 Found 240 hits with Last Name = 'le guevel' and Initial = 'r'
Found 240 hits with Last Name = 'le guevel' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523281

(CHEMBL4583650)Show SMILES O=C(Nc1ccc(cn1)-c1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-16-15-22(20-37-31)29-21-43(42-41-29)19-17-36-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,36,38)(H2,37,39,40,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523276

(CHEMBL4443989)Show SMILES [H][C@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)-c3cn(CCNc4c5CCCCc5nc5ccccc45)nn3)c21 |r| Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46)/t30-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523265

(CHEMBL4531222)Show SMILES O=C(Nc1cc(COCc2cn(CCCc3ccccc3)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C31H33N7O3/c39-30-25-11-6-12-26(29(25)27-13-4-5-17-38(27)30)33-31(40)34-28-18-23(14-15-32-28)20-41-21-24-19-37(36-35-24)16-7-10-22-8-2-1-3-9-22/h1-3,6,8-9,11-12,14-15,18-19,27H,4-5,7,10,13,16-17,20-21H2,(H2,32,33,34,40) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50435473
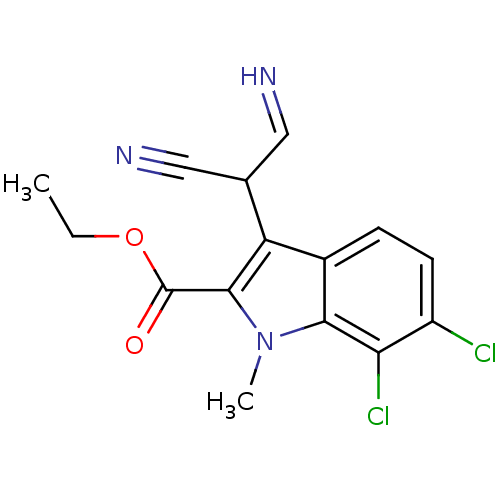
(CHEMBL1236620)Show InChI InChI=1S/C15H13Cl2N3O2/c1-3-22-15(21)14-11(8(6-18)7-19)9-4-5-10(16)12(17)13(9)20(14)2/h4-6,8,18H,3H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK1 (130 to end residues) using ERMRPRKRQGSVRRRV as substrate incubated for 40 mins in presence of [gamma33P-ATP] by... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115962
BindingDB Entry DOI: 10.7270/Q2KD22JS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha/p85alpha by fluorescence based immunoassay |
Eur J Med Chem 57: 225-33 (2012)
Article DOI: 10.1016/j.ejmech.2012.09.001
BindingDB Entry DOI: 10.7270/Q2RF5W4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523276

(CHEMBL4443989)Show SMILES [H][C@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)-c3cn(CCNc4c5CCCCc5nc5ccccc45)nn3)c21 |r| Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46)/t30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523269

(CHEMBL4446287)Show SMILES CN(Cc1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Cc1ccnc(NC(=O)Nc2cccc3C(=O)N4CCCCC4c23)c1 Show InChI InChI=1S/C38H42N10O2/c1-46(23-26-24-47(45-44-26)20-18-40-36-27-9-2-4-12-30(27)41-31-13-5-3-10-28(31)36)22-25-16-17-39-34(21-25)43-38(50)42-32-14-8-11-29-35(32)33-15-6-7-19-48(33)37(29)49/h2,4,8-9,11-12,14,16-17,21,24,33H,3,5-7,10,13,15,18-20,22-23H2,1H3,(H,40,41)(H2,39,42,43,50) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523277
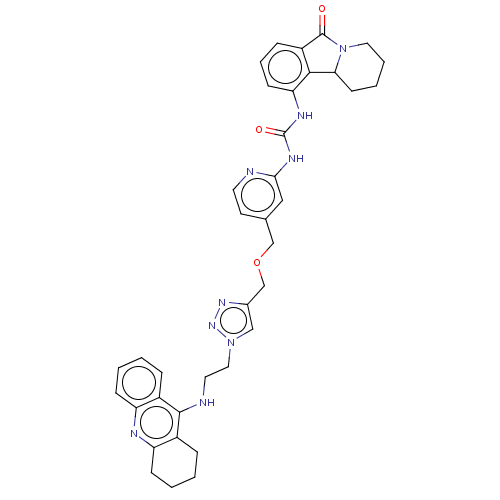
(CHEMBL4516356)Show SMILES O=C(Nc1cc(COCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O3/c47-36-28-10-7-13-31(34(28)32-14-5-6-18-46(32)36)41-37(48)42-33-20-24(15-16-38-33)22-49-23-25-21-45(44-43-25)19-17-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,15-16,20-21,32H,2,4-6,9,12,14,17-19,22-23H2,(H,39,40)(H2,38,41,42,48) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523266

