 Found 224 hits with Last Name = 'morales' and Initial = 'r'
Found 224 hits with Last Name = 'morales' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50571877
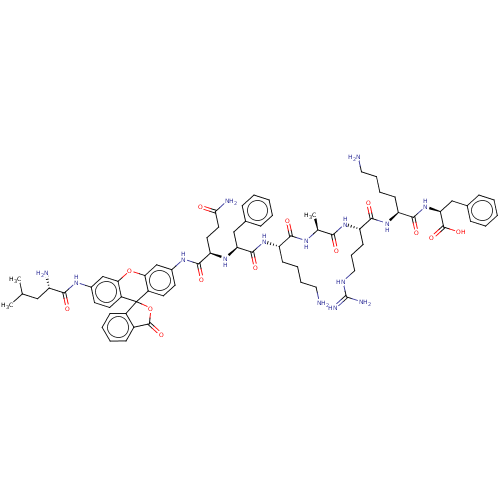
(CHEMBL4878272)Show SMILES CC(C)C[C@H](N)C(=O)Nc1ccc2c(Oc3cc(NC(=O)[C@@H](CCC(N)=O)N[C@@H](Cc4ccccc4)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc4ccccc4)C(O)=O)ccc3C22OC(=O)c3ccccc23)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ERAP1 (unknown origin) using L-Rho-Succ-FKARKF as substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50571875
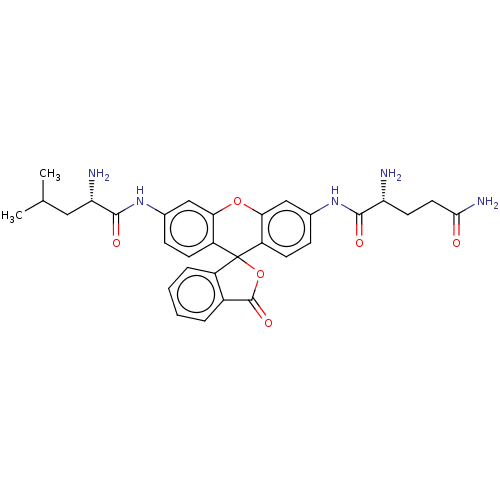
(CHEMBL4847486)Show SMILES CC(C)C[C@H](N)C(=O)Nc1ccc2c(Oc3cc(NC(=O)[C@H](N)CCC(N)=O)ccc3C22OC(=O)c3ccccc23)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ERAP1 (unknown origin) using L-Rho-(D)-Q as substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50571878

(CHEMBL4855021)Show SMILES CC(C)C[C@H](N)C(=O)Nc1ccc2c(Oc3cc(NC(=O)[C@@H](CCC(N)=O)N[C@@H](C)C(=O)N[C@@H](Cc4ccccc4)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc4ccccc4)C(O)=O)ccc3C22OC(=O)c3ccccc23)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ERAP1 (unknown origin) using L-Rho-Succ-AFKARKF as substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50571876

(CHEMBL4875911)Show SMILES CC(C)C[C@H](N)C(=O)Nc1ccc2c(Oc3cc(NC(=O)[C@@H](CCC(N)=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc4ccccc4)C(O)=O)ccc3C22OC(=O)c3ccccc23)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ERAP1 (unknown origin) using L-Rho-Succ-KARKF as substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50534338
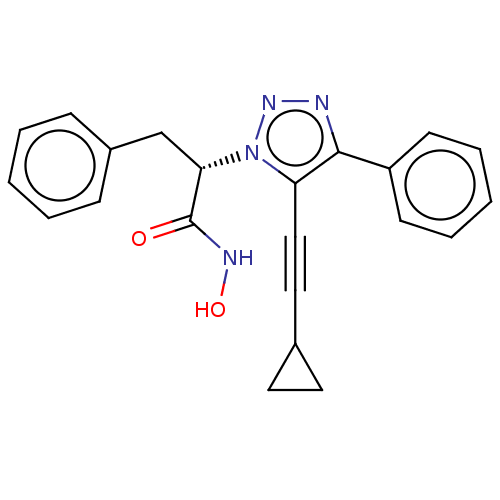
(CHEMBL4534486)Show SMILES ONC(=O)[C@H](Cc1ccccc1)n1nnc(c1C#CC1CC1)-c1ccccc1 |r| Show InChI InChI=1S/C22H20N4O2/c27-22(24-28)20(15-17-7-3-1-4-8-17)26-19(14-13-16-11-12-16)21(23-25-26)18-9-5-2-6-10-18/h1-10,16,20,28H,11-12,15H2,(H,24,27)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC8 using H2N-Arg-His-Lys(Ac)-Lys(Ac)-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50076744

