 Found 55 hits with Last Name = 'ramajayam' and Initial = 'r'
Found 55 hits with Last Name = 'ramajayam' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049642
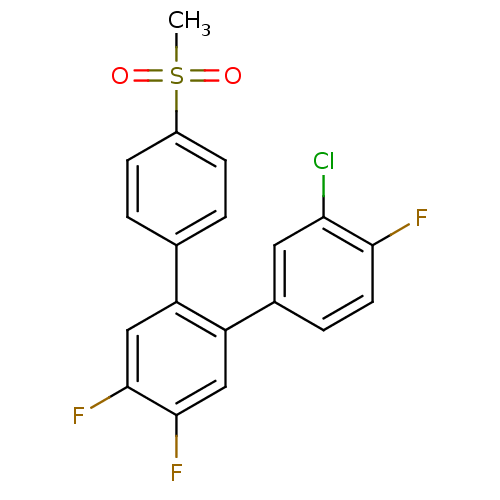
(3-Chloro-4,4',5'-trifluoro-4''-methanesulfonyl-[1,...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C19H12ClF3O2S/c1-26(24,25)13-5-2-11(3-6-13)14-9-18(22)19(23)10-15(14)12-4-7-17(21)16(20)8-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478032
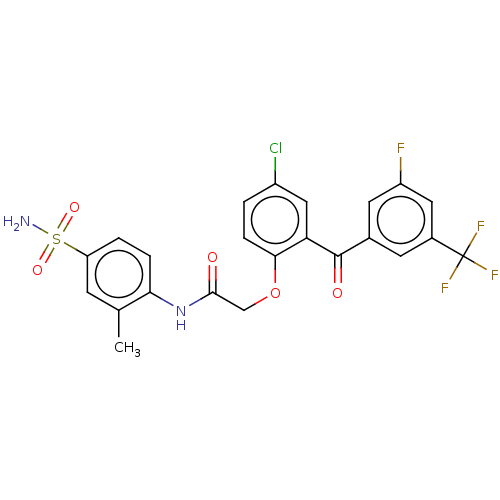
(CHEMBL275658 | GW4511 | GW564511 | GW69564)Show SMILES Cc1cc(ccc1NC(=O)COc1ccc(Cl)cc1C(=O)c1cc(F)cc(c1)C(F)(F)F)S(N)(=O)=O Show InChI InChI=1S/C23H17ClF4N2O5S/c1-12-6-17(36(29,33)34)3-4-19(12)30-21(31)11-35-20-5-2-15(24)10-18(20)22(32)13-7-14(23(26,27)28)9-16(25)8-13/h2-10H,11H2,1H3,(H,30,31)(H2,29,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of wild-type HIV1 reverse transcriptase |
Bioorg Med Chem 17: 5744-62 (2009)
Article DOI: 10.1016/j.bmc.2009.06.060
BindingDB Entry DOI: 10.7270/Q20G3NXJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50288291
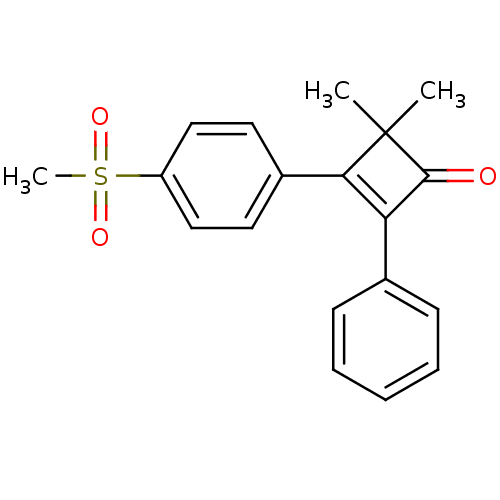
(3-(4-Methanesulfonyl-phenyl)-4,4-dimethyl-2-phenyl...)Show SMILES CC1(C)C(=O)C(=C1c1ccc(cc1)S(C)(=O)=O)c1ccccc1 |c:5| Show InChI InChI=1S/C19H18O3S/c1-19(2)17(14-9-11-15(12-10-14)23(3,21)22)16(18(19)20)13-7-5-4-6-8-13/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in human whole blood |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50289328