(CHEMBL4444585)Show SMILES CN(CC#C)Cc1ccnc(NC(=O)Nc2cccc3C(=O)N4CCCCC4c23)c1 Show InChI InChI=1S/C23H25N5O2/c1-3-12-27(2)15-16-10-11-24-20(14-16)26-23(30)25-18-8-6-7-17-21(18)19-9-4-5-13-28(19)22(17)29/h1,6-8,10-11,14,19H,4-5,9,12-13,15H2,2H3,(H2,24,25,26,30) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523267

(CHEMBL4559936)Show SMILES O=C(Nc1cc(ccn1)-c1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50552555

(CHEMBL4783398)Show SMILES Cc1ccc(c(c1)[N+]([O-])=O)-n1cc(COc2cccc3cnc(Nc4cccc(Cl)c4)nc23)nn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK1 (130 to end residues) using ERMRPRKRQGSVRRRV as substrate incubated for 40 mins in presence of [gamma33P-ATP] by... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115962
BindingDB Entry DOI: 10.7270/Q2KD22JS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523262

(CHEMBL4443468)Show SMILES [H][C@@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)-c3cn(CCNc4c5CCCCc5nc5ccccc45)nn3)c21 |r| Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523282

(CHEMBL4515295)Show SMILES O=C(Nc1cc(CCC#C)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C22H22N4O2/c1-2-3-7-15-11-12-23-19(14-15)25-22(28)24-17-9-6-8-16-20(17)18-10-4-5-13-26(18)21(16)27/h1,6,8-9,11-12,14,18H,3-5,7,10,13H2,(H2,23,24,25,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523267

(CHEMBL4559936)Show SMILES O=C(Nc1cc(ccn1)-c1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50523278
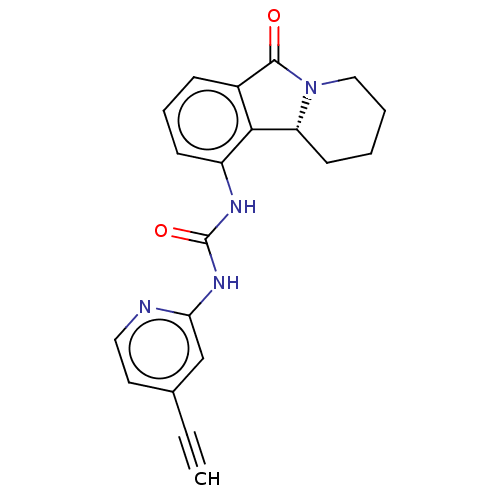
(CHEMBL4463060)Show SMILES [H][C@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)C#C)c21 |r| Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523262

(CHEMBL4443468)Show SMILES [H][C@@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)-c3cn(CCNc4c5CCCCc5nc5ccccc45)nn3)c21 |r| Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46)/t30-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK4
(Homo sapiens (Human)) | BDBM50552555

(CHEMBL4783398)Show SMILES Cc1ccc(c(c1)[N+]([O-])=O)-n1cc(COc2cccc3cnc(Nc4cccc(Cl)c4)nc23)nn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK4 (128 to end residues) using YRRAAVPPSPSLSRHSSPHQS(p)EDEEE as substrate incubated for 40 mins in presence of [gam... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115962
BindingDB Entry DOI: 10.7270/Q2KD22JS |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50523275

(CHEMBL4537026)Show SMILES O=C(Nc1cccc(n1)-c1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C35H35N9O2/c45-34-24-11-7-15-28(32(24)30-16-5-6-19-44(30)34)39-35(46)40-31-17-8-14-27(38-31)29-21-43(42-41-29)20-18-36-33-22-9-1-3-12-25(22)37-26-13-4-2-10-23(26)33/h1,3,7-9,11-12,14-15,17,21,30H,2,4-6,10,13,16,18-20H2,(H,36,37)(H2,38,39,40,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using BTCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellman's me... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523277