(CHEMBL3416733)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using Ala-AMC as substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392456
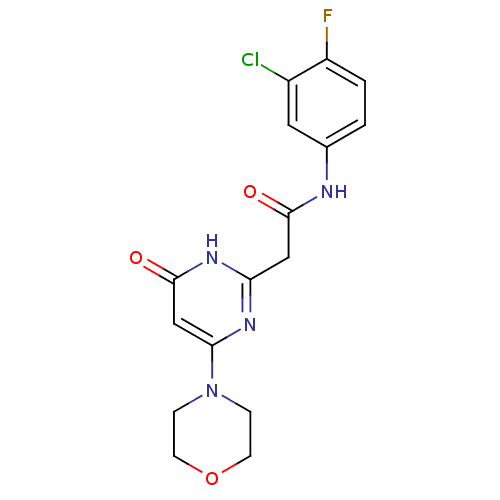
(CHEMBL2151926)Show SMILES Fc1ccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C16H16ClFN4O3/c17-11-7-10(1-2-12(11)18)19-15(23)8-13-20-14(9-16(24)21-13)22-3-5-25-6-4-22/h1-2,7,9H,3-6,8H2,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392457
(CHEMBL2151927)Show SMILES COc1cc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)ccc1F Show InChI InChI=1S/C17H19FN4O4/c1-25-13-8-11(2-3-12(13)18)19-16(23)9-14-20-15(10-17(24)21-14)22-4-6-26-7-5-22/h2-3,8,10H,4-7,9H2,1H3,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392453
(CHEMBL2151923)Show SMILES Brc1cccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)c1 Show InChI InChI=1S/C16H17BrN4O3/c17-11-2-1-3-12(8-11)18-15(22)9-13-19-14(10-16(23)20-13)21-4-6-24-7-5-21/h1-3,8,10H,4-7,9H2,(H,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50510962
(CHEMBL4454646)Show SMILES Cc1noc(C)c1-c1ccc2n(CCCCCCC(=O)NO)cc(Cc3ccc(F)cc3)c2c1 Show InChI InChI=1S/C27H30FN3O3/c1-18-27(19(2)34-30-18)21-10-13-25-24(16-21)22(15-20-8-11-23(28)12-9-20)17-31(25)14-6-4-3-5-7-26(32)29-33/h8-13,16-17,33H,3-7,14-15H2,1-2H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50252396

(CHEMBL4077645)Show SMILES ONC(=O)c1ccc(CC(=O)NC2=CC(=O)c3ccccc3C2=O)cc1 |t:12| Show InChI InChI=1S/C19H14N2O5/c22-16-10-15(18(24)14-4-2-1-3-13(14)16)20-17(23)9-11-5-7-12(8-6-11)19(25)21-26/h1-8,10,26H,9H2,(H,20,23)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Z-(Ac)Lys-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392455
(CHEMBL2151925)Show SMILES Cc1cc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)ccc1F Show InChI InChI=1S/C17H19FN4O3/c1-11-8-12(2-3-13(11)18)19-16(23)9-14-20-15(10-17(24)21-14)22-4-6-25-7-5-22/h2-3,8,10H,4-7,9H2,1H3,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392469
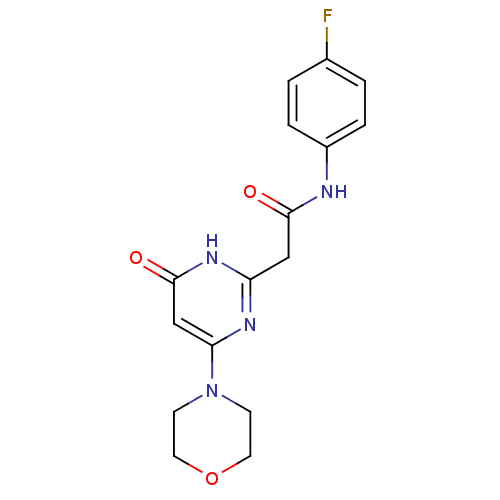
(CHEMBL2151821)Show SMILES Fc1ccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)cc1 Show InChI InChI=1S/C16H17FN4O3/c17-11-1-3-12(4-2-11)18-15(22)9-13-19-14(10-16(23)20-13)21-5-7-24-8-6-21/h1-4,10H,5-9H2,(H,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50538729