(5-(4-Methanesulfonyl-phenyl)-6-phenyl-2-trifluorom...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1sc2nc(nn2c1-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C18H12F3N3O2S2/c1-28(25,26)13-9-7-12(8-10-13)15-14(11-5-3-2-4-6-11)24-17(27-15)22-16(23-24)18(19,20)21/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in CHO-K1 cells using arachidonic acid as substrate preincubated for 15 mins followed by substrate ad... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Mus musculus) | BDBM50474760
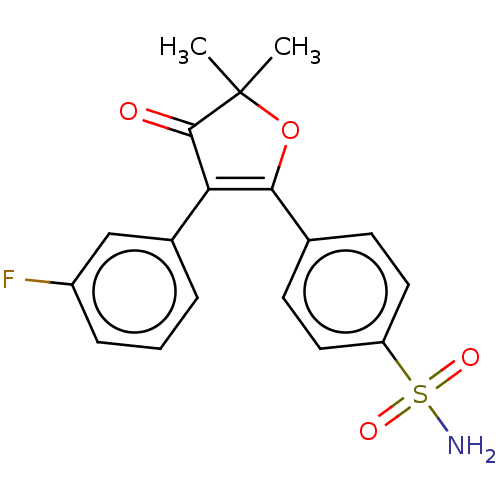
(CG-100649 | CG100649 | Polmacoxib)Show SMILES CC1(C)OC(=C(C1=O)c1cccc(F)c1)c1ccc(cc1)S(N)(=O)=O |c:4| Show InChI InChI=1S/C18H16FNO4S/c1-18(2)17(21)15(12-4-3-5-13(19)10-12)16(24-18)11-6-8-14(9-7-11)25(20,22)23/h3-10H,1-2H3,(H2,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-1 in LPS-induced mouse peritoneal macrophages |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50153982
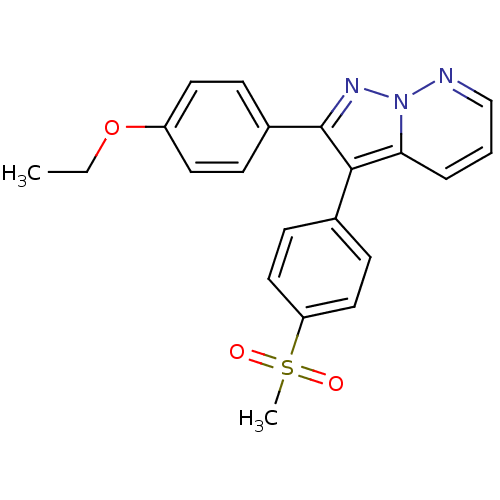
(2-(4-Ethoxy-phenyl)-3-(4-methanesulfonyl-phenyl)-p...)Show SMILES CCOc1ccc(cc1)-c1nn2ncccc2c1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C21H19N3O3S/c1-3-27-17-10-6-16(7-11-17)21-20(19-5-4-14-22-24(19)23-21)15-8-12-18(13-9-15)28(2,25)26/h4-14H,3H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human COX-2 expressed in human COS cells using arachidonic acid as substrate |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50131593
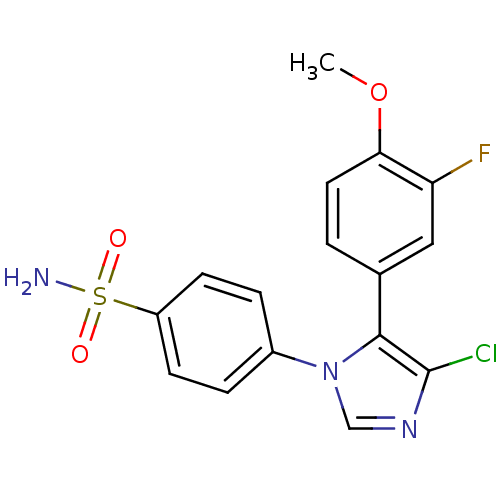
(4-[4-Chloro-5-(3-fluoro-4-methoxy-phenyl)-imidazol...)Show SMILES COc1ccc(cc1F)-c1c(Cl)ncn1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H13ClFN3O3S/c1-24-14-7-2-10(8-13(14)18)15-16(17)20-9-21(15)11-3-5-12(6-4-11)25(19,22)23/h2-9H,1H3,(H2,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in human 143982 cells |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049041
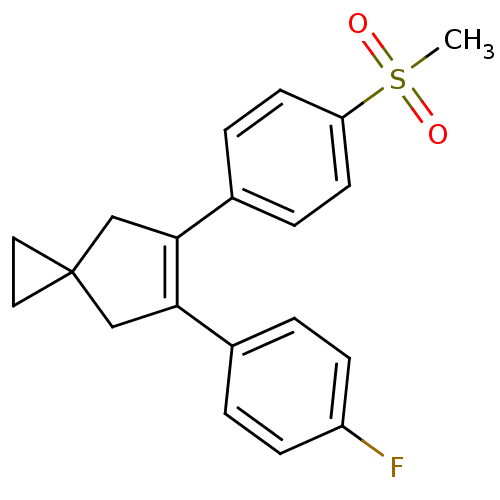
(5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC2(CC2)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C20H19FO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478032

(CHEMBL275658 | GW4511 | GW564511 | GW69564)Show SMILES Cc1cc(ccc1NC(=O)COc1ccc(Cl)cc1C(=O)c1cc(F)cc(c1)C(F)(F)F)S(N)(=O)=O Show InChI InChI=1S/C23H17ClF4N2O5S/c1-12-6-17(36(29,33)34)3-4-19(12)30-21(31)11-35-20-5-2-15(24)10-18(20)22(32)13-7-14(23(26,27)28)9-16(25)8-13/h2-10H,11H2,1H3,(H,30,31)(H2,29,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase Y181C mutant |
Bioorg Med Chem 17: 5744-62 (2009)
Article DOI: 10.1016/j.bmc.2009.06.060
BindingDB Entry DOI: 10.7270/Q20G3NXJ |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50478032