(CHEMBL4516356)Show SMILES O=C(Nc1cc(COCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O3/c47-36-28-10-7-13-31(34(28)32-14-5-6-18-46(32)36)41-37(48)42-33-20-24(15-16-38-33)22-49-23-25-21-45(44-43-25)19-17-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,15-16,20-21,32H,2,4-6,9,12,14,17-19,22-23H2,(H,39,40)(H2,38,41,42,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523268

(CHEMBL4593316)Show SMILES O=C(Nc1cc(CCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O2/c47-36-28-10-7-13-31(34(28)32-14-5-6-20-46(32)36)41-37(48)42-33-22-24(17-18-38-33)15-16-25-23-45(44-43-25)21-19-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,17-18,22-23,32H,2,4-6,9,12,14-16,19-21H2,(H,39,40)(H2,38,41,42,48) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50399310

(CHEMBL2180854)Show InChI InChI=1S/C18H17BrN4O2/c19-11-7-8-20-15(10-11)22-18(25)21-13-5-3-4-12-16(13)14-6-1-2-9-23(14)17(12)24/h3-5,7-8,10,14H,1-2,6,9H2,(H2,20,21,22,25) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50161356
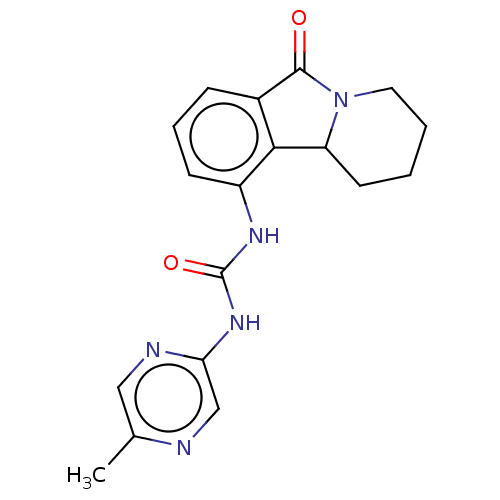
(CHEMBL3786536)Show InChI InChI=1S/C16H12BrN3O/c1-8-2-3-9-7-11-14(10(9)6-8)19-16(18)20-15(11)12-4-5-13(17)21-12/h2-6H,7H2,1H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523268

(CHEMBL4593316)Show SMILES O=C(Nc1cc(CCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O2/c47-36-28-10-7-13-31(34(28)32-14-5-6-20-46(32)36)41-37(48)42-33-22-24(17-18-38-33)15-16-25-23-45(44-43-25)21-19-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,17-18,22-23,32H,2,4-6,9,12,14-16,19-21H2,(H,39,40)(H2,38,41,42,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523274

(CHEMBL4537142)Show SMILES Cn1cc(COCc2ccnc(NC(=O)Nc3cccc4C(=O)N5CCCCC5c34)c2)nn1 Show InChI InChI=1S/C23H25N7O3/c1-29-12-16(27-28-29)14-33-13-15-8-9-24-20(11-15)26-23(32)25-18-6-4-5-17-21(18)19-7-2-3-10-30(19)22(17)31/h4-6,8-9,11-12,19H,2-3,7,10,13-14H2,1H3,(H2,24,25,26,32) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50161347
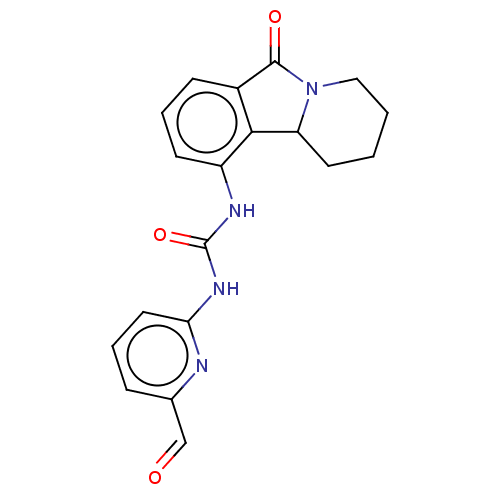
(CHEMBL3786624)Show SMILES O=Cc1cccc(NC(=O)Nc2cccc3C(=O)N4CCCCC4c23)n1 Show InChI InChI=1S/C16H13N3O/c1-9-4-5-10-8-12-14(11(10)7-9)18-16(17)19-15(12)13-3-2-6-20-13/h2-7H,8H2,1H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50399310