(CHEMBL4649617)Show InChI InChI=1S/C16H14N2O2S/c19-16(18-20)11-5-7-13(8-6-11)17-9-12-10-21-15-4-2-1-3-14(12)15/h1-8,10,17,20H,9H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using Z-(Ac)Lys-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50170205
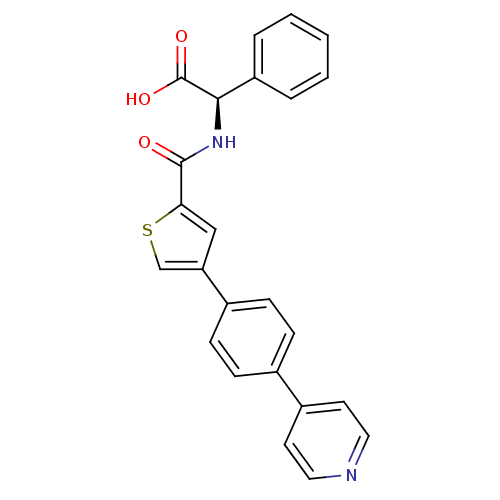
((R)-Phenyl-{[4-(4-pyridin-4-yl-phenyl)-thiophene-2...)Show SMILES OC(=O)[C@H](NC(=O)c1cc(cs1)-c1ccc(cc1)-c1ccncc1)c1ccccc1 Show InChI InChI=1S/C24H18N2O3S/c27-23(26-22(24(28)29)19-4-2-1-3-5-19)21-14-20(15-30-21)17-8-6-16(7-9-17)18-10-12-25-13-11-18/h1-15,22H,(H,26,27)(H,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-12 in presence of 5 nM acetohydroximate |
Bioorg Med Chem Lett 15: 3787-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.079
BindingDB Entry DOI: 10.7270/Q2736QFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392452
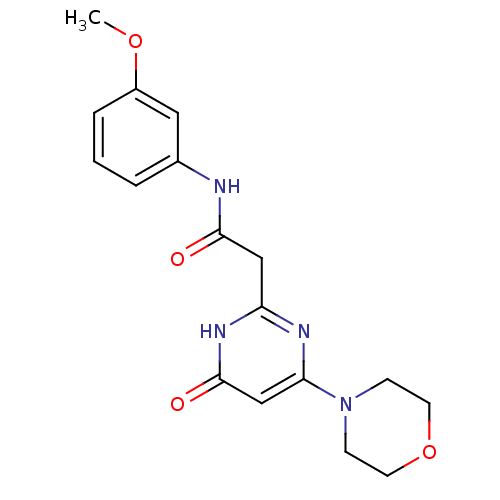
(CHEMBL2151922)Show SMILES COc1cccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)c1 Show InChI InChI=1S/C17H20N4O4/c1-24-13-4-2-3-12(9-13)18-16(22)10-14-19-15(11-17(23)20-14)21-5-7-25-8-6-21/h2-4,9,11H,5-8,10H2,1H3,(H,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392449
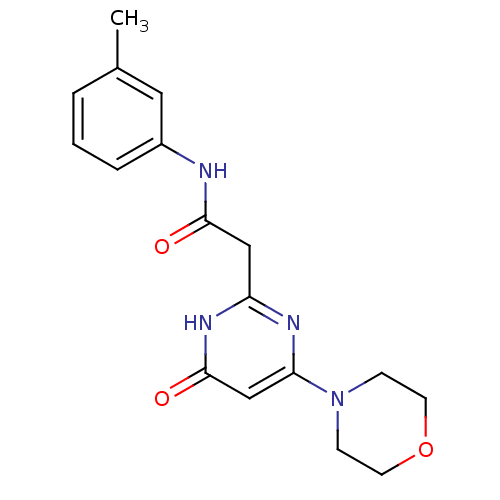
(CHEMBL2151919)Show SMILES Cc1cccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)c1 Show InChI InChI=1S/C17H20N4O3/c1-12-3-2-4-13(9-12)18-16(22)10-14-19-15(11-17(23)20-14)21-5-7-24-8-6-21/h2-4,9,11H,5-8,10H2,1H3,(H,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392458
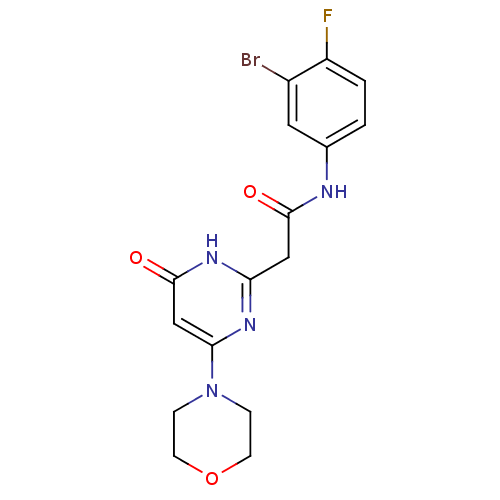
(CHEMBL2151048)Show SMILES Fc1ccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)cc1Br Show InChI InChI=1S/C16H16BrFN4O3/c17-11-7-10(1-2-12(11)18)19-15(23)8-13-20-14(9-16(24)21-13)22-3-5-25-6-4-22/h1-2,7,9H,3-6,8H2,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50246866