(CHEMBL275658 | GW4511 | GW564511 | GW69564)Show SMILES Cc1cc(ccc1NC(=O)COc1ccc(Cl)cc1C(=O)c1cc(F)cc(c1)C(F)(F)F)S(N)(=O)=O Show InChI InChI=1S/C23H17ClF4N2O5S/c1-12-6-17(36(29,33)34)3-4-19(12)30-21(31)11-35-20-5-2-15(24)10-18(20)22(32)13-7-14(23(26,27)28)9-16(25)8-13/h2-10H,11H2,1H3,(H,30,31)(H2,29,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase K103N mutant |
Bioorg Med Chem 17: 5744-62 (2009)
Article DOI: 10.1016/j.bmc.2009.06.060
BindingDB Entry DOI: 10.7270/Q20G3NXJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50507137
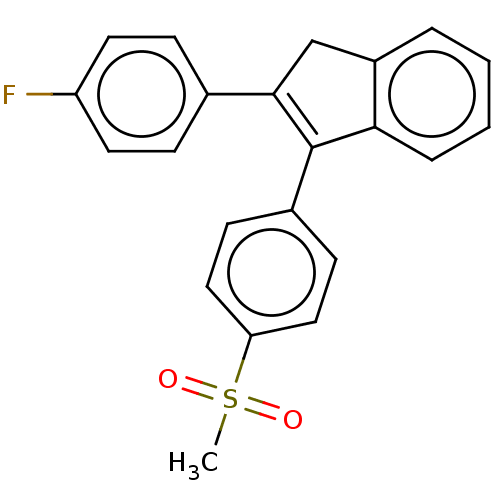
(CHEMBL4533150)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(Cc2ccccc12)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C22H17FO2S/c1-26(24,25)19-12-8-16(9-13-19)22-20-5-3-2-4-17(20)14-21(22)15-6-10-18(23)11-7-15/h2-13H,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50102311
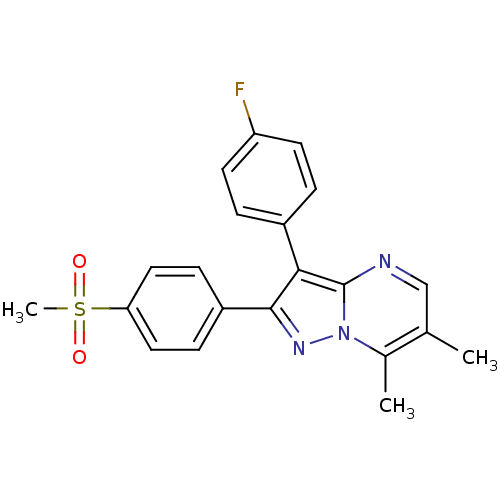
(3-(4-Fluoro-phenyl)-2-(4-methanesulfonyl-phenyl)-6...)Show SMILES Cc1cnc2c(c(nn2c1C)-c1ccc(cc1)S(C)(=O)=O)-c1ccc(F)cc1 Show InChI InChI=1S/C21H18FN3O2S/c1-13-12-23-21-19(15-4-8-17(22)9-5-15)20(24-25(21)14(13)2)16-6-10-18(11-7-16)28(3,26)27/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in human 143982 cells using arachidonic acid as substrate preincubated for 15 mins followed by substrate addition and measured af... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029600
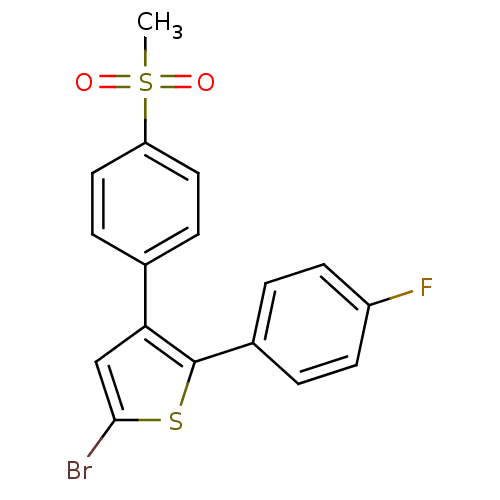
(5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(Br)sc1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-16(18)22-17(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50289303
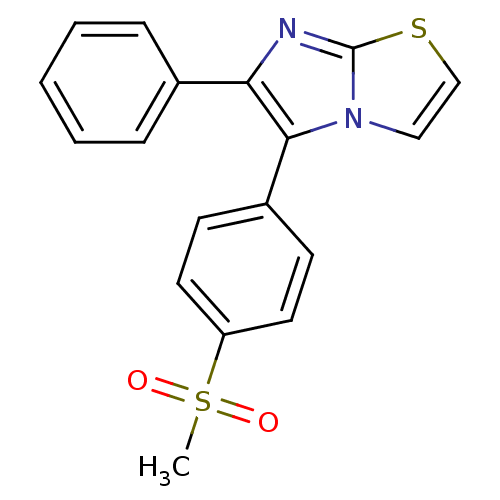
(5-(4-Methanesulfonyl-phenyl)-6-phenyl-3,5-dihydro-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1c(nc2sccn12)-c1ccccc1 Show InChI InChI=1S/C18H14N2O2S2/c1-24(21,22)15-9-7-14(8-10-15)17-16(13-5-3-2-4-6-13)19-18-20(17)11-12-23-18/h2-12H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human COX-2 transfected in CHO cells using arachidonic acid as substrate |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50507138
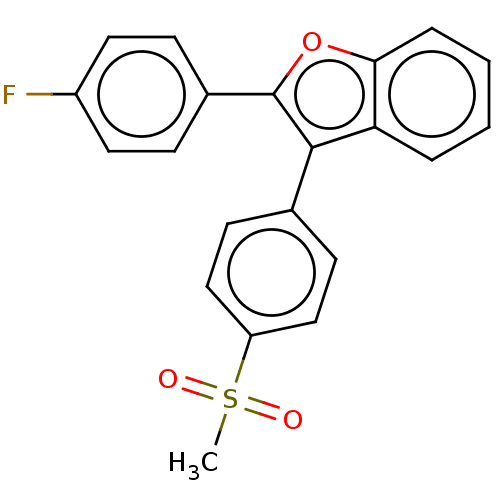
(CHEMBL4473838)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1c(oc2ccccc12)-c1ccc(F)cc1 Show InChI InChI=1S/C21H15FO3S/c1-26(23,24)17-12-8-14(9-13-17)20-18-4-2-3-5-19(18)25-21(20)15-6-10-16(22)11-7-15/h2-13H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049026