(CHEMBL2180854)Show InChI InChI=1S/C18H17BrN4O2/c19-11-7-8-20-15(10-11)22-18(25)21-13-5-3-4-12-16(13)14-6-1-2-9-23(14)17(12)24/h3-5,7-8,10,14H,1-2,6,9H2,(H2,20,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50552555

(CHEMBL4783398)Show SMILES Cc1ccc(c(c1)[N+]([O-])=O)-n1cc(COc2cccc3cnc(Nc4cccc(Cl)c4)nc23)nn1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CLK2 (138 to end residues) using YRRAAVPPSPSLSRHSSPHQS(p)EDEEE as substrate incubated for 40 mins in presence of [gam... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115962
BindingDB Entry DOI: 10.7270/Q2KD22JS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523273

(CHEMBL4459540)Show SMILES O=C(Nc1cc(COCc2cn(CCNc3ccncc3)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C29H31N9O3/c39-28-23-4-3-5-24(27(23)25-6-1-2-14-38(25)28)33-29(40)34-26-16-20(7-12-32-26)18-41-19-22-17-37(36-35-22)15-13-31-21-8-10-30-11-9-21/h3-5,7-12,16-17,25H,1-2,6,13-15,18-19H2,(H,30,31)(H2,32,33,34,40) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523278