(CHEMBL4078458 | US11505523, Compound 22d)Show SMILES COc1ccc(\C=C\C(=O)NO)c(c1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H19NO3/c1-26-20-13-11-19(12-14-22(24)23-25)21(15-20)18-9-7-17(8-10-18)16-5-3-2-4-6-16/h2-15,25H,1H3,(H,23,24)/b14-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC8 using H2N-Arg-His-Lys(Ac)-Lys(Ac)-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50260597
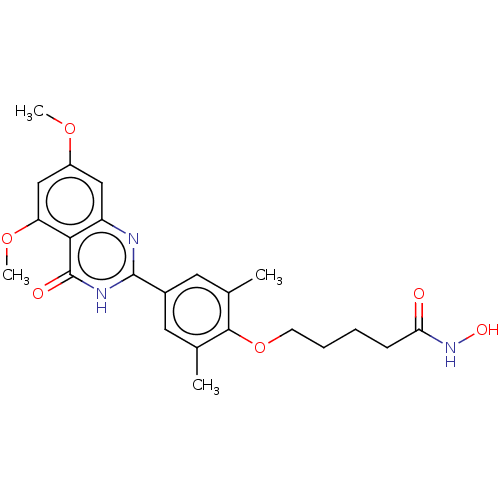
(CHEMBL4079901)Show SMILES COc1cc(OC)c2c(c1)nc([nH]c2=O)-c1cc(C)c(OCCCCC(=O)NO)c(C)c1 Show InChI InChI=1S/C23H27N3O6/c1-13-9-15(10-14(2)21(13)32-8-6-5-7-19(27)26-29)22-24-17-11-16(30-3)12-18(31-4)20(17)23(28)25-22/h9-12,29H,5-8H2,1-4H3,(H,26,27)(H,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC1 using Z-(Ac)Lys-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392451
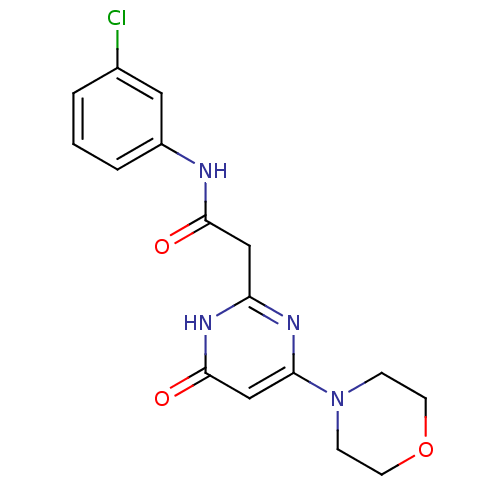
(CHEMBL2151921)Show SMILES Clc1cccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)c1 Show InChI InChI=1S/C16H17ClN4O3/c17-11-2-1-3-12(8-11)18-15(22)9-13-19-14(10-16(23)20-13)21-4-6-24-7-5-21/h1-3,8,10H,4-7,9H2,(H,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50571866
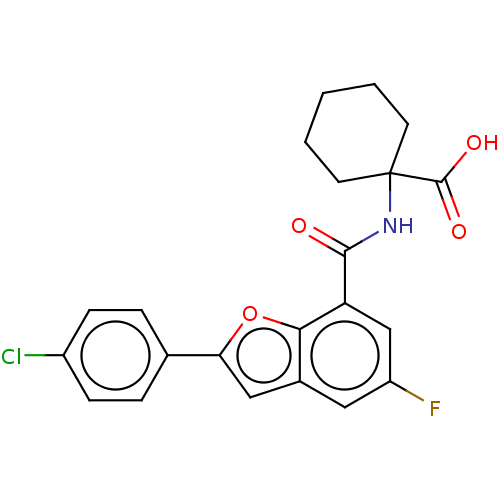
(CHEMBL4862557)Show SMILES OC(=O)C1(CCCCC1)NC(=O)c1cc(F)cc2cc(oc12)-c1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type ERAP1 (unknown origin) using L-Rho-Succ-FKARKF as substrate preincubated for 15 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50389035
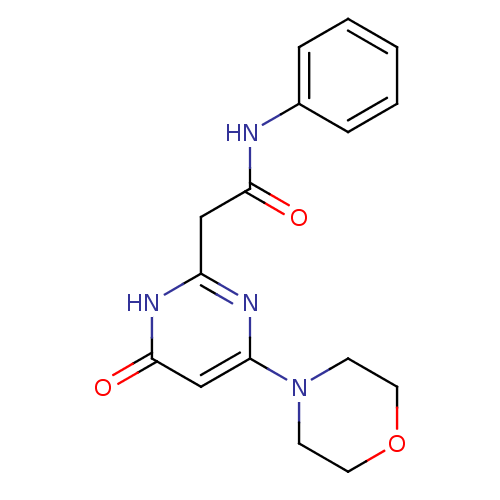
(CHEMBL2064419)Show InChI InChI=1S/C16H18N4O3/c21-15(17-12-4-2-1-3-5-12)10-13-18-14(11-16(22)19-13)20-6-8-23-9-7-20/h1-5,11H,6-10H2,(H,17,21)(H,18,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392466
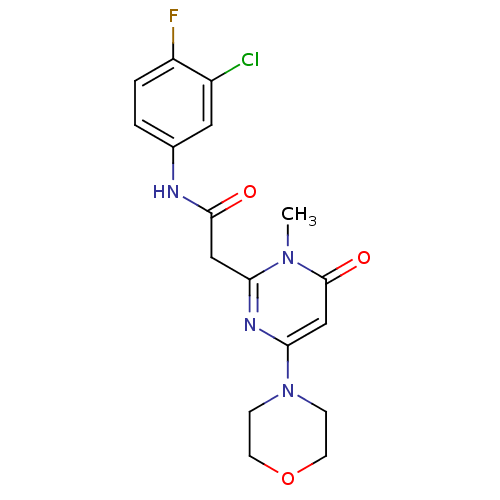
(CHEMBL2151935)Show SMILES Cn1c(CC(=O)Nc2ccc(F)c(Cl)c2)nc(cc1=O)N1CCOCC1 Show InChI InChI=1S/C17H18ClFN4O3/c1-22-14(9-16(24)20-11-2-3-13(19)12(18)8-11)21-15(10-17(22)25)23-4-6-26-7-5-23/h2-3,8,10H,4-7,9H2,1H3,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392460
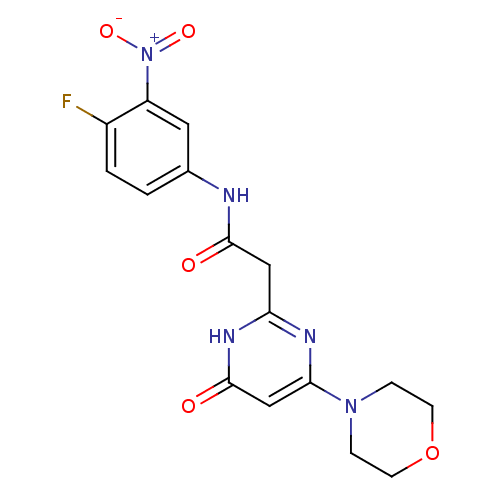
(CHEMBL2151929)Show SMILES [O-][N+](=O)c1cc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)ccc1F Show InChI InChI=1S/C16H16FN5O5/c17-11-2-1-10(7-12(11)22(25)26)18-15(23)8-13-19-14(9-16(24)20-13)21-3-5-27-6-4-21/h1-2,7,9H,3-6,8H2,(H,18,23)(H,19,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50559724
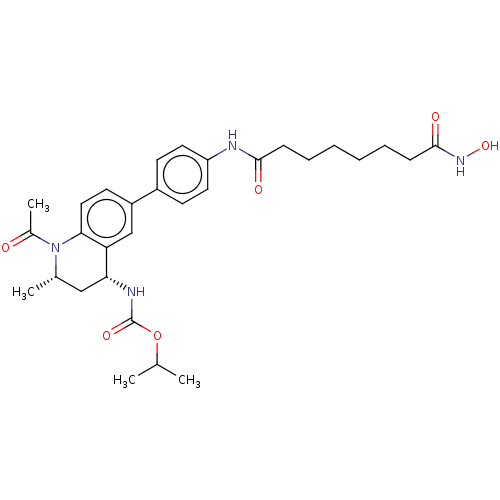
(CHEMBL3220922)Show SMILES CC(C)OC(=O)N[C@@H]1C[C@H](C)N(C(C)=O)c2ccc(cc12)-c1ccc(NC(=O)CCCCCCC(=O)NO)cc1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BRD4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50458430
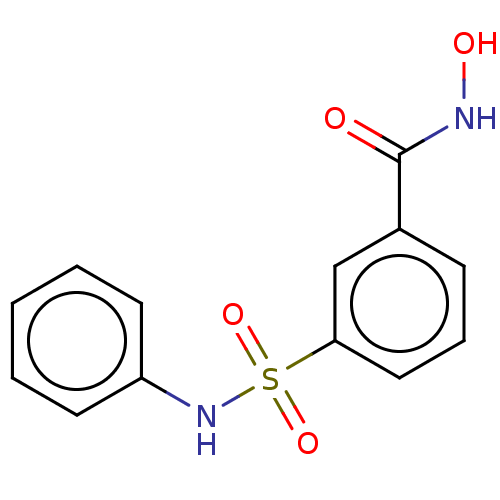
(CHEMBL4206936)Show InChI InChI=1S/C13H12N2O4S/c16-13(14-17)10-5-4-8-12(9-10)20(18,19)15-11-6-2-1-3-7-11/h1-9,15,17H,(H,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC8 using H2N-Arg-His-Lys(Ac)-Lys(Ac)-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50076744