(5-(4-Fluoro-phenyl)-6-(4-methanesulfonyl-phenyl)-s...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=CC2(CC2)C=C1c1ccc(F)cc1 |c:17,t:11| Show InChI InChI=1S/C20H17FO2S/c1-24(22,23)17-8-4-15(5-9-17)19-13-20(10-11-20)12-18(19)14-2-6-16(21)7-3-14/h2-9,12-13H,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029614
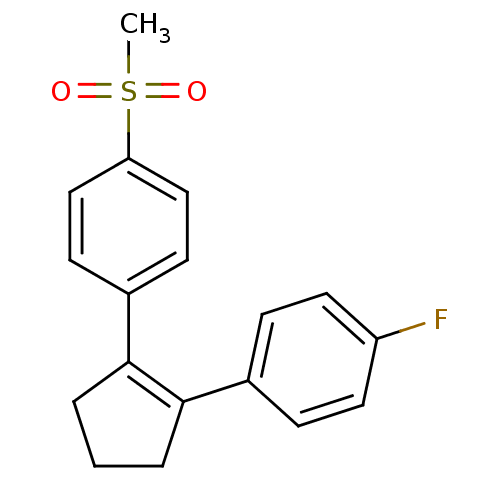
((SC-57666)1-[2-(4-fluorophenyl)-1-cyclopentenyl]-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C18H17FO2S/c1-22(20,21)16-11-7-14(8-12-16)18-4-2-3-17(18)13-5-9-15(19)10-6-13/h5-12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50103314
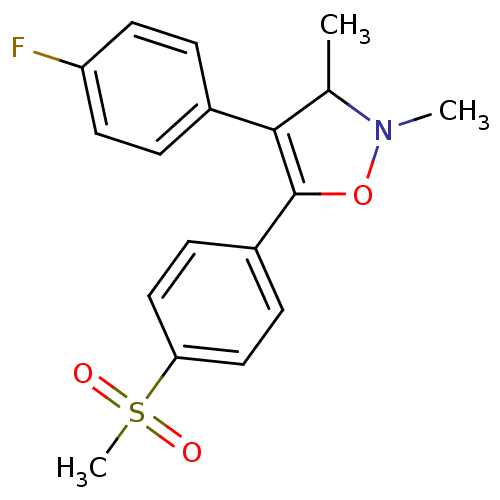
(4-(4-Fluoro-phenyl)-5-(4-methanesulfonyl-phenyl)-2...)Show SMILES CC1N(C)OC(=C1c1ccc(F)cc1)c1ccc(cc1)S(C)(=O)=O |c:5| Show InChI InChI=1S/C18H18FNO3S/c1-12-17(13-4-8-15(19)9-5-13)18(23-20(12)2)14-6-10-16(11-7-14)24(3,21)22/h4-12H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-2 using arachidonic acid as substrate by EIA |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50076873
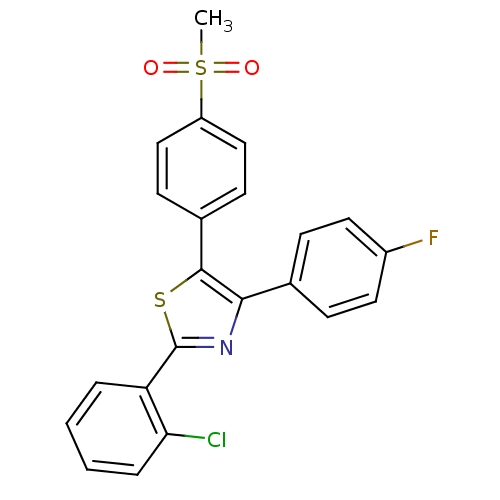
(2-(2-Chloro-phenyl)-4-(4-fluoro-phenyl)-5-(4-metha...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1sc(nc1-c1ccc(F)cc1)-c1ccccc1Cl Show InChI InChI=1S/C22H15ClFNO2S2/c1-29(26,27)17-12-8-15(9-13-17)21-20(14-6-10-16(24)11-7-14)25-22(28-21)18-4-2-3-5-19(18)23/h2-13H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human COX-2 |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50286048
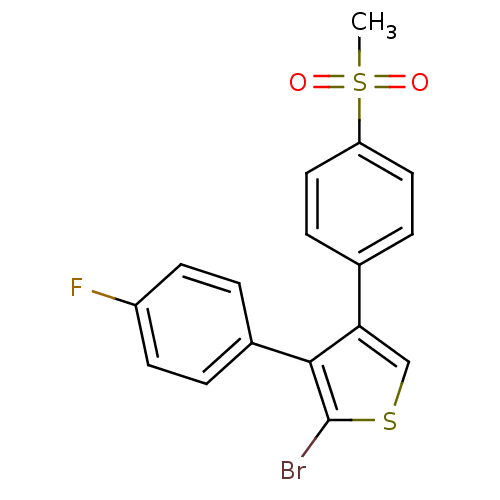
(2-Bromo-3-(4-fluoro-phenyl)-4-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1csc(Br)c1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-22-17(18)16(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50286048