(CHEMBL4463060)Show SMILES [H][C@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)C#C)c21 |r| Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26)/t16-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using BTCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellman's me... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50161348
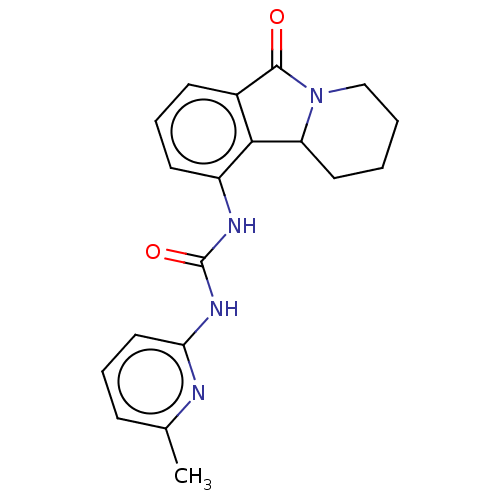
(CHEMBL3785408)Show InChI InChI=1S/C17H14N4/c1-10-2-3-12-9-14-15(11-4-6-19-7-5-11)20-17(18)21-16(14)13(12)8-10/h2-8H,9H2,1H3,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50161338
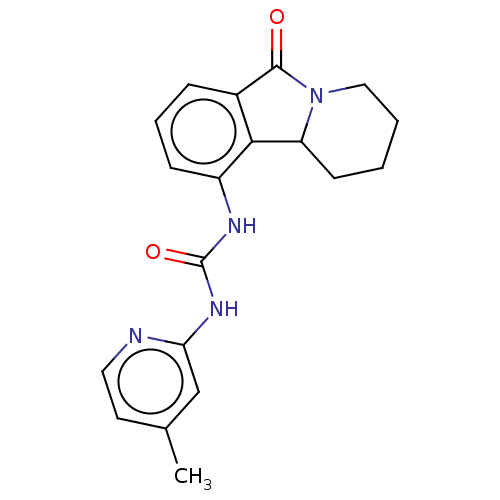
(CHEMBL3787480)Show InChI InChI=1S/C17H15N3O2/c1-9-2-3-10-7-13-15(12(10)6-9)19-17(18)20-16(13)14-5-4-11(8-21)22-14/h2-6,21H,7-8H2,1H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523271
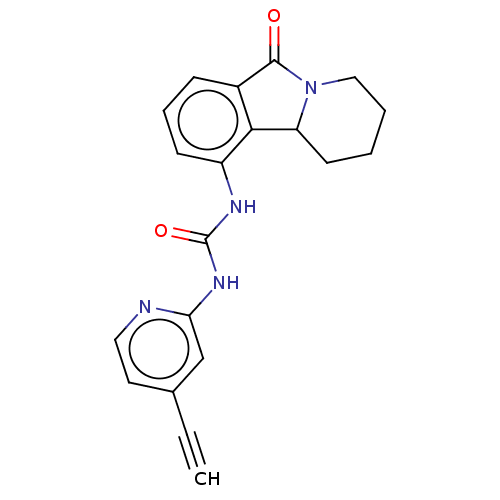
(CHEMBL4566076)Show SMILES O=C(Nc1cc(ccn1)C#C)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50161339
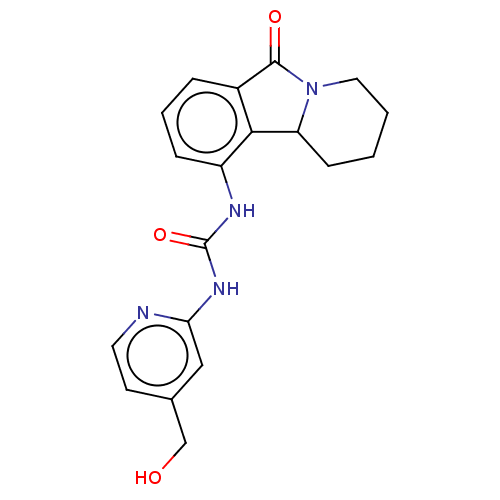
(CHEMBL3787512)Show InChI InChI=1S/C15H9BrClN3O/c16-12-4-3-11(21-12)14-10-5-7-1-2-8(17)6-9(7)13(10)19-15(18)20-14/h1-4,6H,5H2,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
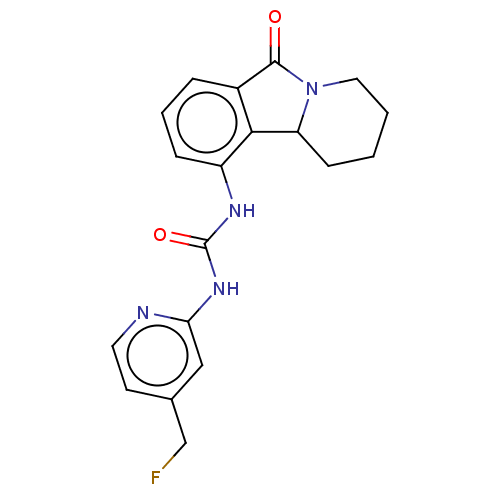
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50161398
(CHEMBL3787292)Show InChI InChI=1S/C19H19FN4O2/c20-11-12-7-8-21-16(10-12)23-19(26)22-14-5-3-4-13-17(14)15-6-1-2-9-24(15)18(13)25/h3-5,7-8,10,15H,1-2,6,9,11H2,(H2,21,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
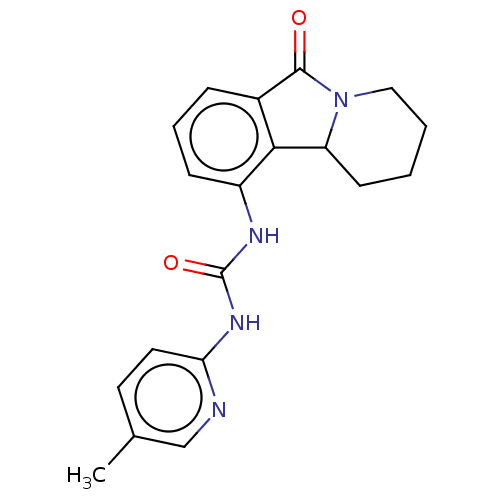
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50161345
(CHEMBL3785575)Show InChI InChI=1S/C16H12BrN3O2/c1-21-9-3-2-8-6-11-14(10(8)7-9)19-16(18)20-15(11)12-4-5-13(17)22-12/h2-5,7H,6H2,1H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
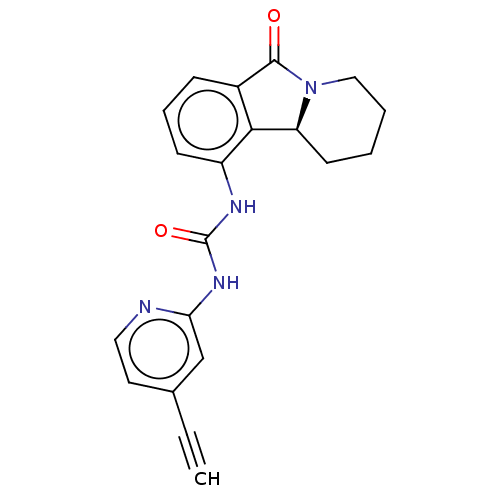
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50523263
(CHEMBL4483607)Show SMILES [H][C@@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)C#C)c21 |r| Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523269