(CHEMBL3416733)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type ERAP1 (unknown origin) using L-Rho-Succ-FKARKF as substrate preincubated for 15 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392454
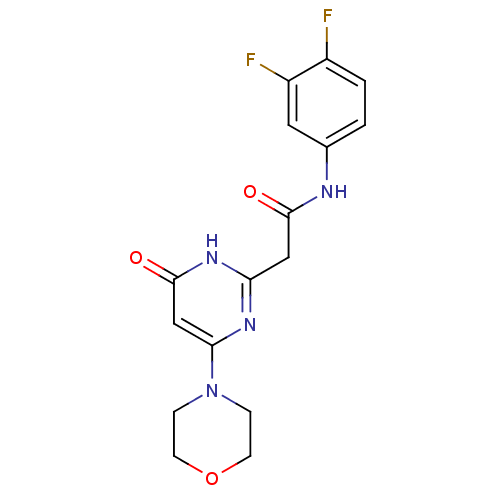
(CHEMBL2151924)Show SMILES Fc1ccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)cc1F Show InChI InChI=1S/C16H16F2N4O3/c17-11-2-1-10(7-12(11)18)19-15(23)8-13-20-14(9-16(24)21-13)22-3-5-25-6-4-22/h1-2,7,9H,3-6,8H2,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50170206
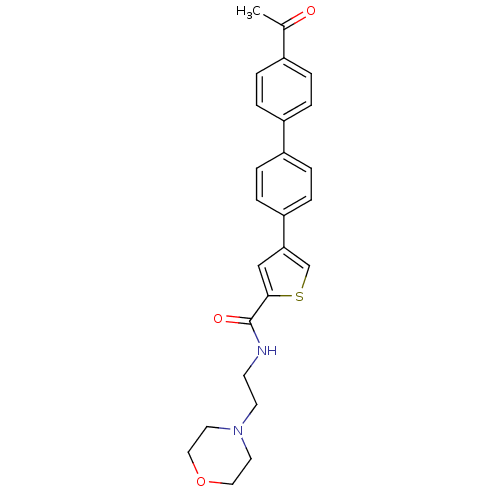
(4-(4'-Acetyl-biphenyl-4-yl)-thiophene-2-carboxylic...)Show SMILES CC(=O)c1ccc(cc1)-c1ccc(cc1)-c1csc(c1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C25H26N2O3S/c1-18(28)19-2-4-20(5-3-19)21-6-8-22(9-7-21)23-16-24(31-17-23)25(29)26-10-11-27-12-14-30-15-13-27/h2-9,16-17H,10-15H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-12 in presence of 5 nM acetohydroximate |
Bioorg Med Chem Lett 15: 3787-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.079
BindingDB Entry DOI: 10.7270/Q2736QFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392455