(2-Bromo-3-(4-fluoro-phenyl)-4-(4-methanesulfonyl-p...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1csc(Br)c1-c1ccc(F)cc1 Show InChI InChI=1S/C17H12BrFO2S2/c1-23(20,21)14-8-4-11(5-9-14)15-10-22-17(18)16(15)12-2-6-13(19)7-3-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human COX-2 transfected in CHO cells using arachidonic acid as substrate |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50125624

(3-(4-Chloro-phenyl)-5-hydroxy-4-(4-methanesulfonyl...)Show SMILES CC1(O)OC(=O)C(=C1c1ccc(cc1)S(C)(=O)=O)c1ccc(Cl)cc1 |c:6| Show InChI InChI=1S/C18H15ClO5S/c1-18(21)16(12-5-9-14(10-6-12)25(2,22)23)15(17(20)24-18)11-3-7-13(19)8-4-11/h3-10,21H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) expressed in CHO cells |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50091181

(4-[2-(4-Methyl-thiazol-2-yl)-4-trifluoromethyl-imi...)Show SMILES Cc1csc(n1)-c1nc(cn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C14H11F3N4O2S2/c1-8-7-24-13(19-8)12-20-11(14(15,16)17)6-21(12)9-2-4-10(5-3-9)25(18,22)23/h2-7H,1H3,(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50103510
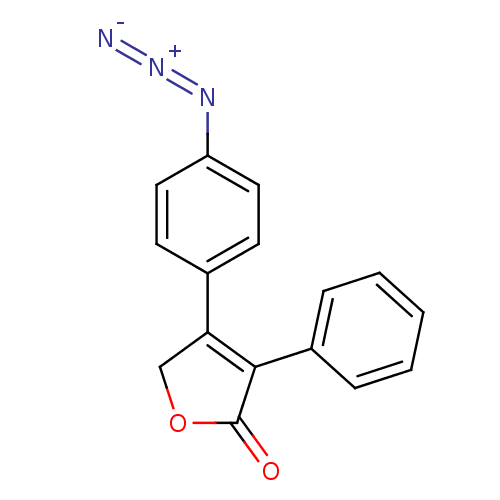
(4-(4-Azido-phenyl)-3-phenyl-5H-furan-2-one | CHEMB...)Show SMILES [N-]=[N+]=Nc1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:10| Show InChI InChI=1S/C16H11N3O2/c17-19-18-13-8-6-11(7-9-13)14-10-21-16(20)15(14)12-4-2-1-3-5-12/h1-9H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-2 |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in human whole blood |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50075664
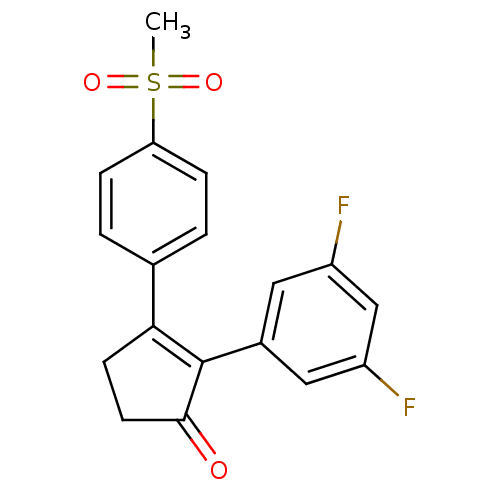
(2-(3,5-Difluoro-phenyl)-3-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)CC1)c1cc(F)cc(F)c1 |t:11| Show InChI InChI=1S/C18H14F2O3S/c1-24(22,23)15-4-2-11(3-5-15)16-6-7-17(21)18(16)12-8-13(19)10-14(20)9-12/h2-5,8-10H,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in human whole blood |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50150218
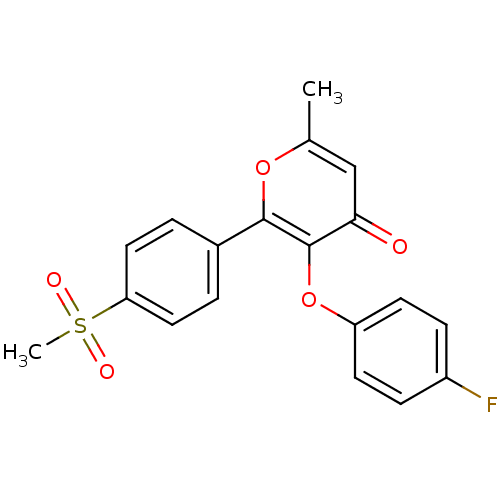
(3-(4-Fluoro-phenoxy)-2-(4-methanesulfonyl-phenyl)-...)Show SMILES Cc1cc(=O)c(Oc2ccc(F)cc2)c(o1)-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C19H15FO5S/c1-12-11-17(21)19(25-15-7-5-14(20)6-8-15)18(24-12)13-3-9-16(10-4-13)26(2,22)23/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 (unknown origin) |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50129503
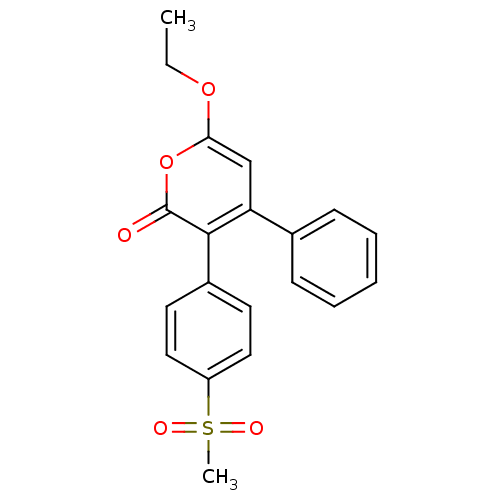
(6-Ethoxy-3-(4-methanesulfonyl-phenyl)-4-phenyl-pyr...)Show SMILES CCOc1cc(-c2ccccc2)c(-c2ccc(cc2)S(C)(=O)=O)c(=O)o1 Show InChI InChI=1S/C20H18O5S/c1-3-24-18-13-17(14-7-5-4-6-8-14)19(20(21)25-18)15-9-11-16(12-10-15)26(2,22)23/h4-13H,3H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-2 using arachidonic acid as substrate preincubated for 2 mins followed by substrate addition and measured after 2 mins by EIA |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50084361