(CHEMBL4446287)Show SMILES CN(Cc1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Cc1ccnc(NC(=O)Nc2cccc3C(=O)N4CCCCC4c23)c1 Show InChI InChI=1S/C38H42N10O2/c1-46(23-26-24-47(45-44-26)20-18-40-36-27-9-2-4-12-30(27)41-31-13-5-3-10-28(31)36)22-25-16-17-39-34(21-25)43-38(50)42-32-14-8-11-29-35(32)33-15-6-7-19-48(33)37(29)49/h2,4,8-9,11-12,14,16-17,21,24,33H,3,5-7,10,13,15,18-20,22-23H2,1H3,(H,40,41)(H2,39,42,43,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523280
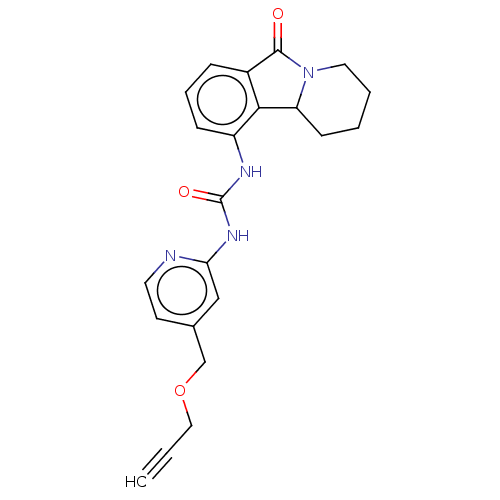
(CHEMBL4435354)Show SMILES O=C(Nc1cc(COCC#C)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C22H22N4O3/c1-2-12-29-14-15-9-10-23-19(13-15)25-22(28)24-17-7-5-6-16-20(17)18-8-3-4-11-26(18)21(16)27/h1,5-7,9-10,13,18H,3-4,8,11-12,14H2,(H2,23,24,25,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM100152
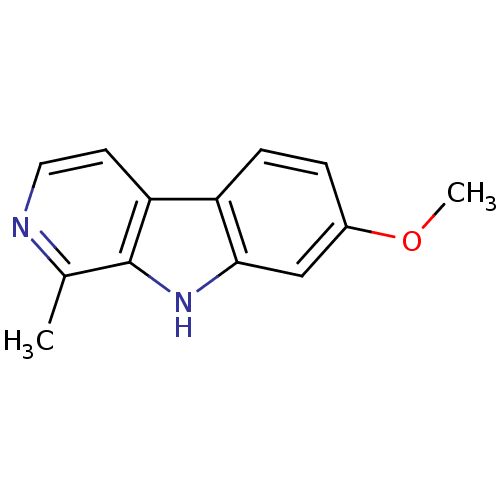
(7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...)Show InChI InChI=1S/C13H12N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-7,15H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of DYRK1A (unknown origin) |
Bioorg Med Chem Lett 24: 5037-40 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.017
BindingDB Entry DOI: 10.7270/Q27H1M51 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50523268

(CHEMBL4593316)Show SMILES O=C(Nc1cc(CCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O2/c47-36-28-10-7-13-31(34(28)32-14-5-6-20-46(32)36)41-37(48)42-33-22-24(17-18-38-33)15-16-25-23-45(44-43-25)21-19-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,17-18,22-23,32H,2,4-6,9,12,14-16,19-21H2,(H,39,40)(H2,38,41,42,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using BTCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellman's me... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50161344

(CHEMBL3786276)Show SMILES O=C(Nc1cc(CN2CCOCC2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C18H15N3/c19-18-20-16(13-7-2-1-3-8-13)15-11-10-12-6-4-5-9-14(12)17(15)21-18/h1-9H,10-11H2,(H2,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50523271

(CHEMBL4566076)Show SMILES O=C(Nc1cc(ccn1)C#C)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50399301
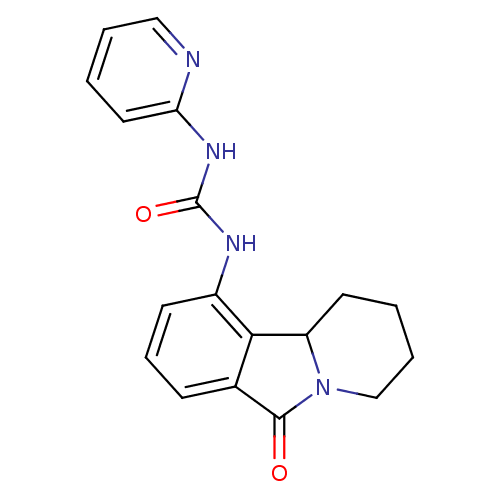
(CHEMBL2180862)Show InChI InChI=1S/C18H18N4O2/c23-17-12-6-5-7-13(16(12)14-8-2-4-11-22(14)17)20-18(24)21-15-9-1-3-10-19-15/h1,3,5-7,9-10,14H,2,4,8,11H2,(H2,19,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50523282