(CHEMBL2151925)Show SMILES Cc1cc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)ccc1F Show InChI InChI=1S/C17H19FN4O3/c1-11-8-12(2-3-13(11)18)19-16(23)9-14-20-15(10-17(24)21-14)22-4-6-25-7-5-22/h2-3,8,10H,4-7,9H2,1H3,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50564920
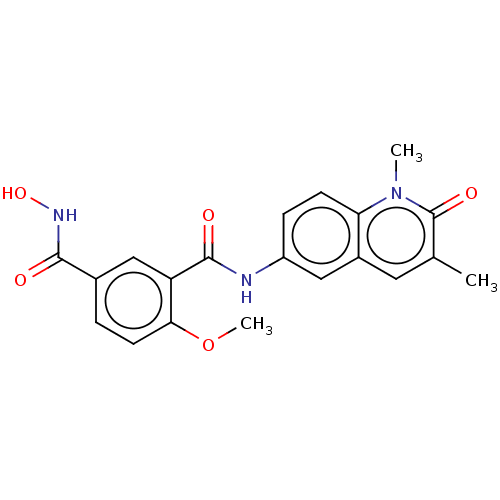
(CHEMBL4789607)Show SMILES COc1ccc(cc1C(=O)Nc1ccc2n(C)c(=O)c(C)cc2c1)C(=O)NO | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC8 using H2N-Arg-His-Lys(Ac)-Lys(Ac)-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50170203

((4-Biphenyl-4-yl-thiophene-2-carbonyl)-carbamic ac...)Show SMILES Cc1ccc(COC(=O)NC(=O)c2cc(cs2)-c2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C26H21NO3S/c1-18-7-9-19(10-8-18)16-30-26(29)27-25(28)24-15-23(17-31-24)22-13-11-21(12-14-22)20-5-3-2-4-6-20/h2-15,17H,16H2,1H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-12 in presence of 5 nM acetohydroximate |
Bioorg Med Chem Lett 15: 3787-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.079
BindingDB Entry DOI: 10.7270/Q2736QFM |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50170207
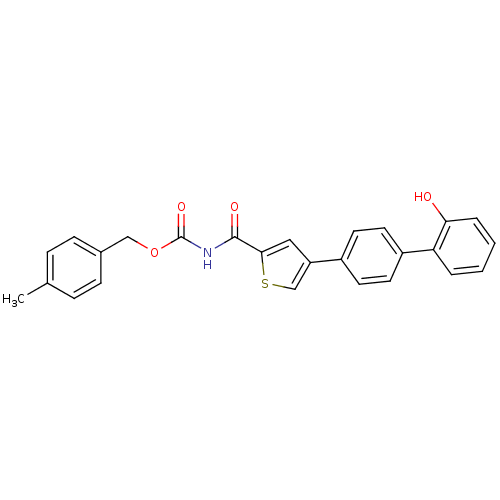
(CHEMBL180762 | [4-(2'-Hydroxy-biphenyl-4-yl)-thiop...)Show SMILES Cc1ccc(COC(=O)NC(=O)c2cc(cs2)-c2ccc(cc2)-c2ccccc2O)cc1 Show InChI InChI=1S/C26H21NO4S/c1-17-6-8-18(9-7-17)15-31-26(30)27-25(29)24-14-21(16-32-24)19-10-12-20(13-11-19)22-4-2-3-5-23(22)28/h2-14,16,28H,15H2,1H3,(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-12 in presence of 5 nM acetohydroximate |
Bioorg Med Chem Lett 15: 3787-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.079
BindingDB Entry DOI: 10.7270/Q2736QFM |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50246846
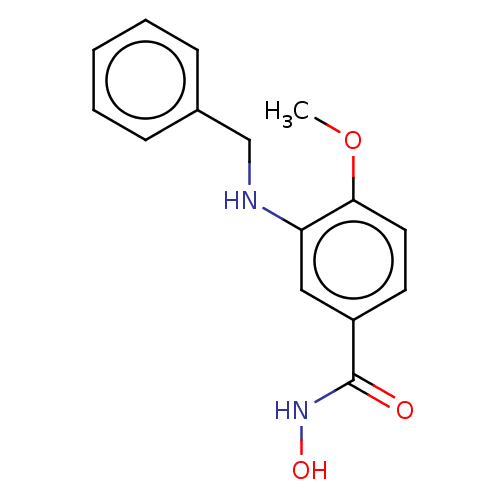
(CHEMBL4105068)Show InChI InChI=1S/C15H16N2O3/c1-20-14-8-7-12(15(18)17-19)9-13(14)16-10-11-5-3-2-4-6-11/h2-9,16,19H,10H2,1H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC8 using H2N-Arg-His-Lys(Ac)-Lys(Ac)-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392468
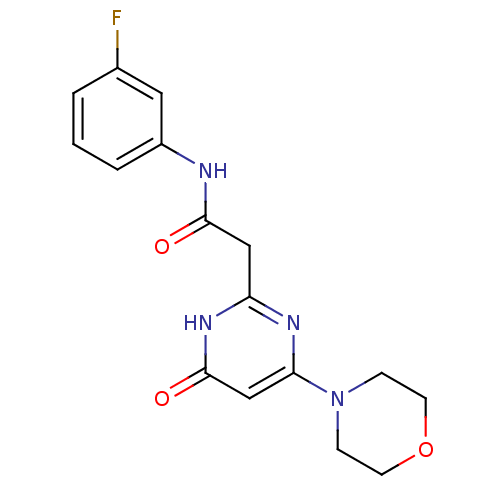
(CHEMBL2151820)Show SMILES Fc1cccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)c1 Show InChI InChI=1S/C16H17FN4O3/c17-11-2-1-3-12(8-11)18-15(22)9-13-19-14(10-16(23)20-13)21-4-6-24-7-5-21/h1-3,8,10H,4-7,9H2,(H,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392469