(4-[3-(4-Fluoro-phenyl)-2-oxo-2,3-dihydro-oxazol-4-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1coc(=O)n1-c1ccc(F)cc1 Show InChI InChI=1S/C15H11FN2O4S/c16-11-3-5-12(6-4-11)18-14(9-22-15(18)19)10-1-7-13(8-2-10)23(17,20)21/h1-9H,(H2,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in LPS-stimulated human whole blood after 24 hrs by ELISA |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50103509

(1-(4-Azido-phenyl)-5-p-tolyl-3-trifluoromethyl-1H-...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)N=[N+]=[N-])C(F)(F)F Show InChI InChI=1S/C17H12F3N5/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)23-25(15)14-8-6-13(7-9-14)22-24-21/h2-10H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-2 |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50091171

(3-[1-(4-Methanesulfonyl-phenyl)-4-trifluoromethyl-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1cc(nc1-c1cccnc1)C(F)(F)F Show InChI InChI=1S/C16H12F3N3O2S/c1-25(23,24)13-6-4-12(5-7-13)22-10-14(16(17,18)19)21-15(22)11-3-2-8-20-9-11/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50483060
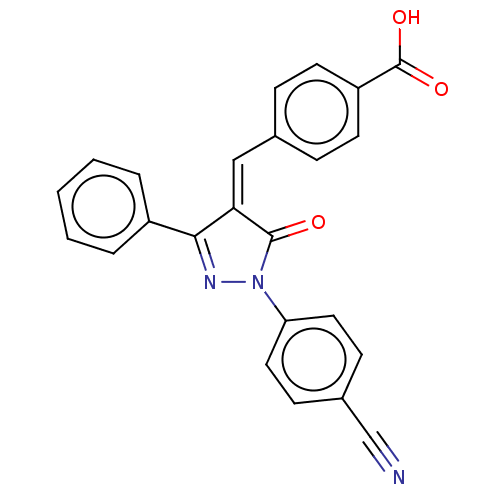
(CHEMBL1277046 | acs.jmedchem.1c00409_ST.374)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2ccc(cc2)C#N)cc1 |c:12| Show InChI InChI=1S/C24H15N3O3/c25-15-17-8-12-20(13-9-17)27-23(28)21(22(26-27)18-4-2-1-3-5-18)14-16-6-10-19(11-7-16)24(29)30/h1-14H,(H,29,30)/b21-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of SARS coronavirus 3C-like protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50483064
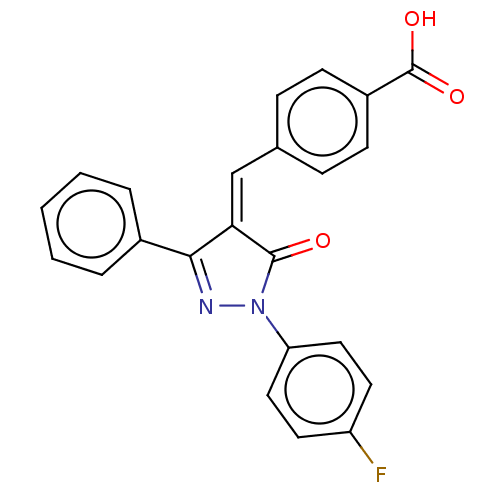
(CHEMBL1277227)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2ccc(F)cc2)cc1 |c:12| Show InChI InChI=1S/C23H15FN2O3/c24-18-10-12-19(13-11-18)26-22(27)20(21(25-26)16-4-2-1-3-5-16)14-15-6-8-17(9-7-15)23(28)29/h1-14H,(H,28,29)/b20-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of SARS coronavirus 3C-like protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50483065