(CHEMBL4515295)Show SMILES O=C(Nc1cc(CCC#C)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C22H22N4O2/c1-2-3-7-15-11-12-23-19(14-15)25-22(28)24-17-9-6-8-16-20(17)18-10-4-5-13-26(18)21(16)27/h1,6,8-9,11-12,14,18H,3-5,7,10,13H2,(H2,23,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50399326
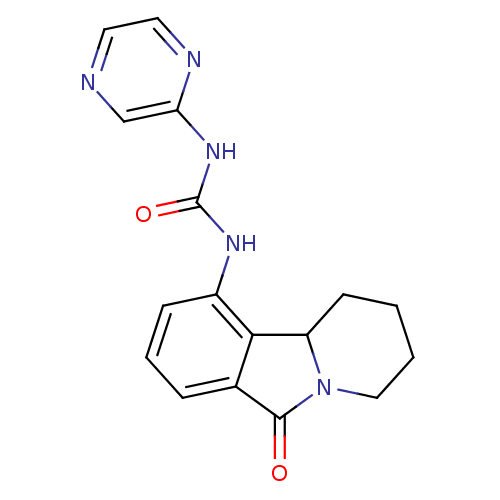
(CHEMBL2180863)Show InChI InChI=1S/C17H17N5O2/c23-16-11-4-3-5-12(15(11)13-6-1-2-9-22(13)16)20-17(24)21-14-10-18-7-8-19-14/h3-5,7-8,10,13H,1-2,6,9H2,(H2,19,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate |
Eur J Med Chem 115: 311-25 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.072
BindingDB Entry DOI: 10.7270/Q2JM2CHT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50399301

(CHEMBL2180862)Show InChI InChI=1S/C18H18N4O2/c23-17-12-6-5-7-13(16(12)14-8-2-4-11-22(14)17)20-18(24)21-15-9-1-3-10-19-15/h1,3,5-7,9-10,14H,2,4,8,11H2,(H2,19,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50523270
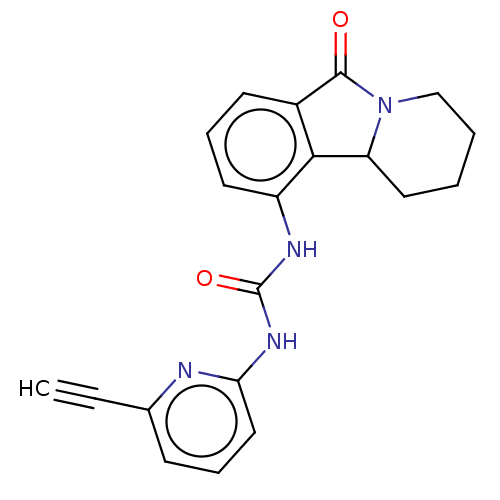
(CHEMBL4475474)Show SMILES O=C(Nc1cccc(n1)C#C)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C20H18N4O2/c1-2-13-7-5-11-17(21-13)23-20(26)22-15-9-6-8-14-18(15)16-10-3-4-12-24(16)19(14)25/h1,5-9,11,16H,3-4,10,12H2,(H2,21,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523263

(CHEMBL4483607)Show SMILES [H][C@@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)C#C)c21 |r| Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26)/t16-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50523266

(CHEMBL4444585)Show SMILES CN(CC#C)Cc1ccnc(NC(=O)Nc2cccc3C(=O)N4CCCCC4c23)c1 Show InChI InChI=1S/C23H25N5O2/c1-3-12-27(2)15-16-10-11-24-20(14-16)26-23(30)25-18-8-6-7-17-21(18)19-9-4-5-13-28(19)22(17)29/h1,6-8,10-11,14,19H,4-5,9,12-13,15H2,2H3,(H2,24,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data