(CHEMBL2151821)Show SMILES Fc1ccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)cc1 Show InChI InChI=1S/C16H17FN4O3/c17-11-1-3-12(4-2-11)18-15(22)9-13-19-14(10-16(23)20-13)21-5-7-24-8-6-21/h1-4,10H,5-9H2,(H,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50571865
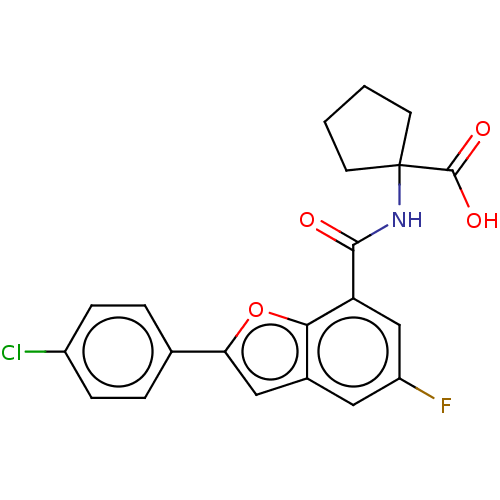
(CHEMBL4863142)Show SMILES OC(=O)C1(CCCC1)NC(=O)c1cc(F)cc2cc(oc12)-c1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type ERAP1 (unknown origin) using L-Rho-Succ-FKARKF as substrate preincubated for 15 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50170201
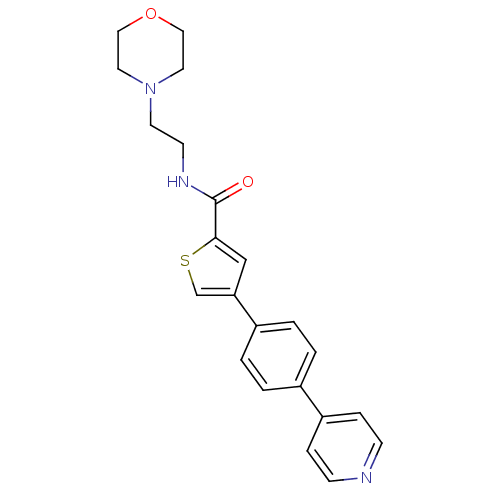
(4-(4-Pyridin-4-yl-phenyl)-thiophene-2-carboxylic a...)Show SMILES O=C(NCCN1CCOCC1)c1cc(cs1)-c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C22H23N3O2S/c26-22(24-9-10-25-11-13-27-14-12-25)21-15-20(16-28-21)18-3-1-17(2-4-18)19-5-7-23-8-6-19/h1-8,15-16H,9-14H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-12 |
Bioorg Med Chem Lett 15: 3787-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.079
BindingDB Entry DOI: 10.7270/Q2736QFM |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50170201