(CHEMBL1277228)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2cccc(c2)[N+]([O-])=O)cc1 |c:12| Show InChI InChI=1S/C23H15N3O5/c27-22-20(13-15-9-11-17(12-10-15)23(28)29)21(16-5-2-1-3-6-16)24-25(22)18-7-4-8-19(14-18)26(30)31/h1-14H,(H,28,29)/b20-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of SARS coronavirus 3C-like protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Coxsackievirus B3 (strain Nancy)) | BDBM50483065

(CHEMBL1277228)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2cccc(c2)[N+]([O-])=O)cc1 |c:12| Show InChI InChI=1S/C23H15N3O5/c27-22-20(13-15-9-11-17(12-10-15)23(28)29)21(16-5-2-1-3-6-16)24-25(22)18-7-4-8-19(14-18)26(30)31/h1-14H,(H,28,29)/b20-13- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of coxsackievirus B3 3C protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057877

(4-[2-(4-Fluoro-phenyl)-pyrrol-1-yl]-benzenesulfona...)Show InChI InChI=1S/C16H13FN2O2S/c17-13-5-3-12(4-6-13)16-2-1-11-19(16)14-7-9-15(10-8-14)22(18,20)21/h1-11H,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX-2 expressed in Baculovirus infected sf9 cells using arachidonic acid as substrate preincubated for 10 mins follow... |
Eur J Med Chem 162: 1-17 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.054
BindingDB Entry DOI: 10.7270/Q2FX7DSW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50483062
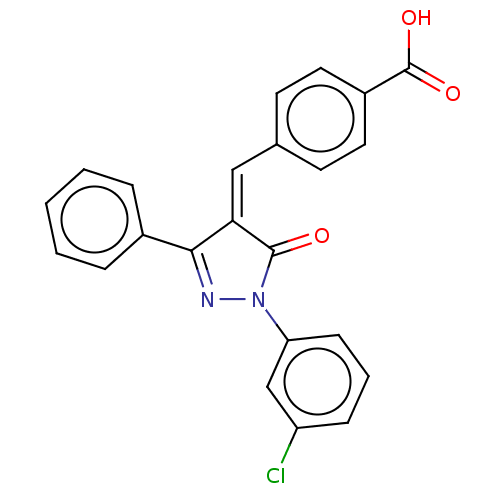
(CHEMBL1277135)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2cccc(Cl)c2)cc1 |c:12| Show InChI InChI=1S/C23H15ClN2O3/c24-18-7-4-8-19(14-18)26-22(27)20(21(25-26)16-5-2-1-3-6-16)13-15-9-11-17(12-10-15)23(28)29/h1-14H,(H,28,29)/b20-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of SARS coronavirus 3C-like protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50483059
(CHEMBL1276871)Show SMILES COc1ccc(cc1)N1N=C(\C(=C\c2ccc(cc2)C(O)=O)C1=O)c1ccccc1 |c:10| Show InChI InChI=1S/C24H18N2O4/c1-30-20-13-11-19(12-14-20)26-23(27)21(22(25-26)17-5-3-2-4-6-17)15-16-7-9-18(10-8-16)24(28)29/h2-15H,1H3,(H,28,29)/b21-15- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of SARS coronavirus 3C-like protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
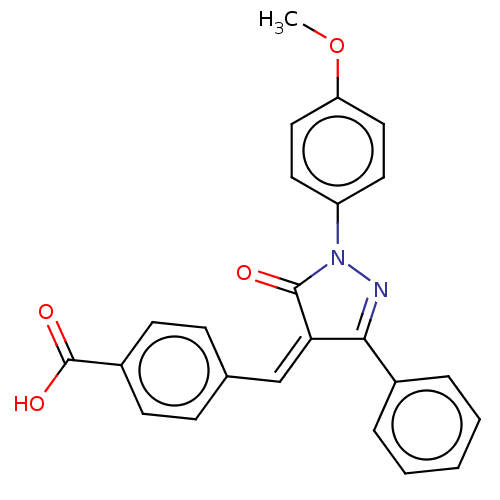
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50483058

(CHEMBL1278125)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2ccc(Cl)cc2)cc1 |c:12| Show InChI InChI=1S/C23H15ClN2O3/c24-18-10-12-19(13-11-18)26-22(27)20(21(25-26)16-4-2-1-3-5-16)14-15-6-8-17(9-7-15)23(28)29/h1-14H,(H,28,29)/b20-14- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of SARS coronavirus 3C-like protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Coxsackievirus B3 (strain Nancy)) | BDBM50483059

(CHEMBL1276871)Show SMILES COc1ccc(cc1)N1N=C(\C(=C\c2ccc(cc2)C(O)=O)C1=O)c1ccccc1 |c:10| Show InChI InChI=1S/C24H18N2O4/c1-30-20-13-11-19(12-14-20)26-23(27)21(22(25-26)17-5-3-2-4-6-17)15-16-7-9-18(10-8-16)24(28)29/h2-15H,1H3,(H,28,29)/b21-15- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of coxsackievirus B3 3C protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Coxsackievirus B3 (strain Nancy)) | BDBM50483062

