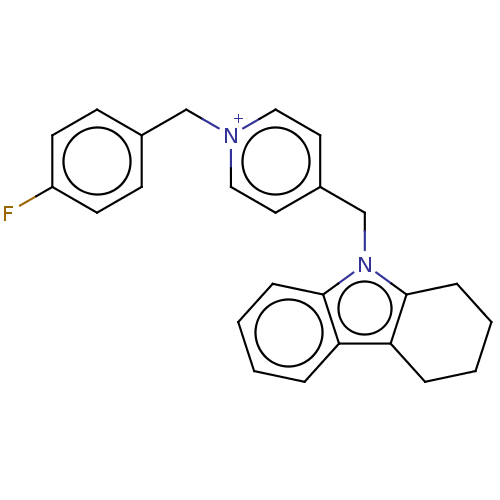 (CHEMBL4175199) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464036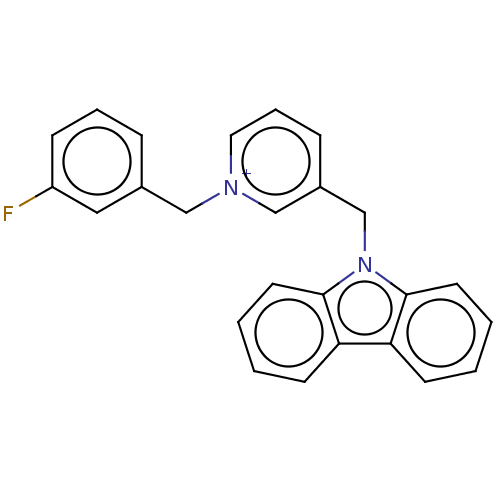 (CHEMBL4240265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50422393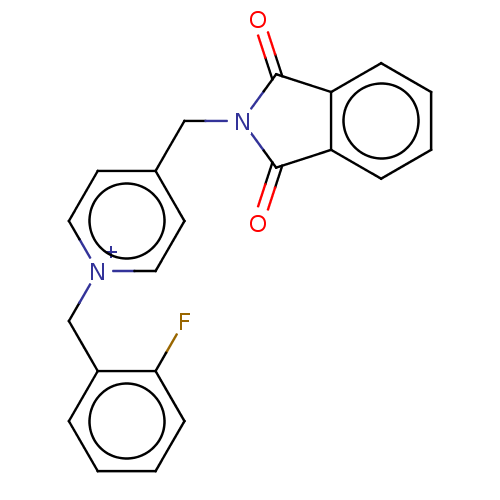 (CHEMBL4170878) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Torpedo californica AChE using acetylthiocholine iodide as substrate preincubated for 15 mins followed by substrate addition by Ellman'... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464032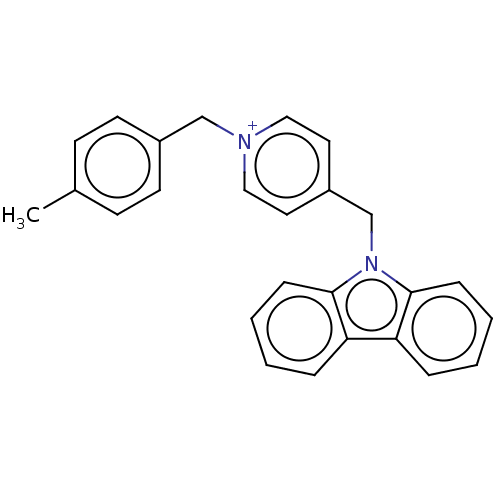 (CHEMBL4247394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464024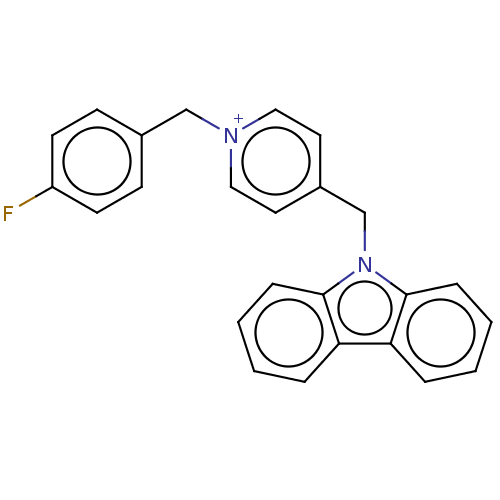 (CHEMBL4246944) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50421754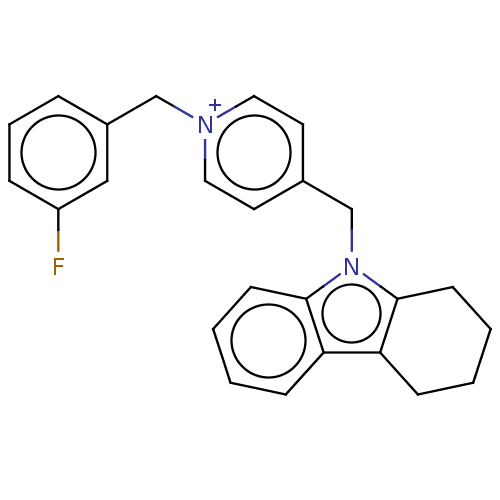 (CHEMBL4172701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 m... | Eur J Med Chem 155: 49-60 (2018) Article DOI: 10.1016/j.ejmech.2018.05.031 BindingDB Entry DOI: 10.7270/Q2765HWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464035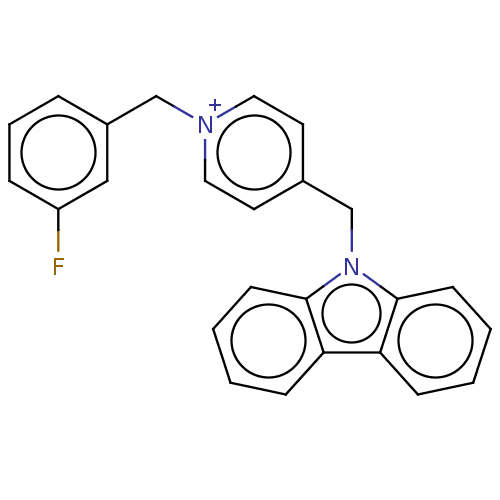 (CHEMBL4249779) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50464023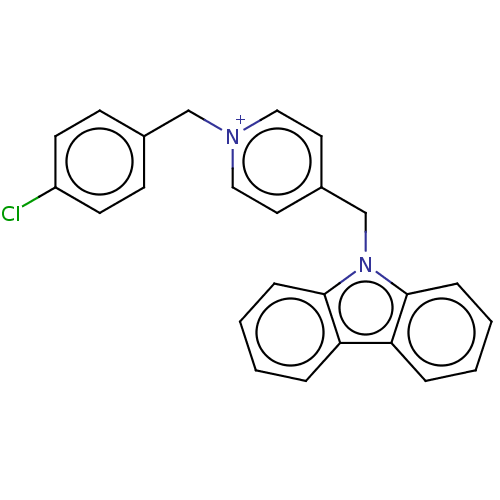 (CHEMBL4239383) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 ... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured for 5 mi... | Bioorg Med Chem 26: 4952-4962 (2018) Article DOI: 10.1016/j.bmc.2018.08.035 BindingDB Entry DOI: 10.7270/Q2NS0XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 98 total ) | Next | Last >> |