(4-(4-Pyridin-4-yl-phenyl)-thiophene-2-carboxylic a...)Show SMILES O=C(NCCN1CCOCC1)c1cc(cs1)-c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C22H23N3O2S/c26-22(24-9-10-25-11-13-27-14-12-25)21-15-20(16-28-21)18-3-1-17(2-4-18)19-5-7-23-8-6-19/h1-8,15-16H,9-14H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-12 in presence of 5 nM acetohydroximate |
Bioorg Med Chem Lett 15: 3787-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.079
BindingDB Entry DOI: 10.7270/Q2736QFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392465
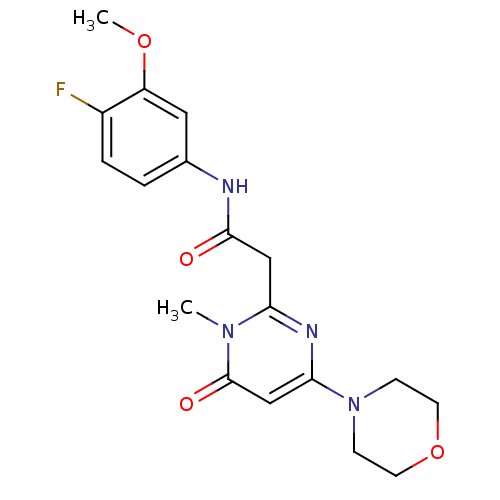
(CHEMBL2151934)Show SMILES COc1cc(NC(=O)Cc2nc(cc(=O)n2C)N2CCOCC2)ccc1F Show InChI InChI=1S/C18H21FN4O4/c1-22-15(21-16(11-18(22)25)23-5-7-27-8-6-23)10-17(24)20-12-3-4-13(19)14(9-12)26-2/h3-4,9,11H,5-8,10H2,1-2H3,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50564924
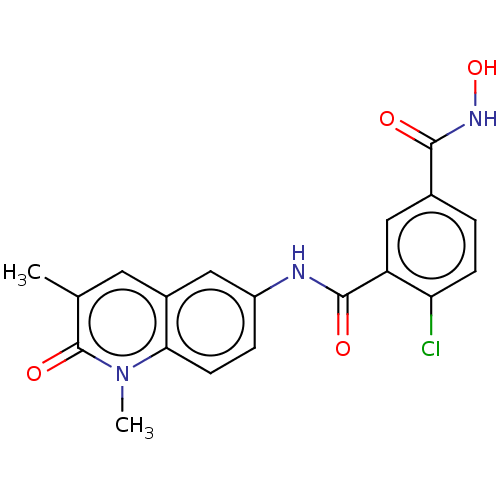
(CHEMBL4790363)Show SMILES Cc1cc2cc(NC(=O)c3cc(ccc3Cl)C(=O)NO)ccc2n(C)c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant HDAC8 using H2N-Arg-His-Lys(Ac)-Lys(Ac)-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50571867
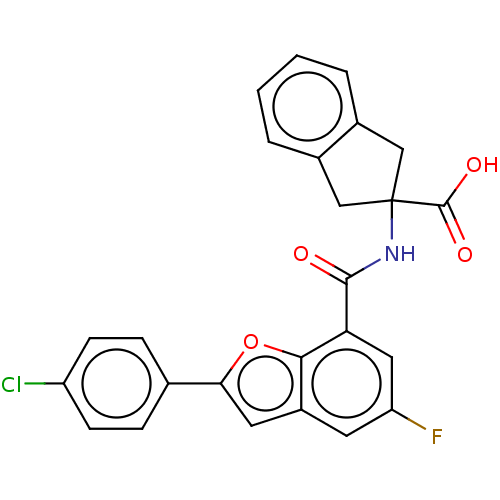
(CHEMBL4875461)Show SMILES OC(=O)C1(Cc2ccccc2C1)NC(=O)c1cc(F)cc2cc(oc12)-c1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type ERAP1 (unknown origin) using L-Rho-Succ-FKARKF as substrate preincubated for 15 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50389035

(CHEMBL2064419)Show InChI InChI=1S/C16H18N4O3/c21-15(17-12-4-2-1-3-5-12)10-13-18-14(11-16(22)19-13)20-6-8-23-9-7-20/h1-5,11H,6-10H2,(H,17,21)(H,18,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 1
(Homo sapiens (Human)) | BDBM50571872
(CHEMBL4864508)Show SMILES OC(=O)C1(CCCCC1)NC(=O)c1cccc2cc(oc12)-c1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild type ERAP1 (unknown origin) using L-Rho-Succ-FKARKF as substrate preincubated for 15 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00235
BindingDB Entry DOI: 10.7270/Q2QJ7N22 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392454

(CHEMBL2151924)Show SMILES Fc1ccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)cc1F Show InChI InChI=1S/C16H16F2N4O3/c17-11-2-1-10(7-12(11)18)19-15(23)8-13-20-14(9-16(24)21-13)22-3-5-25-6-4-22/h1-2,7,9H,3-6,8H2,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392456

(CHEMBL2151926)Show SMILES Fc1ccc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)cc1Cl Show InChI InChI=1S/C16H16ClFN4O3/c17-11-7-10(1-2-12(11)18)19-15(23)8-13-20-14(9-16(24)21-13)22-3-5-25-6-4-22/h1-2,7,9H,3-6,8H2,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50392467
(CHEMBL2151819)Show InChI InChI=1S/C16H17FN4O3/c17-11-3-1-2-4-12(11)18-15(22)9-13-19-14(10-16(23)20-13)21-5-7-24-8-6-21/h1-4,10H,5-9H2,(H,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392457

(CHEMBL2151927)Show SMILES COc1cc(NC(=O)Cc2nc(cc(=O)[nH]2)N2CCOCC2)ccc1F Show InChI InChI=1S/C17H19FN4O4/c1-25-13-8-11(2-3-12(13)18)19-16(23)9-14-20-15(10-17(24)21-14)22-4-6-26-7-5-22/h2-3,8,10H,4-7,9H2,1H3,(H,19,23)(H,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 22: 6381-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.072
BindingDB Entry DOI: 10.7270/Q2FN179H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50180980
(CHEMBL3818594)Show SMILES Cc1noc(C)c1-c1cc(NC(=O)CCCCCCC(=O)NO)cc(c1)C(=O)c1ccccc1 Show InChI InChI=1S/C26H29N3O5/c1-17-25(18(2)34-29-17)20-14-21(26(32)19-10-6-5-7-11-19)16-22(15-20)27-23(30)12-8-3-4-9-13-24(31)28-33/h5-7,10-11,14-16,33H,3-4,8-9,12-13H2,1-2H3,(H,27,30)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC1 using Z-(Ac)Lys-AMC as substrate after 90 mins by fluorescence based micro plate assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112338
BindingDB Entry DOI: 10.7270/Q2VD7363 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data