(CHEMBL1277135)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2cccc(Cl)c2)cc1 |c:12| Show InChI InChI=1S/C23H15ClN2O3/c24-18-7-4-8-19(14-18)26-22(27)20(21(25-26)16-5-2-1-3-6-16)13-15-9-11-17(12-10-15)23(28)29/h1-14H,(H,28,29)/b20-13- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of coxsackievirus B3 3C protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50483057
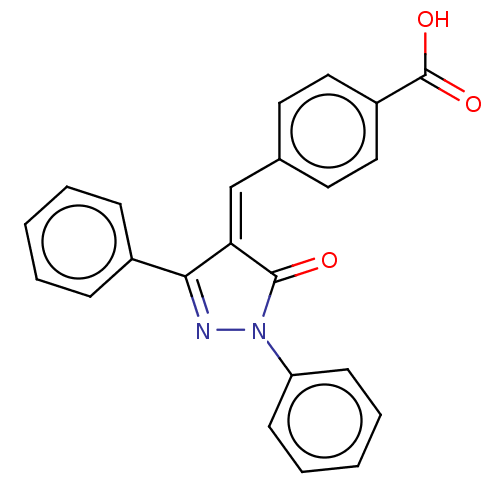
(CHEMBL1277944)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2ccccc2)cc1 |c:12| Show InChI InChI=1S/C23H16N2O3/c26-22-20(15-16-11-13-18(14-12-16)23(27)28)21(17-7-3-1-4-8-17)24-25(22)19-9-5-2-6-10-19/h1-15H,(H,27,28)/b20-15- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of SARS coronavirus 3C-like protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Coxsackievirus B3 (strain Nancy)) | BDBM50483060

(CHEMBL1277046 | acs.jmedchem.1c00409_ST.374)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2ccc(cc2)C#N)cc1 |c:12| Show InChI InChI=1S/C24H15N3O3/c25-15-17-8-12-20(13-9-17)27-23(28)21(22(26-27)18-4-2-1-3-5-18)14-16-6-10-19(11-7-16)24(29)30/h1-14H,(H,29,30)/b21-14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of coxsackievirus B3 3C protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50470153
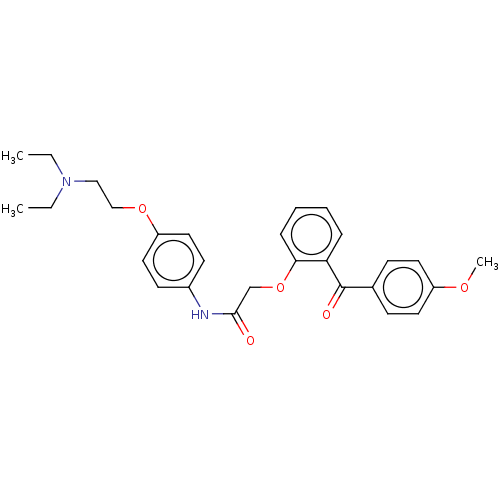
(CHEMBL415908)Show SMILES CCN(CC)CCOc1ccc(NC(=O)COc2ccccc2C(=O)c2ccc(OC)cc2)cc1 Show InChI InChI=1S/C28H32N2O5/c1-4-30(5-2)18-19-34-24-16-12-22(13-17-24)29-27(31)20-35-26-9-7-6-8-25(26)28(32)21-10-14-23(33-3)15-11-21/h6-17H,4-5,18-20H2,1-3H3,(H,29,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
Bioorg Med Chem 17: 5744-62 (2009)
Article DOI: 10.1016/j.bmc.2009.06.060
BindingDB Entry DOI: 10.7270/Q20G3NXJ |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Coxsackievirus B3 (strain Nancy)) | BDBM50483064

(CHEMBL1277227)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2ccc(F)cc2)cc1 |c:12| Show InChI InChI=1S/C23H15FN2O3/c24-18-10-12-19(13-11-18)26-22(27)20(21(25-26)16-4-2-1-3-5-16)14-15-6-8-17(9-7-15)23(28)29/h1-14H,(H,28,29)/b20-14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of coxsackievirus B3 3C protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50483063

(CHEMBL1277136)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2ccc(Cl)c(Cl)c2)cc1 |c:12| Show InChI InChI=1S/C23H14Cl2N2O3/c24-19-11-10-17(13-20(19)25)27-22(28)18(21(26-27)15-4-2-1-3-5-15)12-14-6-8-16(9-7-14)23(29)30/h1-13H,(H,29,30)/b18-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of SARS coronavirus 3C-like protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Coxsackievirus B3 (strain Nancy)) | BDBM50483058

(CHEMBL1278125)Show SMILES OC(=O)c1ccc(\C=C2/C(=O)N(N=C2c2ccccc2)c2ccc(Cl)cc2)cc1 |c:12| Show InChI InChI=1S/C23H15ClN2O3/c24-18-10-12-19(13-11-18)26-22(27)20(21(25-26)16-4-2-1-3-5-16)14-15-6-8-17(9-7-15)23(28)29/h1-14H,(H,28,29)/b20-14- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Academia Sinica
Curated by ChEMBL
| Assay Description
Inhibition of coxsackievirus B3 3C protease by fluorescence plate reader analysis |
Bioorg Med Chem 18: 7849-54 (2010)
Article DOI: 10.1016/j.bmc.2010.09.050
BindingDB Entry DOI: 10.7270/Q2GQ71KW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data